Plastination - an Innovative Preservative Technique In Anatomy
*Corresponding Author(s):
Anas Sarwar QureshiDepartment Of Anatomy, University Of Agriculture Faisalabad, Faisalabad, Pakistan
Tel:+92 041 920016170,
Email:anas-sarwar@uaf.edu.pk
Abstract
Preserving the cadavers from ongoing natural processes of decomposition and putrefaction have always been the focus of medical professionals especially anatomists, in order to use them for future studies and research. Embalming is the most commonly used procedure to prevent decomposition/putrefaction of cadavers. However, there are certain limitations/disadvantages ascribed to traditional “Embalming” procedures. Among these disadvantages, the most obvious ones are the repulsiveness of the students due to the smell and the formation of irritant fumes from the embalming fluid. Use of formaldehyde and methanol is also not considered environment friendly. In contrast, plastination technique has several advantages like making cadaver easy to handle, easy storage, being cheaper, odorless presentation and presents more anatomical details. Customization of the technique has been done for particular organs and according to the environmental conditions but still the basic techniques considered as pioneers for plastination are the Silicone (S10) and Sheet Plastination (P40).Though plastination has been established as an excellent preservation method but the ethical issues are still debatable. The moral dilemma has been under discussion since the development of this procedure. The major reason for this ethical and moral discussion is the social and cultural restraints towards the depiction of such exhibitions. In this article, a comprehensive detail history, methodology, advantages/limitations and a brief discussion on the ethical and moral dilemma have been presented.
INTRODUCTION
Preserving the cadavers from ongoing natural processes of decomposition and putrefaction have always been the focus of medical professionals especially anatomists, in order to use them for future studies and research. Embalming is the most commonly used procedure to prevent decomposition/putrefaction of cadavers. However, there are certain limitations/disadvantages ascribed to traditional “Embalming” procedures. Among these disadvantages, the most obvious ones are the repulsiveness of the students due to the smell and the formation of irritant fumes from the embalming fluid. Use of formaldehyde and methanol is also not considered environment friendly. In contrast, plastination technique has several advantages like making cadaver easy to handle, easy storage, being cheaper, odorless presentation and presents more anatomical details. Customization of the technique has been done for particular organs and according to the environmental conditions but still the basic techniques considered as pioneers for plastination are the Silicone (S10) and Sheet Plastination (P40).Though plastination has been established as an excellent preservation method but the ethical issues are still debatable. The moral dilemma has been under discussion since the development of this procedure. The major reason for this ethical and moral discussion is the social and cultural restraints towards the depiction of such exhibitions. In this article, a comprehensive detail history, methodology, advantages/limitations and a brief discussion on the ethical and moral dilemma have been presented.
PLASTINATION
It is a procedure through which living specimen can be processed and preserved in their near-natural state. In this procedure, the lipids and the water present in the cadaver is replaced with synthetic components like polyester or silicone etc., and then after the hardening of these materials, we obtain a natural looking and durable anatomical specimen [9]. These specimens can be specified organs or entire organisms (if required) [10]. The most striking feature of this technique over conventional formalin based preservation is the anatomical detail and ease of handling of bodies after the procedure [11].
While classifying the plastination procedures, we can use the type of end-product as the reference point. Specimen can either be preserved as a whole in 3-D matter or slices of the processed specimen can be made for illustration. These two procedures are termed as silicone (S10) and sheet plastination (P40), respectively. A wide number of resins have been tested over the past 30 years for their potential use in the plastination procedure. Resins that are commonly used for the plastination are Silicone, Epoxy resin and Polyester. Now-a-days different variants of these chemicals are used by different laboratories and museums each having their own advantages and disadvantages.
SILICONE PLASTINATION (P10)
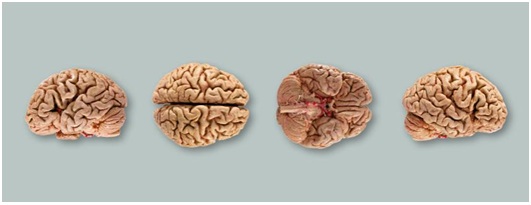 Figure 1: Brain models preserved through plastination showing various sides (Source. von Hagen Plastination [8]).
Figure 1: Brain models preserved through plastination showing various sides (Source. von Hagen Plastination [8]).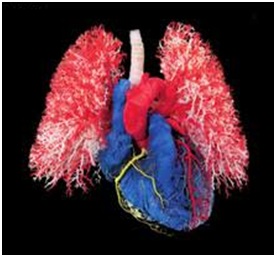 Figure 2: The silicon plasination models of blood vessels of heart and lungs used for human body (Source: von Hagen Plastination [8]).
Figure 2: The silicon plasination models of blood vessels of heart and lungs used for human body (Source: von Hagen Plastination [8]). Figure 3: Anatomical plastination models of camels showing inside out (Source: von Hagens Plastination [8]).
Figure 3: Anatomical plastination models of camels showing inside out (Source: von Hagens Plastination [8]).Procedure
Dehydration: Water in the specimen is substituted with acetone at -25ºC. Usually, acetone is changed up to three times. Ethanol can also be used but is not very preferable in routine procedures [15].
Defatting: In this step, lipid is removed by transferring the specimen from -25ºC to room temperature. As soon as the lipid starts turning opaque, procedure is stopped [16].
Forced impregnation: The basic principle is to replace the volatile solvent (acetone etc.,) with the silicone reaction mixture. Vacuum is required so that the viscous mixture can come in equilibrium with the dehydration agent. Forced impregnation ensures that the specimen will not be shrunk [17]. Although studies indicate that shrinkage of tissues actually takes place even with forced impregnation. But with the use of proper vacuum pressure, this shrinkage can be minimized [18].
Hardening: Curing or hardening is obtained by exposure of specimen to the curing gas, heat or ultra-violet light in an enclosed chamber. This leads to chain extension and cross linkage of the polymer [19]. After drying of the specimen surface, it is placed in an air tight bag which ensures the internal hardening of polymer [20].
SHEET PLASTINATION (P40)
 Figure 4: Sheet (slice) plastinates are 1.5 mm thin slices of real bodies, impregnated with resins and subsequently hardened. Sheet plastinates illustrate anatomical structures, in color, either transparent or translucent. The method of plastination facilitates the production and preservation of thin, transparent body slices of unparalleled transparency, vibrant colors, and size. (Source: Von Hagens Plastination).
Figure 4: Sheet (slice) plastinates are 1.5 mm thin slices of real bodies, impregnated with resins and subsequently hardened. Sheet plastinates illustrate anatomical structures, in color, either transparent or translucent. The method of plastination facilitates the production and preservation of thin, transparent body slices of unparalleled transparency, vibrant colors, and size. (Source: Von Hagens Plastination).Fixation
Cold dehydration
Defatting
Forced impregnation
Hardening
ADVANTAGES OVER FORMALIN-BASED PRESERVATION
1. Storage of the preserved samples is easier as we can store the plastinated samples even in a shopping bag with appropriate labeling [27].
2. This procedure offers completely preserved specimen without any toxic fumes or foul smell [28].
3. It is relatively cheaper than the conventional formalin based method in a longer run [29].
4. It is sometimes difficult for some students to study due to the smell of formalin, this in turn leads to impairment of interest of the student [30].
5. Specimen can be preserved for up-to 40 years if plastination is performed which is almost 10 times more than that of conventional method [31].
6. Plastination offers relatively more detailed features as all the structures are fully preserved in their near-natural state [30].
7. With the help of sheet plastination, it becomes easier to study the topographical anatomy in detail [32].
8. We can preserve the parasites present in the flesh such as larvae in the putrid flesh can be preserved for demonstration [33].
9. Fragile tissue sample such as intra-cerebral hematoma can be preserved perfectly and made durable for the future use [34].
LIMITATIONS OF PLASTINATION
Although plastination is a good technique for the preservation of specimen but, like any other procedure, it also has certain limitations. Few of these cons are that plastinated specimen are relatively inflexible (due to the presence of silicone in the tissue) so it becomes troublesome to reflect the specimen and demonstrate the deeper anatomical features. This inflexibility is the major problem that the scientists have to face while using plastinated specimen for clinical practices such as ultrasonography and endoscopy [35]. Thus for the clinical practices, plastinated specimen are not ideal for use. Similarly, after the completion of plastination, tissue becomes so much hard that further dissection is difficult so, the final dissection has to be done before the procedure. In addition, the process is quite time consuming and sensitive technique so it requires skilled manpower [19]. Plastination laboratory development also requires a huge amount of investment which also poses major bottleneck while developing the laboratory [36].
Chemicals used in the plastination procedure pose a health hazard if they are not properly handled. Acetone used in different variants of plastination techniques can cause respiratory and dermal irritation in case of short term exposure in low concentration (250ppm-1000ppm). High concentration (>12000ppm) can cause more severe symptoms such as vomiting and unconsciousness [37]. Long term exposure to acetone in mice with concentration >19000ppm has shown to produce reversible decrease in the absolute brain weight of cadaver [38]. Like most of the organic solvents, acetone also causes visual impairment and loss of sensitivity if exposed to for a long time [39]. Hydroxyl-terminated polydimethy isiloxane and Ethyl silicate used for the silicone impregnation technique has been reported to cause allergic reactions and dermatitis respectively in case of long term exposure [40,41]. Dibutyltin dilaurate used for sheet plastination not only causes allergic reaction but also can cause asthma if vapors are inhaled by the operator [42].
Another major limitation regarding the plastination protocol is the exposure to pathogens especially during the early processing of samples [43]. This risk factor can be reduced either by the use of protective equipment such as gloves, masks and aprons etc., or by the fixation of specimen in formalin prior to the further processing as formalin effectively neutralizes most of the pathogenic organisms [44].
MORAL AND ETHICAL DILEMMA
Although there are well developed policies regarding the donation of human bodies after death but still there are a number of questions regarding the questionnaire for the consent form [45]. According to a survey, individuals were found more inclined to give consent for whole body plastination instead of the plastination for few body parts only [36]. The reason may be the resentment of the public towards the words “autopsy or dissection”. Similarly, few cultures are more prone towards getting offensive against these body displays in exhibitions [46].
CONCLUSION
Unlike most of the innovations that faced huge criticism in the beginning, plastination was appreciated right from the start. Although it is a much better preservation technique than the conservative methods but room for improvement is still there. Despite the ethical questions being raised, the number of donors, willing to undergo this process, gives a good idea of how this procedure has fascinated the public and this fascination can be used to educate the society for betterment.
REFERENCES
- Jordan G, Thomasius R, Schröder H, Wulf JS, Schlüter O, et al. (2009) Non-invasive mobile monitoring of meat quality. Journal für Verbraucherschutz und Lebensmittelsicherheit 4: 7-14.
- Vass AA (2001) Beyond the grave-understanding human decomposition. Microbiology Today 28: 190-192.
- Saeed M, Rufai AA, Elsayed SE (2001) Mummification to plastination. Saudi Med J 22: 956-959.
- Whitten MB (1928) A review of the technical methods of demonstrating the circulation of the heart. Arch Intern Med 42: 846-864.
- McLachlanJC, Patten D (2006) Anatomy teaching: Ghosts of the past, present and future. Medical Education 40: 243-253.
- Coleman R, Kogan I (1998) An improved low-formaldehyde embalming fluid to preserve cadavers for anatomy teaching. J Anat 192: 443-446.
- Chu WS, Furusato B, Wong K, Sesterhenn IA, Mostofi FK, et al. (2005) Ultrasound-accelerated formalin fixation of tissue improves morphology, antigen and mRNA preservation. Mod Pathol 18: 850-863.
- von Hagens G (1979) Impregnation of soft biological specimens with thermosetting resins and elastomers. Anat Rec 194: 247-255.
- Pashaei S (2010) A brief review on the history, methods and applications of plastination. Int J Morphol 28: 1075-1079.
- Riederer BM (2014) Plastination and its importance in teaching anatomy. Critical points for long-term preservation of human tissue. J Anat 224: 309-315.
- Mantri E, Kataria K, Kumar M, Sushma K, Agarwal R, et al. (2017) Cadaveric study of plastination over formalin. International Journal of Applied Research 3: 531-535.
- ?ora MC (2016) The general protocol for the s10 technique. Research of Clinical Medicine 1: 14-18.
- Ottone NE, Cirigliano V, Bianchi HF, Medan CD, Algieri RD, et al. (2015) New contributions to the development of a plastination technique at room temperature with silicone. Anat Sci Int 90: 126-135.
- Ulmer D (1995) FIXATION: The Key to Good Tissue Preservation. Time 3-6.
- Pereira-Sampaio MA, von Horst C, Marques-Sampaio BPS, Smodlaka H, Favorito LA, et al. (2006) Theoretical considerations and preliminary studies on alcohol as an intermediary solvent. J Int Soc Plastination 21: 27-28.
- Dejong K, Henry RW (2007) Silicone plastination of biological tissue: Cold temperature technique BiodurTM S10/S15 technique and products. Journal of International Society for Plastination 22: 2-14.
- Singh NN, Chaudhary A, Nair S, Kumar S, Mustaqueem M, et al. (2015) Non-perishable museum specimens?: Redefined plastination technique. The Journal of Plastination 27: 20-24.
- Manjunatha K, Prasad RV, Jamuna KV, Placid ED, Rao S, et al. (2014) Comparison of histological architecture of paraffin embedded and indigenously plastinated tissues. Indian Journal of Veterinary Anatomy 26: 132-133.
- Ravi SB, Bhat VM (2011) Plastination: A novel, innovative teaching adjunct in oral pathology. J Oral Maxillofac Pathol 15: 133-137.
- Sargon MF, Tatar ? (2014) Plastination: Basic principles and methodology. Anatomy 8.
- Liang L, Diao Y, Xu Q, Zhang M (2014) Transcranial segment of the trigeminal nerve: Macro-/microscopic anatomical study using sheet plastination. Acta Neurochir (Wien) 156: 605-612.
- Shahar T, Pace C, Henry RW (2007) Epoxy plastination of biological tissue: VisDocta EP73 technique. Journal of International Society for Plastination 22: 46-49.
- Gao H, Liu J, Yu S, Siu, H. (2006) A new polyester technique for sheet plastination. J Int Soc Plastination 21: 7-10.
- Johnson GM, Zhang M (2002) Regional differences within the human supraspinous and interspinous ligaments: A sheet plastination study. Eur Spine J 11: 382-388.
- Sui HJ, Henry RW (2007) Polyester platination for biological tissue: Hoffen P45 technique. J Int Soc Plastination 22: 78-81.
- Sora MC, Cook P (2007) Epoxy Plastination of biological tissue: E12 technique. J Int Soc Plastination 22: 31-39.
- Haque AE (2017) Perception on the Use of Plastinated Specimen in Anatomy Learning Among Preclinical Medical Students of UNIKL RCMP, Malaysia. Journal of Global Pharma Technology 9.
- Kumaraswamy J, Hirusappa R, Naidu J, Raghavendra R (2011) International journal of oral & maxillofacial pathology, (Vol 2). Celesta Software Private Limited, Jaipur, India.
- Valliyate M, Robinson NG, Goodman JR (2012) Current concepts in simulation and other alternatives for veterinary education: A review. Veterinarni Medicina 57: 325-337.
- Fruhstorfer BH, Palmer J, Brydges S, Abrahams PH (2011) The use of plastinated prosections for teaching anatomy-The view of medical students on the value of this learning resource. Clin Anat 24: 246-252.
- Ottone NE, Cirigliano V, Lewicki M, et al. (2014) Plastination technique in laboratory rats: An alternative resource for teaching, surgical training and research development. Int J Morphol 32: 1430-1435.
- Sora MC, Jilavu R, Matusz P (2012) Computer aided three-dimensional reconstruction and modeling of the pelvis, by using plastinated cross sections, as a powerful tool for morphological investigations. Surg Radiol Anat 34: 731-736.
- Kocevski Z, Stefanovska J, Ilieski V, Pendovski L, Atanaskova E (2010) Improved determination of macroscopic parasite preparations using S10 modified plastination procedure. Mac Vet Rev 33: 7-14.
- Prasad G, Karkera B, Pandit S, Desai D, Tonse RG (2015) Preservation of tissue by plastination?: A review. International Journal of Advanced Health Sciences 1: 27-31.
- Ameko E, Achio S, Alhassan S, Sam EE, Quaynor LS, et al. (2013) Further studies on plastinated teaching aids in ghana, three bovine hearts and kidneys. Int J Pure Appl Sci Technol 16: 34-41.
- Bin P, Conti A, Buccelli C, Addeo G, Capasso E, et al. (2016) Plastination: Ethical and medico-legal considerations. Open Med 11: 584–586.
- Dick RB, Setzer JV, Taylor BJ, Shukla R (1989) Neurobehavioural effects of short duration exposures to acetone and methyl ethyl ketone. Occupational and Environmental Medicine 46: 111-121.
- Arts J, Mojet J, Gemert LV, Emmen H, Lammers J, et al. (2002) An analysis of human response to the irritancy of acetone vapors. Crit Rev Toxicol 32: 43-66.
- Costa TL, Barboni MT, Moura AL, Bonci DM, Gualtieri, M, et al. (2012) Long-term occupational exposure to organic solvents affects color vision, contrast sensitivity and visual fields. PLoS ONE 7: 42961.
- Genium (1989) Schenectady. Genium Publishing Corporation, New York, USA.
- Janowsky EC, Kupper LL, Hulka BS (2000) Meta-analysis of the relation between silicone breastimplants and the risk of connective-tissue diseases. N Engl J Med 342: 781-790.
- Yokota K, Johyama Y, Yamaguchi K, Fujiki Y,Takeshita T, et al. (1997) Risk factors for sensitisation to methyltetrahydrophthalic anhydride. Occup Environ Med 54: 667-670.
- Smith BJ, Holladay SD (2001) Risk factors associated with plastination: II. Infectious agent considerations. Journal of the International Society for Plastination 16: 14-18.
- Brown P, Wolff A, Gajdusek C (1990) A simple andeffective method for inactivating virus infectivity informalin-fixed tissue samples from patients with creutzfeldt-jakob disease. Neurology 40: 887-890.
- Chaturvedi RK, Singh A, Chaturvedi P, Mishra A, Mishra SP (2014) Advantages of plastinated human body in medical education and its legal & ethical aspects. Journal of Evolution of Medical and Dental Sciences 3: 2626-2631.
- Giunta LA (2010) The dead on display: A call for the international regulation of plastination exhibits. Columbia Journal of Transnational Law 49.
Citation: Hayat K, Qureshi AS, Rehan S, Rehman T (2018) Plastination - an innovative preservative technique in Anatomy. Trends Anat Physiol 1: 003.
Copyright: © 2018 Khizar Hayat, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.