
Placental Mesenchymal Dysplasia - Prenatal Diagnosis and Clinical Outcome
*Corresponding Author(s):
Mariana TsankovaDepartment Of Obstetrics And Gynecology, Medical University Sofia, University Hospital “Maichin Dom”, Sofia, Bulgaria
Tel:+359 888401144,
Email:drmzankova@medicinabg.com
Abstract
Placental mesenchymal dysplasia is an uncommon vascular anomaly of the placenta with characteristics of placentomegaly and multicystic appearance and with or without association with fetal chromosomal anomaly. In the beginning, it could be confused with a partial hydatidiform mole. In this report, we present a case of a 29 -year old patient, G3P0, with placentomegaly and anatomically and chromosomal normal fetus, following preeclampsia, fetal growth restriction and who gave birth to a structurally normal hypotrophic fetus with an emergency CS. The patient was followed-up by brief ultrasound and clinical investigation from 1st to the beginning of 3rd trimester and sonographic findings showed : thick homogeneous placenta with an area of grape-like appearance, multiple vesicular lesions “swiss-cheese“, similar to a partial mola, subsequently rejected due to the normal levels of hHCG and structurally normal appearing fetus. Finally, according to biomarkers and US images the differential diagnosis was PMD.
The diagnosis was confirmed by 2D Colour Doppler and 3D ultrasound examination which visualised a significant increase of the pathological zone and progressive placentomegaly with the advance of pregnancy, correlating with clinical complications in the early third trimester - preeclampsia and fetal growth restriction. Histopathology after delivery confirmed placental mesenchymal dysplasia. According to literature reviews, this condition is associated with chromosomal abnormalities, Beckwith- Wiedman Syndrome, Fetal Growth Restriction or a structurally normal fetus. We present a short review of literature for PMD illustrated with a clinical case.
Introduction
The placenta is a unique nutritious and endocrine organ serving as an interface between the mother and the fetus. The placenta has both maternal and embryonic components and it gradually increases in a linear progression, approximately 1mm per week and correlates with gestational age. The placenta is also the most vascularized organ in the human body and some of the rare placental abnormalities lead to deviation in the fetal development - chromosomal, structural and growth restriction. The thickness of normal placenta is around 2.5-4 cm, about 22cm in diameter and placental weight of about 400gr. The anterior placenta is 0.7cm thinner than posterior placenta and the maximum thickness of the placenta is 4 cm according to literature [1,2].
Thin placenta most commonly correlates with placental insufficiency, fetal growth restriction and rare abnormalities of the placenta, such as placenta membranacea and some chromosome and structural malformations of the fetus. Some ultrasound and clinical findings of the placenta, umbilical cord and membranes are linked with fetal abnormal development and perinatal morbidity, e.g., anomalies in the form of the placenta like - bilobata placenta with incidence of 2-8%, placenta succenturiata - 5%. In both specific conditions vasa praevia could be observed; marginal cord insertion - 7-9% is observed with fetal growth development [3]. Antenatal sonographic diagnosis of these rare placental malformations could correlate with clinical complications like antepartum and postpartum bleeding, fetal growth restriction, retained placental tissue, preterm labour and fetal distress.
Placentomegaly is a condition in which the placenta has abnormal thickness - more than 5.5.cm. Placentomegaly could be the upper range of normal variation like fetal macrosomia; or be associated with fetal immune or non-immune hydrops, “mirror- syndrome”, TORCH infections, partial molar pregnancy, triploidy, PMD and Beckwith- Wiedemann syndrome - visceromegaly, macroglossia, and omphalocele and maternal-related diseases - gestational diabetes, preeclampsia, anemia [4]. Moscosso recognized the first case of PMD and described it in 1991 as a rare (incidence 0.02%)) [5,6] placental vascular anomaly - mesenchymal hyperplasia of the stem villi in the placenta leading to increased placental volume and exhibiting elevated levels of maternal serum alpha- fetoprotein , with normal or slightly increased elevated hHCG.
The ultrasound differential diagnosis is with molar pregnancy with its specific sonographic feature “snowstorm sign” [7] characterized by the presence of many hydropic villi, which give out ultrasound appearance of central heterogenous mass and multiple cystic area around and elevated levels of β-HCG. Most of the PMD cases correlate with intrauterine fetal death, intrauterine growth restriction, Beckwith-Wiedemann Syndrome (BWS), and preeclampsia, but in rare cases, it can associate with a normal fetus pregnancy [8,9]. Nayeri UA at all find all: PMD base been associated with vaginal bleeding, preterm delivery in 33%, foetal anomalies, foetal growth restriction in 33%, stillbirth in 13% and normal neonatal outcome in 9%; BWS in 23% [10]. Fetal karyotyping is mandatory to rule out fetal aneuploidy and rare fetal anomalies. Pathologically, PMD is characterized by /Pawoo and Heller, 2014/ [11] mesenchymal hyperplasia, dilated blood vessels or aneurysm that may be thrombosed. The stem villi are oedematouse and enlarged with thick- walled vessels, without trophoblastic proliferation.
- Objective
The report illustrates a short review of literature of PMD and a follow-up ultrasound of a clinical case with PMD which finished in the early 3rd trimester with clinical complications specific for placentomegaly - preeclampsia and fetal growth restriction.
Clinical Case
A 29-year old patient with G3A2P0. 2 AB.SPONTANEOUS in the early first trimester, diagnosed with a placental grape-like vesicular type of part of the placenta; at first sight it looks like a partial mola. The patient referred to our service to undergo a routine first trimester combined screening scan at 12.5 weeks of gestation and showed a normal fetal morphology, a nuchal thickness 1.66mm. Placenta was homogeneous and larger with an area with multicystic hypoechoic lesion with lack of high velocity Doppler signals inside the lesion. Additional triple investigation of the hHG levels was normal for the g.w. 150 000 IU - 4 th gestational week. Biochemical screening markers show normal levels of MoM range as hCG and PAPP - hCG- 1.9 MoM. PAPP- 0.8; AFP- 2.4. Free estriol fraction- 1.5; Low biochemical risk of 1st trimester and between 1st and 2nd trimester. The alpha fetoprotein tested independently showed high levels > 20 000 U. Owing to the sonographic findings of placentomegaly, an amniocentesis was discussed in order to rule out genetic mutations but was rejected by the patient.
We present 2D ultrasound images, Colour Doppler and 3D reconstruction of the cystic zone (Figure 1).
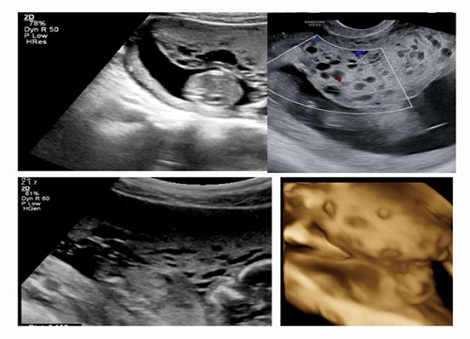 Figure 1: Grey scale picture on the left of multiple cystic appearance of the placenta and homogenuous thick placenta in the rest of; “Moth-eaten” sign of the target zone. Color Doppler image without Doppler signals and Swiss-cheese appearance on 3D image.
Figure 1: Grey scale picture on the left of multiple cystic appearance of the placenta and homogenuous thick placenta in the rest of; “Moth-eaten” sign of the target zone. Color Doppler image without Doppler signals and Swiss-cheese appearance on 3D image.
After discussion with geneticists and the patient, the karyotyping was not made. An ultrasound follow-up in the second trimester was performed to observe the fetal growth and showed a normal fetal anatomy, a thick placenta and progressive enlargement of the cystic zone illustrated below figure 2. Colour Doppler interrogation of the placenta could not identify high velocity blood flow signals within the lesion; weak low velocity signals were noted.
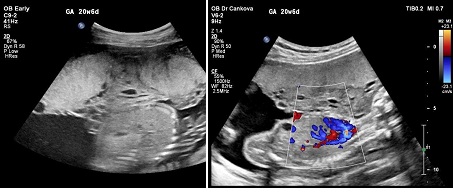 Figure 2: Second trimester- Gray scale and Color Doppler ultrasound images shows thick placenta with grape-like zone (arrow) with Colour Doppler low velocity signals.
Figure 2: Second trimester- Gray scale and Color Doppler ultrasound images shows thick placenta with grape-like zone (arrow) with Colour Doppler low velocity signals.
In the 3rd trimester (31-32 gestational week) the patient went for her routine sonographic examination with - clinical symptoms of preeclampsia of oedema in the face and limbs, high blood pressure- 150/90 mmHg and ultrasound findings of fetal growth restriction of 3 weeks. The estimated fetal weight is 1100 gr, placentomegaly with 7cm thickness including the zone of vesicular appearance; lack of big and small fetal movements. Transabdominal sonography was highly difficult due to the patient's oedema and anterior thick placenta. The Doppler shows high resistance - high pulsative index of umbilical artery and low pulsative index of the middle cerebral artery, correlating with brain sparing.
The patient was referred to an emergency hospital because of severe preeclampsia, poor biophysical profile and Doppler findings of brain sparing. Emergency caesarean section was performed 2 hours after hospitalisation due to ultrasound and monitoring data of fetal distress figure 3.
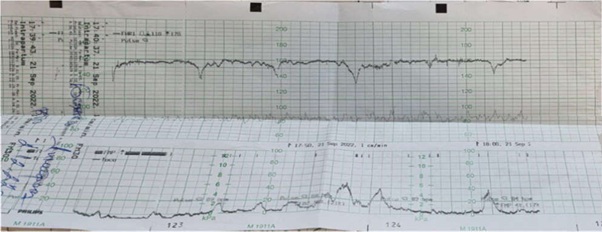 Figure 3: Non- Stress test with spontaneous deceleration.
Figure 3: Non- Stress test with spontaneous deceleration.
- Neonate characteristics
Anatomically normal fetus, fetal weight - 1130 gr, length 35cm; pH after delivery – 7,07; pH 2 hours later - 7,35. Apgar at 1ST MIN – 1; Apgar at 5TH MIN – 5; Placentomegaly - weight - 790 grams.
Pathonatomical speciment of the placenta: Placentomegaly with dilated vessels and blood clots inside, hydropic cysts in part of placenta. Arrow - vesicules; below the same one in formalin figure 4.
Patohystologycal result: Thick-wall vessels with mesenchymal stem villous hyperplasia with myxoid stroma and obliterated blood vessels with thrombs inside (Figure 5).
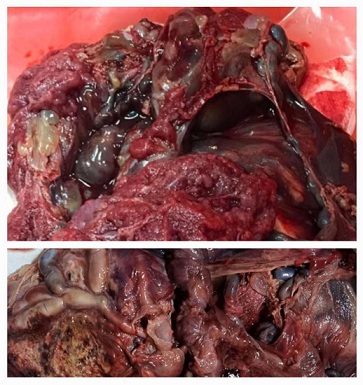 Figure 4: Placentomegaly with delated vessels on the chorionic plate hydropick cyst / vezicular zone and trombosise/Gross specimen of PMD 790 g.
Figure 4: Placentomegaly with delated vessels on the chorionic plate hydropick cyst / vezicular zone and trombosise/Gross specimen of PMD 790 g.
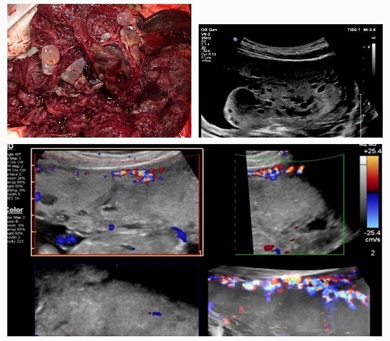 Figure 5: Correlation between the pathoanatomical vesicular part of placenta, ultrasound images in 1st trimester and thick placenta, normal decidual vascularization on Colour Doppler, without Doppler signals inside of the placenta- in 3rd trimester.
Figure 5: Correlation between the pathoanatomical vesicular part of placenta, ultrasound images in 1st trimester and thick placenta, normal decidual vascularization on Colour Doppler, without Doppler signals inside of the placenta- in 3rd trimester.
Discussion
Placentomegaly has been associated with different normal or abnormal maternal and fetal conditions. Polyhydramnion, fetal hydrops, TORCH infections, gestational diabetes, fetal macrosomia partial mole, fetal growth restriction, preeclampsia, placental mesenchymal dysplasia; placentomegaly can mimic placental abruption, transient myometrial contraction. PMD is a rare and fairly new diagnosis termed in 1991 for the first time. With reported incidence of 0.02% - 7 cases among 30,758 placentas over a period of 21 years [5,6]. According to other authors the prevalence is 0.2 %. 50 cases among 7560 [12].
Placental mesenchymal dysplasia is a rare vascular anomaly, benign with abnormal stem villous hyperplasia, placentomegaly that can be misdiagnosed or mistaken easily for hydatidiform mole due to the combination of cysts and normal - appearing parenchyma. According to literature, PMD most often correlates with BWS in 21 - 30% of the cases (hepatic mesenchymal hamartoma or other complications (omphaloceles, fetal anaemia, thrombocytopenia, facial or pulmonary hamartoma) [13].
Ultrasonography is the most accurate method for investigation and diagnosis of PMD. Due to the association with fetal chromosomal abnormalities, karyotyping is necessary to be applied. The presented case illustrates, based on 30-year experience, the first histologically and ultrasound confirmed case of PMD. It is the first 2D and 3D image of placental vascular lesions, characteristic of PMD and supported by colour Doppler investigation [14,15].
Maternal serum biomarkers indicating PMD are an increasing level of maternal serum AFP and normal or slightly increased hCG [10]. According to literature (estimated - 38% of cases) [6], the elevated levels AFP, as a major characteristic for PMD, are the main reason for the adverse fetal outcome correlates with the growth of the placental lesion area causing poor erythropoiesis leading to fetal anaemia, fetal growth restriction, hydrops fetalis, intrauterine fetal death in 10% of the cases [11,12,16,17]. In a report by Pham et al., a fifth (15/81) of the studied cases of PMD were associated with BWS (11 cases of PMD were identified by the author in a 34-year period, and summarised with 71 cases previously reported in literature). After excluding the cases with features of BWS becoming apparent after delivery of a normal appearing newnate, the authors found a risk of 50% for Fetal Growth Restriction, 36% for fetal demise and 7% for neonatal death [18].
Ultrasound Doppler investigation is a basic monitoring tool for a differential diagnosis between PMD and placental tumours on the one hand, and on the other - for fetus condition assessment, in cases of preeclampsia and fetal growth restriction. Chorion angioma is a well circumscribed lesion with different echogenicity than the rest of placenta with increased vascularity and large feeding vessels inside the tumour with the same pulsation rate as the umbilical vessels. Hamartoma is a solid tumour or partial lesion on 2D image without blood flow in the tumour [14]. PMD can correlate also with a normal fetus like in our case. However, clinical abnormal possibilities include FGR, prematurity, preeclampsia, BWS characterised with - macrosomia, exomphalos, hemihyperplasia, neonatal hyperinsulinemic hypoglycemia macroglossia, visceromegaly and increased risk of developing certain tumours. The PMD newborns with normal phenotype should be followed-up for BWS features of the mesenchymal tumour (hepatic mesenchymal hamartoma, congenital adrenal hyperplasia and vascular hamartoma) [9,19].
Maternal complications are preeclampsia, mirror syndrome; 9% of all patients develop gestational hypertension, preeclampsia, eclampsia, or HELLP syndrome. Reactive changes in the placenta villi leading to ischemia along with increased secretion trophoblast-derived proteins in maternal blood such as hCG, activin and inhibin. The further changes lead to an antiangiogenic state and reciprocally trigger preeclampsia. PMD may develop subsequently after diabetes mellitus, TORCH infections [20]. Ultrasound - an essential element for detecting PMD from the beginning of the pregnancy with its specific characteristics of numerous hypoechoic/solucent spaces that are adjacent but not communicating with each other, owing to the dilation of chorionic vessels – so-called - “swiss-cheese” or “moth-eaten placenta” [21,22].
Colour Doppler investigation of the cystic zone in the first two trimesters show low or absent velocity blood flow signals in the lesions. In the third trimester, some articles report a broad vascular zone with a turbulent blood flow with either arterial or venous blood [17] but most often the findings show high velocity blood flow signals. Further, colour Doppler helps to distinguish PMD from other placental abnormalities with similar sonographic vesicular aspects: chorioangioma (large vessel/increased vascularity), spontaneous abortion with hydropic changes (no vessels), molar pregnancy (high velocity with low resistance flow), complete mole with coexisting abnormal fetus (the lesion affects the entire placental thickness, the cysts lack blood flow signal, the lesion is beyond the fetal sac), subchorionic hematoma (no vessels), and partial hydatidiform mole [21,23,24]. Jauniaux et al., using a series of ultrasound and colour Doppler examinations in four pregnancies with PMD did not identify blood flow within the placental lesion in the first five months of pregnancy. However, in the third trimester, large vascular areas with turbulent blood flow were observed, either in arterial or venous patterns, located mainly under and at the level of the chorionic plate. According to the author the changes were due to progressive dilatation of chorionic arteries and veins, thus becoming aneurismal [25].
Three-Dimensional (3D) ultrasound reconstruction usually demonstrates a multicystic placental mass with numerous cysts varying in diameter, not communicating with each other, separate but adjacent to a normal-appearing placenta [14,15]. MRI could be used to support ultrasound diagnosis in the first trimester in cases with complete hydatidiform mole and coexistent fetus or to confirm diagnosis in ultrasound investigation of cases with difficulties stemming from olygohydramnion and thick placenta with inside lesions in the 3rd trimester [26].
- Karyotype
Keiser - Rogers et al., [27] assumed as an etiological factor for PMD the androgenetic/biparental mosaicism, specifically its phenotype which ranges from mild PMD to a complete hydatidiform mole. It depends on the extent and distribution of the androgenetic lineage. The androgenetic cell line is thought to inherit from endoreduplication of the haploid paternal genome, and those cells were confined predominantly to chorionic mesoderm, membranes, and vessels, but absent in the trophoblast. This explains the absence of trophoblast overgrowth in PMD in contrast to complete mole in which androgenetic cells were identified in the trophoblastic cell layer.
The most common chromosome with mosaicism is 11p15.5. Abnormal expression of the imprinted genes on 11p15.5 may result in BWS. It includes epigenetic mutations at one of two imprinting centres. In PMD there is a possibility for fetal congenital malformation of the mesoderm, molecular disruption of the imprinting genes of chromosome 11p15.5. This would influence important decisions including termination of pregnancy. Elevated maternal serum Alpha-fetoprotein is associated with PMD in 70% of the cases. Confirmation of the normal karyotype should be performed by either chorionic villus sampling or amniocentesis to exclude partial molar pregnancy and rare fetal anomalies [28]. Poojary V.G et iology all, “Large placenta and small foetus at early gestational predicts foetal growth restriction due to placental dismorphology”, 2018 JCDR, /37168, 12226.
Congenital malformations of themesoder, molecular disruption of the impriming genes of chromosome 11p15.5 associated with BWS and Androgenetic/Biparietal mosaicism are same of theories explained the etiology of congenital malformations, fetal vascular thrombosis in case of multiple pregnancies, related to PMD Around 15% of PM are familial and theoretically there is a small recurrence risk in the next pregnancies; [29].
Differential Diagnosis - The following differential diagnoses to be considered when PMD is suspected- partial molar pregnancy, complete mole, chorangioma, subchorionic hematoma, confined placental mosaicism and spontaneous abortion with hydropic changes [30]. PMD is not associated with malignant trophoblastic tumours and carries no indications for pregnancy termination.
- Histological
The histological examination shows cistern formation with lax connective tissue, enlarged stem villi and the most important clue for the differential diagnosis from partial hydatidiform mole, the lack of trophoblast proliferation, and the absence of stromal trophoblastic inclusions [27,28]. The histological findings differ depending on gestational age: at the pregnancy inception, the stem villi display dilated cisterns encircled by loose myxomatous stroma, which contains sheer vessels under the trophoblast layer, while in the third trimester, changes comprise enlarged thick-walled vessels in the chorionic plate, with thrombi that obstruct the arterial and venous vessels, and fibromuscular hyperplasia [19]. The terminal villi could exhibit mesenchymal cell hypercellularity and stromal fibrosis [11,15]. PMD does not correlate with malignant trophoblastic tumour and is not directly associated with fetal demise, except for cases of maternal complications [29]. Women with PMD pregnancies can deliver a healthy neonate. Most normal-appearing infants were not observed with any developmental problems secondary at the follow-up in early childhood Subsequent pregnancies show recurrence of PMD within a 5-year follow-up PMD base been associated with vaginal bleeding, preterm delivery in 33%, foetal anomalies, foetal growth restriction in 33%, stillbirth in 13% and normal neonatal outcome in 9% [23,31].
PMD is a challenging diagnosis and possibly misdiagnosed. It is of high probability that this is not the first case at the University hospital, but is definitely the one and only case which was diagnosed, ultrasound followed and patho-histologically confirmed after discussions with pathoanatomists. In our case, we are representing a PMD which goes with 2D ultrasound and 3D reconstruction images of multicystic lesions with lack of Doppler velocity flow inside, morphologically normal fetus, without karyotyping. The case finished with a typical for placentomegaly maternal and fetal complications – preeclampsia, fetal growth restrictions, Doppler findings of brain sparing.
Conclusion
Diagnosing PMD can be confirmed by combining the following: serum biomarkers, 2D ultrasound imaging, Colour Doppler and 3D ultrasound reconstruction, and maternal and fetus clinical symptoms. Ultrasound 2D diagnosis is a basic informative option about location, echogenicity of parenchyma; Doppler investigations show a of blood supply of placental lesions and 3D diagnosis illutstrates a multicystic placental zone, separated, not communicating with each other, fluid- filled surrounded by normal placental tissue. Final diagnosis of PMD is confirmed following a pathology examination of the placenta. PMD has been underdiagnosed because the placenta is not investigated, especially in cases of good perinatal outcome.
Conflict of Interest
No conflict of interest.
References
- Salafia CM, Zhang J, Miller RK, Charles AK, Shrout P, et al. (2007) Placental growth patterns affect birth weight for given placental weight. Birth Defects Res A Clin Mol Teratol 79: 281-288.
- Salafia CM, Zhang J, Charles AK, Bresnahan M, Shrout P, et al. (2008) Placental characteristics and birthweight. Paediatr Perinat Epidemiol 22: 229-239.
- Rathbun KM, Hildebrand JP (2023) Placenta Abnormalities. StatPearls Publishing.
- Sun X, Shen J, Wang L (2021) Insights into the role of placenta thickness as a predictive marker of perinatal outcome. J Int Med Res 49: 300060521990969.
- Guenot C, Kingdom J, De Rham M, Osterheld M, Keating S, et al. (2019) Placental mesenchymal dysplasia: An underdiagnosed placental pathology with various clinical outcomes. Eur J Obstet Gynecol Reprod Biol 234: 155-164.
- Moscoso G, Jauniaux E, Hustin J (1991) Placental vascular anomaly with diffuse mesenchymal stem villous hyperplasia. A new clinico-pathological entity? Pathol Res Pract 187: 324-328.
- Skandhan A, Alrasheed W, Rasuli B (2023) Snowstorm sign (complete hydatiform mole). Radiopaedia.
- Li H, Li L, Tang X, Yang F, Yang KX (2014) Placental mesenchymal dysplasia: A case of a normal-appearing fetus with intrauterine growth restriction. Int J Clin Exp Pathol 7: 5302-5307.
- Gavanier D, Allias F, Huissoud C, Hajri T, Golfier F, et al. (2017) Ultrasound findings and clinical outcomes in 23 cases of placental mesenchymal dysplasia. The Journal of reproductive medicine 62: 366-375.
- Nayeri UA, West AB, Grossetta Nardini HK, Copel JA, Sfakianaki AK (2013) Systematic review of sonographic findings of placental mesenchymal dysplasia and subsequent pregnancy outcome. Ultrasound Obstet Gynecol 41: 366-374.
- Pawoo N, Heller DS (2014) Placental mesenchymal dysplasia. Arch Pathol Lab Med 138: 1247-1249.
- Paradinas FJ, Sebire NJ, Fisher RA, Rees HC, Foskett M, et al. (2001) Pseudo-partial moles: placental stem vessel hydrops and the association with Beckwith-Wiedemann syndrome and complete moles. Histopathology 39: 447-454.
- Umazume T, Kataoka S, Kamamuta K, Tanuma F, Sumie A, et al. (2011) Placental mesenchymal dysplasia, a case of intrauterine sudden death of fetus with rupture of cirsoid periumbilical chorionic vessels. Diagn Pathol 6: 38.
- Vaisbuch E, Romero R, Kusanovic JP, Erez O, Mazaki-Tovi S, et al. (2009) Three-dimensional sonography of placental mesenchymal dysplasia and its differential diagnosis. J Ultrasound Med 28: 359-368.
- Psarris A, Sindos M, Kourtis P, Pampanos A, Antsaklis P, et al. (2020) Placental Mesenchymal Dysplasia: Ultrasound Characteristics and Diagnostic Pitfalls. Ultrasound Int Open 6: 2-3.
- Ishikawa S, Morikawa M, Umazume T, Yamada T, Kanno H, et al. (2016) Anemia in a neonate with placental mesenchymal dysplasia. Clin Case Rep 4: 463-465.
- Kodera C, Aoki S, Ohba T, Higashimoto K, Mikami Y, et al. (2021) Clinical manifestations of placental mesenchymal dysplasia in Japan: A multicenter case series. J Obstet Gynaecol Res 47: 1118-1125.
- Pham T, Steele J, Stayboldt C, Chan L, Benirschke K (2006) Placental mesenchymal dysplasia is associated with high rates of intrauterine growth restriction and fetal demise: A report of 11 new cases and a review of the literature. Am J Clin Pathol 126: 67-78.
- Rosner-Tenerowicz A, Pomorski M, Fuchs T, Sliwa J, Zimmer-Stelmach A, et al. (2020) Placental mesenchymal dysplasia and hepatic cyst. Ginekol Pol 91: 779-780.
- Woo GW, Rocha FG, Gaspar-Oishi M, Bartholomew ML, Thompson KS (2011) Placental mesenchymal dysplasia. Am J Obstet Gynecol 205: 3-5.
- Tasha I, Lazebnik N (2020) Placental Mesenchymal Dysplasia; Current Understanding of the Sonographic, Histologic, and Molecular Findings of a Rare and Challengin Disorder. J Gynecol Womens Health 18: 1-10.
- Cubal A, Carvalho J, Faria B, Rodrigues G, Carmo O, et al. (2015) Placental mesenchymal dysplasia. Acta Obstet Ginecol Port 9: 235-240.
- Parveen Z, Tongson-Ignacio JE, Fraser CR, Killeen JL, Thompson KS (2007) Placental Mesenchymal Dysplasia. Arch Pathol Lab Med 131: 131-137.
- Imafuku H, Miyahara Y, Ebina Y, Yamada H (2018) Ultrasound and MRI Findings of Twin Pregnancies with Complete Hydatidiform Mole and Coexisting Normal Fetus: Two Case Reports. Kobe J Med Sci 64: 1-5.
- Jauniaux E, Ogle R (2000) Color Doppler imaging in the diagnosis and management of chorioangiomas. Ultrasound Obstet Gynecol 15: 463-467.
- Colpaert RM, Ramseyer AM, Luu T, Quick CM, Frye LT, et al. (2019) Diagnosis and Management of Placental Mesenchymal Disease. A Review of the Literature. Obstet Gynecol Surv 74: 611-622.
- Kaiser-Rogers KA, McFadden DE, Livasy CA, Dansereau J, Jiang R, et al. (2006) Androgenetic/biparental mosaicism causes placental mesenchymal dysplasia. J Med Genet 43: 187-192.
- Adelaide C, Joana C, Barbara F, Graca R, Olimpia C (2015) Placental mesenchymal dysplasia- Review article. Acta Obstet Gynecol Port 9: 235-240.
- Poojari VG, Vasudeva A, Shetty J, Kudva R, Solipuram D (2018) Large Placenta and Small Foetus at Early Gestation Predicts Foetal Growth Restriction due to Placental Dysmorphology. Journal of Clinical and Diagnostic Research 12.
- Cohen MC, Roper EC, Sebire NJ, Stanek J, Anumba DO (2005) Placental mesenchymal dysplasia associated with fetal aneuploidy. Prenat Diagn 25: 187-192.
- Matsui H, Iitsuka Y, Yamazawa K, Tanaka N, Mitsuhashi A, et al. (2003) Placental mesenchymal dysplasia initially diagnosed as partial mole. Pathol Int 53: 810-813.
Citation: Tsankova M, Geshev N (2023) Placental Mesenchymal Dysplasia - Prenatal Diagnosis and Clinical Outcome. J Reprod Med Gynecol Obstet 8: 137.
Copyright: © 2023 Mariana Tsankova, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

