
Physiological Effects of Manganese Gluconate and Calcium Combination from Saint-Gervais Mont Blanc Spring Water for Human Keratinocytes Differentiation
Abstract
Alterations of skin barrier function affect quality of life and there is a need to develop dermatological/cosmetic treatments to reinforce or restore it. Inspiring of Hailey-Hailey disease, in which barrier alteration is due to a mutation of a Calcium-transporting protein (ATP2C1), we focused on the role of minerals and more especially those contained in Saint-Gervais Mont Blanc (SGMB) spring water to reinforce barrier function. Objectives: Demonstrate the interest to enrich SGMB spring water with manganese to improve both keratinocytes differentiation and barrier function.
Methods: Effects of treatments on the expression of ATP2C1 and on the expression of key markers in keratinocyte differentiation and barrier function were studied by gene expression analysis on keratinocytes monolayers and also by measuring the protein expression of transglutaminase 1 using in situ immunofluorescence and image analysis in keratinocytes monolayers.
Results: SGMB spring water stimulates transcriptomic expression of key markers involved in keratinocytes differentiation and barrier function while manganese gluconate has no effect. Combination of both dramatically enhances keratinocytes differentiation, in a synergistic way, at both transcriptomic and protein level. None of treatments modulated ATP2C1 expression.
Conclusion: These results highlight the interest to enrich SGMB spring water with manganese to boost keratinocytes differentiation and barrier function.
Keywords
Cell culture; Keratinocyte differentiation; Mineral; Skin barrier;
Introduction
The skin is the most important organ of the body protecting us from external pathogens (Bacteria, fungi, yeast), environmental threats (UV light, chemical) and having also an important role in thermoregulation of the body and the reduction of water loss. The barrier function of the skin is extremely important and is a key player in innate immunity [1]. That is why one of the skin first response after an injury is to restore barrier function [2]. Alterations of skin barrier function are observed in several dermatosis like psoriasis, atopic dermatitis, Hailey-Hailey disease, and the severity of these diseases is associated with the degree of degradation of the barrier function [3-4]. An increase of water loss is also observed with aging, due to skin structure changes as thinning of epidermis and dermis [5]. Environmental stressors (pollution, climatic changes, lifestyle, light exposure, smoke) induce also premature skin aging with alteration of skin barrier function [6]. All these diseases seriously affect the quality of life. These patients may complain of itching or appearance, which may be a social distress to patients’ lives. Thus, there is a need to develop skincare treatments to restore or to reinforce barrier function, which is key for the maintenance of skin health. For some of diseases, barrier alteration is the result of an inflammation status involving or not microbiota [7-8]. Thus, clearance of skin inflammation is often at the center of therapeutic strategy and there is no very efficient solution able to improve directly barrier function [9-11]. We are thus facing to an absence of efficient therapies to treated non inflammatory-induced default of barrier function as observed during skin aging or in the Hailey-Hailey disease. In addition, it is not so clear today how a barrier function default could be a trigger for an inflammatory-induced dermatosis as psoriasis or atopic dermatitis [12]. Thus, even in this case, although a repair of the skin can be observe with anti-inflammatory treatments, a treatment able to improve barrier function could make sense as preventing treatment [13].
In the Hailey-Hailey disease, the alterations of skin integrity (blisters and erosions) are not due to inflammation but due to a default in calcium homeostasis caused by a haploinsufficiency of a Calcium-transporting protein (ATP2C1 for Calcium-transporting ATPase type 2C member 1) resulting of a mutation of ATP2C1 gene [14-16]. ATP2C1 is An Adenosine Triphosphate (ATP)-powered calcium pump, which uses energy from ATP molecules to pump charged calcium atoms (calcium ions) across cell membranes but also manganese. Based on the effect observed in HHD when ATP2C1 is deficient, we hypothesized that inversely, by stimulating ATP2C1 expression/activity we could have a novel way to reinforce barrier function and skin quality. Interestingly, this transporter do not vehicle only calcium but also manganese (Mn2+). If the role of calcium in epidermis differentiation and barrier function genesis is well describe [17], the effect of Mn2+ is less clear. Although some studies show the role of manganese in the skin, none of its effects is linked to barrier function. Manganese is a co-factor of many enzymes in the skin as manganese superoxide dismutase (MnSOD, SOD2) which is the main antioxidant enzyme in the mitochondria. A deficiency of SOD2 is described in aged skin and more particularly in dermal compartment [18]. Additionally, a default of manganese metabolism is associated with abnormal wound healing [19]. Manganese is involved in extracellular matrix synthesis/remodeling by activating prolidase, involved in collagen production and by activating enzymes involved in glycosaminoglycans synthesis such as manganese-activated glycosyltransferase [20,21].
Saint-Gervais Mont-Blanc thermal spring water is naturally strongly mineralized with high rates of calcium and other minerals like manganese, boron and zinc (Table I) and recognized since 1996 by the French National Academy of Medicine for its healing dermatological properties [22]. The thermal center receives patients for the treatment of psoriasis, eczema, burn scars, etc. The composition in minerals of Saint-Gervais Mont Blanc thermal spring water contributes to its benefits. Calcium is well known for being one of the key factors controlling keratinocytes differentiation and the formation of the skin barrier. Boron is known to have benefits on the skin healing process and Chebassier, et al. have presented the stimulatory effect of manganese and boron on keratinocytes migration [23,24]. The benefits of this thermal water and the crenotherapy, treatment developed in Saint-Gervais Mont Blanc thermal center on scar sequelae following burns, had been presented in the review “La Presse Thermale et Climatique” in 1964 [25]. Since, a clinical study had been performed on 106 patients presenting dermatological problems and having undergone a crenotherapy. Each kind of lesion was evaluated with specific and validated scoring systems (The inflammation / Surface / Structure for patients with scars, the Psoriasis Area Severity Index for patients with psoriasis and the Score of Atopic Dermatitis for patients with eczema). Overall, an improvement was noted for at least 98% of patients. All these results show the interest of SGMB spring water for dermatological problems and motivate us to explore its mechanisms of action on the skin barrier repairing and to explore how manganese could improve the efficiency of this spring water. We already know that in vitro SGMB spring water induced keratinocytes differentiation (data not shown) but we assumed that this effect is mainly due to the high content of calcium which is the main mineral compared to other like strontium, boron or manganese (Table 1). As manganese is transported by ATP2C1, like calcium, we hypothesized that it could have a role in keratinocyte differentiation, hence the belief to enrich SGMB spring water with manganese-gluconate. Thus, the objective of this work was first to determine how Mn2+ could affect the expression of ATP2C1 at the transcriptomic level and also to determine, at least, how Mn2+ could enhance calcium efficacy on the induction of keratinocytes differentiation. By this way, we could have some indications on the role of Mn2++ on calcium homeostasis and keratinocytes differentiation and first indications on the interest of Mn2+ in a therapeutic strategy. Beyond HHD, increasing calcium efficacy and increasing barrier function is important for other skin diseases (Wound healing, psoriasis, atopic dermatitis) and also important in cosmetic to prevent skin aging.
Materials and Methods
Actives
Paraformaldehyde (PFA), Triton-X100 and Hoechst 33342 (bis-benzimide) were obtained from Sigma-Aldrich (Merck, Germany). Manganese gluconate and Saint-Gervais Mont Blanc thermal spring water (composition in Table 1) were obtained from the Saint-Gervais Mont Blanc company.
|
pH |
7.8 |
Ammonium (mg/L) |
< 1 |
Cobalt (µg/L) |
< 1 |
|
Conductivity at 20 ° (mS/cm) |
4.95 |
Potassium (mg/L) |
30 |
Copper (µg/L) |
< 2 |
|
Carbonate (HCO3-) (mg/L) |
247 |
Calcium (mg/L) |
234 |
Iron (µg/L) |
< 30 |
|
Chloride (Cl-) (mg/L) |
530 |
Antimony (µg/L) |
< 5 |
Magnesium (mg/L) |
26.8 |
|
Nitrite (NO2-) (mg/L) |
< 1 |
Aluminium (µg/L) |
< 15 |
Manganese (mg/L) |
0.327 |
|
Nitrate (NO3-) (mg/L) |
< 1 |
Arsenic (µg/L) |
< 2 |
Nickel (µg/L) |
< 2 |
|
Sulphate (SO42-) (mg/L) |
1 812 |
Barium (µg/L) |
17 |
Lead (µg/L) |
< 1 |
|
Oxalate (C2O42-) (mg/L) |
< 1 |
Boron (mg/L) |
5.03 |
Selenium (µg/L) |
< 5 |
|
Hydrogen phosphate (HPO42-) (mg/L) |
< 1 |
Cadmium (µg/L) |
< 1 |
Strontium (mg/L) |
8.9 |
|
Sodium (mg/L) |
944 |
Chromium (µg/L) |
< 1 |
Zinc (µg/L) |
57 |
Table 1: Physicochemical analysis of Saint-Gervais Mont Blanc thermal spring water.
Expression of ATP2C1, RTQPCR analysis
Human epidermal keratinocytes were isolated from adult mammary skin obtained from plastic surgery procedures after informed consent and in line with the principles and guidelines of the Declaration of Helsinki (two donors, 18 years old and 41 years old) and cultured in Green medium on a feeder layer of lethally irradiated mouse 3T3-J2 fi-broblasts as described previously [21,22]. The keratinocytes were seeded in 6-well plates (TPP, Switzerland) and cultured until 70% to 80% of confluence. Culture medium was then removed and replaced by assay medium containing or not (Control) 20 µM or 50 µM manganese gluconate (Mn-GLC). Three types of assay medium were used, a first containing no calcium, a second containing 0.8 mM calcium and a third containing 1.5 mM calcium. For this assay medium we used calcium free DMEM powder (MyBioSource, San Diego, USA) dissolved in appropriate proportions of Saint Gervais Thermal Spring Water (SGMB) and deionized water to achieve the expected calcium concentrations. Keratinocytes were then incubated for 24 hours and all the experimental conditions were performed in n=3 and the experiment was performed three times (N=3). After incubation, cells were rinsed with phosphate buffer saline (PBS, Gibco®, Paisley, UK). Ribonucleic Acids (RNA) were extracted using RNeasy Mini (Qiagen, Courtaboeuf, France), and cDNA were prepared with random hexamer priming using SuperScript IV First-Stand Synthesis System (ThermoFisher scientific, Villebon-sur-Yvette, France). The expression of selected genes was measured by real time quantitative polymerase chain reaction (RTQPCR) using an ABI prism 7300 Real Time Detection system (ThermoFisher scientific, Villebon-sur-Yvette, France) and PCR MesaGreen Mastermix (Eurogentec, Seraing, Belgium) and using specific probes ATP2C1 ((5’-3’) forward = CCAGTCGTCTGGGATTGTATTC, reverse = ATTTTCATCTTGTGCCTTGGG), PGK ((5’-3’) forward = GTGTGCCCATGCCTGACA, reverse = TGGGCCTACACAGTCCTTCAA). For each gene, relative expression was normalized to housekeeping genes (PGK) and fold change were calculated form the untreated control. Data are presented as mean +/- standard deviation (sd). Statistical analysis was performed using one-way ANOVA followed by Dunnett’s multiple comparison test.
Keratinocytes differntiation, RTQPCR analysis
Normal human epidermal keratinocytes (NHEK, Lifeline Cell Technology, Frederick, MD, USA) used at passage 4 (P4) were seeded in 48-well plates (Greiner Bio-One, Courtaboeuf, France) and cultured for 48 hours in DermaLife basal medium supplemented with DermaLife K LifeFactors kit (both Lifeline Cell Technology, Frederick, MD, USA), and Penicillin/Streptomycin antibiotics (Gibco, Waltham, MA, USA). Culture medium was replaced after 24 hours of incubation. Keratinocytes were then treated or not (Control) for 24 hours with 10 µg/mL Mn-GLC, or with 12.5% and 25% SGMB spring water, or with the combination 10 µg/mL Mn-GLC + 12.5% SGMB spring water and 10 µg/mL Mn-GLC + 25% SGMB spring water. Final water concentration was harmonized in all conditions by adding the necessary quantity of deionized water to reach a final concentration of 25%. All conditions were performed in triplicate in 2 independent experiments (N=2). After incubation, cells were rinsed with phosphate buffer saline (PBS, Gibco®, Paisley, UK). Ribonucleic Acids (RNA) were extracted using KingFisher Flex and Magmax 96 total RNA isolation kit (both ThermoFisher scientific, Villebon-sur-Yvette, France) and quantified and qualified using a bioanalyseur (LabChip® GX, Perkin Elmer, Villebon-sur-Yvette, France). Reverse transcription was then performed using a QuantiTect Reverse Transcription Kit (Qiagen, Courtaboeuf, France). The expression of selected genes was measured by real time quantitative polymerase chain reaction (RTQPCR) using a LightCylcer® 480 and SybrGreen technology (both Roche LifeScience, Meylan, France) and using specific probes obtained from Qiagen (Courtaboeuf, France): GAPDH (Glyceraldehyde 3-phosphate dehydrogenase; Gene ID:7051; Ref. QT00082320), RPL13A (60S ribosomal protein L13; Gene ID: 23521; Ref. QT00089915), TGM1 (Transglutaminase I; Gene ID:7051; Ref. QT00082320), OCDN (Occludin; Gene ID: 100506658; Ref. QT00081844), TJP1 (Tight junction protein 1; Gene ID:21872; Ref. QT00077308) and CNFN (Cornifelin; Gene ID:84518; Ref. QT00208453). For each gene, relative expression was normalized to housekeeping genes (GAPDH and RPL13A) and fold change were calculated form the untreated control. Data are presented as mean +/- standard deviation (sd). Statistical analysis was performed using one-way ANOVA followed by Dunnett’s multiple comparison test.
Keratinocytes differntiation, Tranglutaminase I protein expression
Normal human epidermal keratinocytes (nHEK, Bioalternatives, Gençay, France) used at passage 3 (P3) were seeded in 96-well plates (ThermoFisher scientific, Villebon-sur-Yvette, France) and cultured for 24 hours in Keratinocyte-SFM basal medium supplemented with Epidermal Growth Factor and Pituitary extract (both ThermoFisher scientific, Villebon-sur-Yvette, France), and Gentamycin (Gibco, Waltham, MA, USA). Keratinocytes were then treated or not (Control) for 72 hours with 10 µg/mL Mn-GLC, or with 5% SGMB spring water, or with the combination 10 µg/mL MN-GLC + 5% SGMB spring water. Final water concentration was harmonized in all conditions by adding the necessary quantity of deionized water to reach a final concentration of 5%. All conditions were performed in triplicate in 3. After incubation, culture medium was removed and cell fixed with 5% Paraformaldehyde (PFA) and permeabilized using 0.1% triton-X100. After washing, cells were incubated for 1 hour with primary antibody (anti-Transglutaminase 1, Novus biologicals, ref. NB100-1844, dilution 1/100). After extensive washing, cells were incubated with secondary antibody (GAR-Alexa 488, Invitrogen, ref. A11008, dilution 1/500) and nuclei were labelled using Hoechst 33342 (bis-benzimide, Sigma, ref. B2261). After incubation and washing, high-resolution images were acquired using an INCell AnalyserTM2200 (GE Healthcare, 5 images/well, lens x20). Relative expression was quantified by image analysis using Developer Toolbox 1.5 (GE Healthcare) and by measuring total fluorescence intensity reported to cell number. Data are presented as mean +/- standard deviation (sd). Statistical analysis was performed using one-way ANOVA followed by Dunnett’s multiple comparison test.
Results
Effects of manganese gluconate and SGMB spring water on ATP2C1 expression
Manganese gluconate (Mn-GLC) tested at 20 µM and 50 µM inhibited in a concentration dependent manner ATP2C1 expression in keratinocytes from both donors (18 y/o and 41 y/o) cultured in low calcium concentration (Figure 1). This effect was less clear in presence of calcium. This inhibitory effect was observed only in the young donor (18 y/o) and mainly in presence of 0.8 mM of calcium (Figure 1). Regarding the own effect of calcium on ATP2C1 expression, no clear effects were observed on both donors (Figure 1). 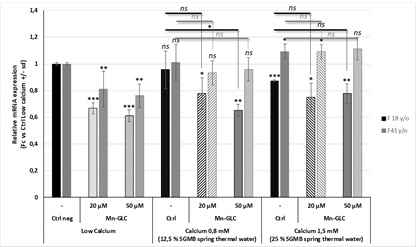
Figure 1: Effects of Saint-Gervais Mont-Blanc thermal spring water, Manganese gluconate and their combination on ATP2C1 expression.
Effects of manganese gluconate and SGMB spring water on keratinocytes differentiation
In order to see how Mn2+ could affect the effect of calcium on keratinocytes differentiation, keratinocytes were cultured with increasing concentrations of Saint-Gervais Mont-Blanc thermal spring water containing or not 10 µg/mL Manganese gluconate. SGMB thermal spring water significantly stimulated the expression of markers involved in keratinocytes differentiation and barrier function (TGM1, TJP1, OCDN and CNFN, Figure 2). The adding of 6.25% SGMB thermal spring water is sufficient to initiate keratinocytes differentiation (Figure 2). Manganese gluconate tested alone at 10 µg/mL, did not modulate clearly the expression of the selected markers (Figure 2) while tested in presence of SGMB thermal spring water, Manganese gluconate clearly potentiate the effects of SGMB thermal spring water on the expression of these selected markers (Figure 2).
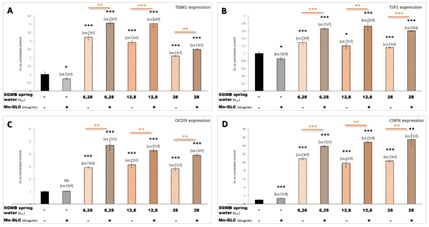 Figure 2: Effects of Saint-Gervais Mont-Blanc spring water, Manganese gluconate and their combination on keratinocytes differen-tiation: (A) RTQPCR analysis on TGM1; (B) RTQPCR analysis on TJP1; (C) RTQPCR analysis on OCDN and (D) RTQPCR analysis on CNFN.
Figure 2: Effects of Saint-Gervais Mont-Blanc spring water, Manganese gluconate and their combination on keratinocytes differen-tiation: (A) RTQPCR analysis on TGM1; (B) RTQPCR analysis on TJP1; (C) RTQPCR analysis on OCDN and (D) RTQPCR analysis on CNFN.
To reinforce our first observations on the effect of Mn-GLC on the calcium-effect potentiation, the expression of transglutaminase 1 (TGM1) was measured at the protein level. In these conditions, both SGMB thermal spring water at 5% and Mn-GLC at 10 µg/mL did not increased significantly TGM1 expression by human keratinocytes while a clear stimulation was observed with the combination of both (Figure 3). The effect of the combination tended to be higher or at least equivalent to that of 1.5 mM CaCl2 used as pro-differentiating reference.
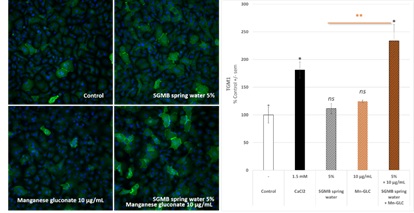
Figure 3: Effects of Saint-Gervais Mont-Blanc thermal spring water, Manganese gluconate and their combination on transglutami-nase 1 expression in human keratinocytes.
Discussion
The skin barrier function is extremely important; it is the first bulwark of protection of the body against external aggressions. As such, the barrier function is an integral part of innate immunity [1]. To help the skin to repair it after an injury or in response to a dermatosis (i.e., psoriasis, atopic dermatitis, HHD) is capital as well as to reinforce barrier function to maintain the health of the skin and thus the health of the organism is key. Today, clearance of skin inflammation is often at the center of therapeutic strategy and there is no very efficient pharmacological treatment targeting keratinocyte differentiation and barrier function enhancement [9-11]. Even if by inhibiting inflammation, a quick repair of the skin is observed in main cases, anti-inflammatory molecules are non-efficient on others pathologies such as ichtyosis, Hailey-Hayley disease or non-efficient on some skin behavior where the involvement of inflammation is not so clear (i.e., skin aging). Today, thermal bath using thermal spring water is mainly used to treated skin diseases and the interest of Saint-Gervais Mont Blanc thermal water for dermatological problems and the crenotherapy [16], treatment developed in Saint-Gervais Mont Blanc thermal center, was demonstrated in a clinical study performed on 106 patients. Some of these effects were related to the composition of this thermal water. SGMB thermal spring water is naturally and strongly mineralized with high rates of calcium, sulfates and other minerals like manganese, boron and zinc (Table 1). It was demonstrated that manganese, boron and zinc stimulate the wound healing process by stimulating keratinocytes migration and proliferation by acting on the expression of some integrins [24,26-30].
In the Hailey-Hailey disease, the alterations of skin integrity (blisters and erosions) are caused by a mutation of ATP2C1 gene coding a Calcium-transporting protein able to transport also manganese [14-16]. We thus do the link between this protein and SGMB thermal water naturally rich in calcium and containing manganese. If the effect of calcium on the improvement of keratinocytes differentiation is expected [17], the effect of manganese on the establishment of barrier function need to be clarify and deepened. Excepted the effects of manganese on keratinocytes wound healing, by acting on keratinocytes migration and matrix synthesis [19-21], and excepted the description of manganese in the activity of enzymes involved in detoxification [18], there is no data able to make a link between manganese and keratinocyte differentiation and more globally with the skin barrier function. Based on the effects observed in HHD when ATP2C1 is deficient, we hypothesized that inversely, by stimulating ATP2C1 expression/activity we could enhance barrier function and skin quality. In addition, considering that this transporter is able to transport calcium and manganese, we hypothesized also that both could be involved in keratinocytes differentiation. We first try to understand how manganese could act on ATP2C1 expression or activity. Considering that it is challenging to study specifically the activity of this type a transporter, located in the Golgi apparatus, we consider that at least, by having information on effects of manganese on the potentiation of the effect of calcium could be a first indication on an effect of manganese on ATP2C1 activity. In our study, we assessed the effect of Mn2+ on ATP2C1 expression in human keratinocytes in a proliferating status, cultured without calcium, or in a differentiating status by culturing them with increasing calcium concentration. In all of the cases, we did not observe any effect of Mn-GLC on ATP2C1 transcript expression. On the contrary, its expression tended to be decreased. We also did not observe any effect of calcium-induced keratinocytes differentiation on the expression of this gene despite ATP2C1 is consider as a differentiation marker. We cannot verify our hypothesis on the involvement of manganese on ATP2C1 expression but it is important to keep in mind that for this type of markers, encoding an ATPase calcium-pump, the expression of the transcript does not reflect the activity of the protein. We cannot thus exclude that Mn2+ could enhance ATP2C1 activity or at least, independently of the role or not of ATP2C1, we cannot exclude that Mn2+ could be an actor of barrier function establishment. In order to have first proofs on the interest on Mn2+ to improve keratinocytes differentiation, we measured the expression of other differentiation markers, important for the barrier function, at the mRNA level. Keratinocytes were treated with increasing concentrations of SGMB thermal water, with Mn-GLC or with the combination of both. One more time, we did not observe any effect of Mn-GLC on keratinocytes differentiation while the SGMB thermal spring water increased dramatically the expression of all. Nevertheless, a synergistic effect between SGMB thermal water and Mn-GLC was clearly observed. The potency of the combination of Mn-GLC with SGMB thermal water was confirmed at the protein level. The combination clearly stimulated TGM1 expression by keratinocytes monolayers, while tested alone, both did not have any effect. Although we don’t have any indications on a possible mechanism of action, or a possible implication of ATP2C1, these results clearly demonstrate the interest to combine Mn2+ and calcium to empower keratinocytes differentiation and to stimulate barrier function. Additional works could be performed to make a link between manganese and ATP2C1 activity and to demonstrate, in a functional point of view using full-thickness reconstructed skin, the effect of the combination on barrier function but also a wound repair. Nevertheless, to date, this new finding could help to enhance skin quality in several skin disorders in which an alteration of barrier function is observed due or not to an inflammation status.
Author Contribution
Conceptualization, F.J.; methodology, F.J.; validation, F.J. and D.F.; investigation, J.A.; D.F.; S.M.; data curation, F.J.; D.F.; S.B.; M.V.; writing-original draft preparation, F.J.;S.B.; writing-review and editing, F.J.; visualization, F.J.; supervision, F.J.; project administration, M.V.
Funding
This research received no external funding.
Acknowledgment
We are thankful to our colleague Jean Baptiste Galey who helped us to review the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elias PM (2007) The skin barrier as an innate immune element. Semin Immunopathol 29: 3-14.
- Madison KC (2003) Barrier function of the skin: "la raison d'être" of the epidermis. J Invest Dermatol 121: 231-241.
- Segre JA (2006) Epidermal barrier formation and recovery in skin disorders. The Journal of clinical investigation 116: 1150-1158.
- Erturan I, Ceyhan AM, Meriç G, Akkaya VA (2012) Case of Hailey-Hailey disease that responds dramatically to acyclovir treatment. Turkish Journal of Dermatology 6: 175-177.
- Chambers ES, Vukmanovic-Stejic M (2020) Skin barrier immunity and ageing. Immunology 160: 116-125.
- Parrado C, Mercado-Saenz S, Perez-Davo A, Gilaberte Y, Gonzalez S, et al. (2019) Environmental stressors on skin aging: Mechanistic insights. Front Pharmacol 10: 759-761.
- Dainichi T, Kitoh A, Otsuka A, Nakajima S, Nomura T, et al. (2018) The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat Immunol 19: 1286-1298.
- Reich K (2012) The concept of psoriasis as a systemic inflammation: Implications for disease management. J Eur Acad Dermatol Venereol 26: 3-11.
- De Benedetto A, Agnihothri R, McGirt LY, Bankova LG, Beck LA (2009) Atopic dermatitis: a disease caused by innate immune defects? J Invest Dermatol 129: 14-30.
- Kastarinen H, Okokon EO, Verbeek JH (2015) Topical anti-inflammatory agents for seborrheic dermatitis of the face or scalp: summary of a Cochrane Review. JAMA Dermatol 151: 221-222.
- Heratizadeh A, Werfel T (2016) Anti-inflammatory therapies in atopic dermatitis. Allergy 71: 1666-1675.
- Shigetoshi S (2015) Psoriasis as a barrier disease. Dermatologica Sinica 2015, 33: 10-16.
- Wang Z, Man MQ, Li T, Elias PM, Mauro TM (2020) Aging-associated alterations in epidermal function and their clinical significance. Aging, 12: 5551-5565.
- Li X, Zhang D, Ding J, Li L, Wang Z (2020) Identification of ATP2C1 mutations in the patients of Hailey-Hailey disease. BMC medical genetics 21: 120.
- Hu Z, Bonifas JM, Beech J, Bench G, Shigihara T, et al. (2000) Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet 24: 61-65.
- Sudbrak R, Brown J, Dobson-Stone C, Carter S, Ramser J, et al (2000) Hailey-Hailey disease is caused by mutations in ATP2C1 encoding a novel Ca(2+) pump. Hum Mol Genet 9: 1131-1140.
- Daniel B, Zhongjian X, Chia-Ling T (2012) Calcium regulation of keratinocyte differentiation. Expert Rev Endocrinol Metab 7: 461-472.
- Treiber N, Maity P, Singh K, Ferchiu F, Wlaschek M, et al. (2012) The role of manganese superoxide dismutase in skin aging. Dermatoendocrinol 4: 232-235.
- Keen CL, Zidenberg-Cherr S (1996) “Manganese” In Present Knowledge in Nutrition, 7th ed., Ziegler, E.E.; Filer L.J. Eds.;ILSI Press, Washington, DC, USA, Page no:. 334-343.
- Muszynska A, Palka J, Gorodkiewicz E (2000) The mechanism of daunorubicin-induced inhibition of prolidase activity in human skin fibroblasts and its implication to impaired collagen biosynthesis. Exp Toxicol Pathol 52: 149-155.
- Shetlar MR, Shetlar CL (1994) The role of manganese in wound healing. In Klimis-Tavantzis DL, ed. Manganese in health and disease. Boca Raton (ed.). CRC Press, Inc., Page no: 145-157.
- Bulletin de l’Académie Nationale de Médecine (1996, 180, n°2, 419-436, séance du 13 février 1996.
- Blech MF, Martin C, Borrely J, Hertemann P (1990) Traitement des plaies profondes avec perte de substance, intérêt d’une solution d’acide borique à 3%. Presse Med 19: 1050-1052.
- Chebassier N, Ouijja EH, Viegas I, Dréno B (2004) Stimulatory effect of boron and manganese salts on keratinocyte migration. Acta Derm Venereol 84: 191-194.
- Lepinay E, Hardy P, Gate A (1964) Bilan et enseignement de quatre années d’application de la crénothérapie aux séquelles cica-tricielles de brûlures. La Presse Thermale et Climatique 2: 106-116.
- Barrandon Y, Green H (1987) Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA 84: 2302-2306.
- Rochat A, Kobayashi K, Barrandon Y (1994) Location of stem cells of human hair follicles by clonal analysis. Cell 76: 1063-1073.
- Tenaud I, Sainte-Marie I, Jumbou O, Litoux P, Dréno B (1999) In vitro modulation of keratinocyte wound healing integrins by zinc, copper and manganese. Br J Dermatol 140: 26-34.
- Tenaud I, Leroy S, Chebassier N, Dreno B (2000) Zinc, copper and manganese enhanced keratinocyte migration through a functional modulation of keratinocyte integrins. Exp Dermatol 9: 407-416.
- Tenaud I, Saiagh I, Dreno B (2009) Addition of zinc and manganese to a biological dressing. The Journal of dermatological treat-ment 20: 90-93.
Citation: Juchaux F, Albaud J, Fagot D, Miskinyte S, Benosmane S, et al. (2021) Physiological Effects of Manganese Gluconate and Calcium Combination from Saint-Gervais Mont Blanc Spring Water for Human Keratinocytes Differentiation. J Clin Dermatol Ther 7: 080.
Copyright: © 2021 Franck Juchaux, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

