
Physiologic Testing at Discharge Improves Chronic Lung Disease Classification in Very Low Birth weight Infants
*Corresponding Author(s):
Dale R GerstmannDepartment Of Neonatology, Timpanogos Regional Hospital, Orem, Utah, United States
Tel:801-369-6129,
Email:dale.gerstmann@pediatrix.com
Abstract
Background
Classification of Chronic Lung Disease (CLD) based on oxygen need at 36 weeks post-menstrual age has been the de facto standard for designating neonatal pulmonary morbidity in Very Low Birth Weight (VLBW) infants. Physiologic measurements to improve this designation have been promoted but infrequently implemented. The hypothesis for this study was that physiologic testing at discharge using a modified hypoxia altitude simulation test would improve identification of ongoing pulmonary dysfunction in VLBW infants.
Methods
A query was performed on our facility’s Vermont Oxford dataset for VLBW infants discharged home from January 2012 through August 2021. Patient demographic information and VON CLD classification were obtained from the VON dataset; discharge physiologic tests and laboratory results were retrieved from patients’ electronic records. The frequency of CLD classification was then compared to physiologic testing at discharge.
Results
134 VLBW patients met query criteria. By VON definition, 69.4% (93/134) were classified as having CLD. By physiologic testing at discharge, 97.8% (131/134) were breathing at or below sea-level equivalent partial pressure of oxygen (160 mmHg).
Conclusion
Physiologic testing at discharge was consistent with sea-level equivalent oxygenation in nearly all infants. Oxygen need at 36 weeks over-designates the classification of CLD.
Keywords
Bronchopulmonary dysplasia; Chronic lung disease; Infant; Lung diseases; Morbidity; Newborn; Oxygen; Patient discharge; Partial pressure; Very low birth weight.
Abbreviations
GA: Gestational Age
CLD: Chronic Lung Disease
NPM: Neonatal Pulmonary Morbidity
NICU: Neonatal Intensive Care Unit
VON: Vermont Oxford Network
VON VLBW: Very Low Birth Weight 401-1500g or gestational age 22-29 weeks
PiO2: Partial pressure of inspired oxygen
HCT: Hematocrit
WT: Weight
SpO2: Pulse oximeter oxygen saturation
FiO2: Fractional inspired oxygen concentration;
RDS: Respiratory distress syndrome
HAST: Hypoxia altitude simulation test
Introduction
Different methods have been used to classify Neonatal Pulmonary Morbidity (NPM). From the early 1960s to the present, this evolution is well detailed in a recent article by Ibrahim and Bhandariby [1]. For the past 30 years, oxygen need at 36 weeks (O2 at 36wk) has been the accepted clinical definition for neonatal Chronic Lung Disease (CLD) [1]. This definition has routinely been used by the Vermont Oxford Network (VON) for outcomes reporting [2]. Ballard, Walsh, and others have championed using physiologic measurement in the form of an oxygen withdrawal protocol to better identify pulmonary impairment [3-5]. This practice has not been widely accepted. The Neonatal Research Network recently proposed a stratified classification for NPM based only on degrees of respiratory support [6]. However; even this new definition did not consider three factors directly relevant to VLBW infants in our NICU: altitude correction, use of micro-flow nasal oxygen, and physiologic testing. The hypothesis for this study was that classification of NPM at discharge using an evaluation that included these factors would provide a more accurate assessment of ongoing pulmonary impairment.
Methods
Following hospital IRB study approval, a query was performed on our nursery’s VON dataset to identify VLBW infants admitted to our NICU from January 2012 through August 2021 and discharged to home. The VON definition for VLBW was used: birthweight 401-1500g or gestational age 22-29 weeks. This list was then used to query patients’ electronic medical records. Data retrieved were birth and discharge weight (WT), Gestational Age (GA) at birth and discharge, hematocrit (HCT) within two weeks of discharge, and O2 at 36wk (CLD) status by VON.
Our physiologic testing procedure is an adaptation of the Hypoxia Altitude Simulation Test (HAST) [7-8]. Using local altitude as the hypoxia challenge. An ambient room air breathing test is performed 48-72 hours before discharge. If the infant cannot maintain oxygen saturation (SpO2 >89%), the infant is placed in an oxygen hood with 10 LPM flow. Hood oxygen concentration is titrated to achieve infant SpO2 92-95%. Using this titrated FiO2, the infant’s partial pressure of inspired oxygen (PiO2) is calculated utilizing the station pressure at our NICU location (645 mmHg at 4774 ft altitude). If oxygen is needed to maintain SpO2 within this range, nasal cannula micro-flow oxygen is provided at discharge (100% O2 at flow < 0.10 LPM). The physiologic test process is recorded on a report form and then included in the infant’s medical record. These data were recovered from the electronic record and tabulated for analysis.
Each infant's discharge PiO2 was graphically compared to their O2 at 36wk status with reference to sea level equivalent oxygen partial pressure. In addition, birth and discharge EGA and WT and discharge HCT were compared by Student’s T-test for infants who did or did not need micro-flow oxygen at discharge. Values are shown as mean ± standard deviation.
Results
The query identified 134 VLBW infants whose birth GA and WT were 29.8 ± 2.3wk and 1.20 ± 0.25kg, respectively. By O2 at 36wk criteria, 69.4% (93/134) were classified as having CLD. At discharge, 26.1% (35/134) needed micro-flow oxygen support at 0.03 ± 0.02 LPM. The measured PiO2 for the study group was 148 ± 8 mmHg. PiO2 vs. O2 at 36wk is shown. The percentage of infants breathing at or below sea-level equivalent oxygen partial pressure (160 mmHg) was 97.8% (131/134).
There was no difference in discharge GA or HCT based on whether micro-flow oxygen was or was not needed, namely 38.9 ± 1.8wk vs. 38.8 ± 1.7wk and 32.4 ± 3.2% vs. 32.7 ± 4.4%, respectively. However, birth GA and WT differed significantly, 28.7±2.6wk vs. 30.1±2.1wk (p < 0.001) and 1.07 ± 0.25kg vs. 1.27 ± 0.23kg (p=0.002), respectively. Three infants required discharge FiO2 greater than 21% but less than 23% (sea level equivalent). Using O2 at 36wk as criteria for assigning CLD over-designated NPM by a factor of 31 (93/3) as shown in (figure 1).
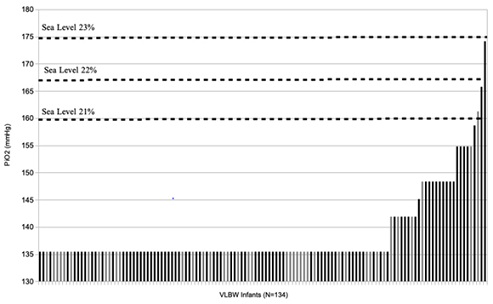 Figure 1: PiO2 at Discharge in VLBW Infants - PiO2 by physiologic testing at discharge is shown for each infant (N=134), ordered left to right. Black bars indicate the infant was assigned as having CLD by O2 at 36wk (VON). Corresponding FiO2 at sea level is shown for reference.
Figure 1: PiO2 at Discharge in VLBW Infants - PiO2 by physiologic testing at discharge is shown for each infant (N=134), ordered left to right. Black bars indicate the infant was assigned as having CLD by O2 at 36wk (VON). Corresponding FiO2 at sea level is shown for reference.
Discussion
In this single site retrospective cohort of VLBW infants, the O2 at 36wk classification for CLD did not correspond to our results by physiologic testing at discharge. Though 68% of the study group were labeled as having CLD using the O2 at 36wk criteria, 98% had normal oxygen saturation readings at discharge while breathing PiO2 at or below 160 mmHg. We have previously reported that premature infants treated for RDS breathing oxygen at or below 160 mmHg PiO2 had normal pulmonary function indices at five years of age as measured by whole-body plethysmography [9].Thus, the likelihood of chronic pulmonary impairment in the current cohort of VLBW infants is low.
Though discharge GA and HCT were not different, infants discharged with micro-flow were slightly smaller and more premature at birth. In the normal fetus, saccular lung development continues up to 38 weeks as alveolarization begins around 36 weeks [10]. Using the time point of 36 weeks for NPM assessment may be too early to reflect any alveolarization failure due to lung injury. In addition, late initiation of alveolarization, whether from maturational arrest [11] or delay [12] also makes evaluating NPM after 36 weeks a potentially better strategy for improving estimates of NPM. Those more immature infants may need additional time to progress through their pulmonary maturational potential, which may explain the more frequent need for discharge micro-flow oxygen at PiO2 < 160 mmHg in those younger infants in the current cohort. It is also possible that those infants in the present study with measured PiO2 between 135-160 mmHg may have a mild residual pulmonary impairment identifiable through this type of physiologic testing. Though not part of the current study, serial testing after discharge would discriminate between delayed pulmonary maturation and chronic or static pulmonary morbidity.
The ability of the lung to transport oxygen is a primary pulmonary function, dependent on alveolar number and volume, alveolar architecture, ventilation-perfusion matching, blood-oxygen characteristics, end-expiratory volumes, and respiration. A hypoxia test evaluates this entire complex. If done to find the minimum PiO2 which allows normal and stable blood oxygen saturation, the test can provide a measurable entity of lung oxygenation capacity. The HAST is typically performed at a PiO2 to simulate a flight cabin altitude of 8000-10000 feet, thus identifying persons who may need supplemental oxygen to prevent desaturation during a commercial airline flight. We have performed this test at discharge in our NICU to give parents who reside out-of-state the choice of returning home by air or by automobile. Located in the Rocky Mountains, we have also used this test to decide whether an infant needs supplemental oxygen as parents drive home through high mountain passes ranging up to 11,000 feet. Using a variable PiO2, that is, titrating the oxygen/nitrogen gas ratio during the test, the minimum tolerated PiO2 can be identified and thus the maximum tolerated altitude. As described above, we adapted the HAST to evaluate pulmonary function in VLBW infants at discharge. As a measure of the infant’s total oxygenation capacity, it is a relatively easy pulmonary test to perform by respiratory therapy staff, yielding a measurable description of lung function.
In summary, for this single site cohort of VLBW infants, results of physiologic testing at discharge (38-39 weeks) using a modified hypoxia challenge test did not correlate with CLD assigned using the criteria of O2 at 36wk. The later markedly over-designated NPM in 98% of infants at discharge breathing oxygen with PiO2 at or below 160 mmHg. A modified hypoxia challenge test provides a measurable descriptor for assessing the degree of pulmonary impairment in premature infants at risk for NPM.
Impact
Physiologic measurement improves classification of neonatal pulmonary morbidity. A hypoxia challenge test performs well as a physiologic pulmonary measurement at discharge. Classification of chronic lung disease by oxygen need at 36 weeks post-menstrual age over-designates neonatal pulmonary morbidity.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
The author wishes to express appreciation to the Timpanogos Regional Hospital administration for their support of this project.
Funding
No financial assistance was received in support of this study.
Author Contributions
Dale Gerstmann MD provided the following author contributions: study concept and design; data acquisition, analysis, interpretation; manuscript drafting and revision; final approval for the published version.
Competing Interests
There are no potential conflicts of interest.
Consent Statement
Patient consent was not required for this study.
References
- Ibrahim J, Bhandari V (2018) The definition of Broncho pulmonary dysplasia: An evolving dilemma. Pediatr Res84: 586-588.
- Kim F, Bateman DA, Goldshtrom N, Sahni R, Wung JT, et al. (2021) Revisiting the definition of bronchopulmonary dysplasia in premature infants at a single center quaternary neonatal intensive care unit. J Perinatol 41: 756-763.
- Ballard RA, Keller RL, Black DM, Ballard PL, Merrill JD, et al. (2016) Randomized Trial of Late Surfactant Treatment in Ventilated Preterm Infants Receiving Inhaled Nitric Oxide. J of Pediatrics 168: 23-29.
- Walsh MC, Yao Q, Gettner P, Hale E, Collins M, et al. (2004) Impact of a physiologic definition on bronchopulmonary dysplasia rates. J of Pediatrics 114: 1305-1311.
- Walsh MC, Wilson-Costello D, Zadell A, Newman N, Fanaroff A (2003) Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol 23: 451-456.
- Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, et al. (2019) The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am J Respir Crit Care Med 200: 751-759.
- Dine CJ, Kreider ME (2008) Hypoxia altitude simulation test. Chest 133: 1002-1005.
- Vetter-Laracy S, Osona B, Peña-Zarza JA, Gil JA, Figuerola J (2016) Hypoxia Challenge Testing in Neonates for Fitness to Fly. Pediatrics 137: e20152915.
- Gerstmann DR, Wood K, Miller A, Steffen M, Ogden B, et al. (2001) Childhood outcome after early high-frequency oscillatory ventilation for neonatal respiratory distress syndrome. Pediatrics 108: 617-623.
- Fraga MV, Guttentag S (2012) Lung Development: Embryology, Growth, Maturation, and Developmental Biology.
- Baker CD, Alvira CM (2014) Disrupted lung development and bronchopulmonary dysplasia: Opportunities for lung repair and regeneration. Curr Opin Pediatr 26: 306-314.
- Salaets T, Aertgeerts M, Gie A, Vignero M, de Winter D, et al. (2020) Preterm birth impairs postnatal lung development in the neonatal rabbit model. Respir Res21: 59.
Citation: Gerstmann DR (2022) Physiologic Testing at Discharge Improves Chronic Lung Disease Classification in Very Low Birth weight Infants. J Neonatol Clin Pediatr 10: 99.
Copyright: © 2022 Dale R Gerstmann, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

