
Podocyte Infolding Glomerulopathy (PIG): Two Case Reports and Review of the Literature
*Corresponding Author(s):
Bin SunDepartment Of Nephrology, Jiangsu Province Hospital, The First Affiliated Hospital Of Nanjing Medical University, China
Email:sunbing@jsph.org.cn
Jingfeng Zhu
Department Of Nephrology, Jiangsu Province Hospital, The First Affiliated Hospital Of Nanjing Medical University, China
Email:cyxing62@126.com
Abstract
Objective: Podocyte Infolding Glomerulopathy (PIG) is a described pathologic entity characterized by diffuse podocyte infolding into the Glomerular Basement Membrane (GBM) associated with ultra structurally demonstrable microspherular aggregates. The objective of this study was to report the clinical characteristics of two PIG patients and to investigate the pathological changes of nephrotic syndrome caused by immune disease, which may be the abnormal morphology of podocytes entrapment into the glomerular basement membrane. This pathological change is closely related to the immune disease, but is not related to the prognosis.
Case: In case report 1, a 17-year-old China female was admitted due to intermittent lower limb edema for more than 4 years. The patient showed proteinuria without renal functional impairment, and Systemic Lupus Erythematosus other systemic diseases diagnosis for 4 years. Repeated renal biopsy was performed after admission due to poor treatment of repeated proteinuria and unexplained decrease albumin. GBMs were diffusibility and mildly thickened and showed abubbly appearance with Periodic Acid Methenamine Silver (PAMS) staining. Immunofluorescence showed tracea mounts of IgA, IgM and C4d deposition in the mesangium, but was negative for IgG, C3, C1q , κ,λ, HBsAg , HBcAg , PLA2R, IgG1, IgG2, IgG3, IgG4 and fibrinogen. Electron microscopy showe microspheres of different sizes and microtubular membranous structures are seen in the basement membrane of the glomerular capillary loop. Additional serologic tests revealed positive antinuclear antibodies (antinuclear antibody was detected: 1/3200homogeneous/cytoplasm). The diagnosis was lupus nephritis with PIG. The patient was treated with Prednisone Acetate Tablets, tacrolimus, hydroxychloroquine and mycophenolate mofetil for three months, after which proteinuria decreased to the normal range and albumin increased. In case report 2, a 64-year-old China man was admitted to the hospital because he found foam urine for more than 2 months and edema of both lower limbs for 2 weeks. Proteinuria: 3+, Albumin: 18.6g/L, The clinical diagnosis was nephrotic syndrome. The Renal biopsy pathology showed Membrane nephropathy with PIG. Immunofluorescence results showed that IgG and IgG3 were positive, IgG1, IgG2, IgG4, IgA, IgM, CLQ, C3, C4, TG, K chain, λ chain, HBsAg, HBcAg and PLA2R were negative.Congo red staining was negative. Electron microscopic examination showed microspheres of different sizes and microtubular membranous structures were observed in the basement membrane. The diagnosis revealed PIG.The patient was treated with oral methylprednisolone.
Conclusion: Characteristic clustering of these microparticles near the invaginated tips of podocyte foot processes in the GBM was observed on transmission electron microscopy. The clinical, light microscopic, and diagnostic electron microscopic features of this condition are highlighted in this report in an attempt to contribute some insights into the possible pathogenetic mechanisms of this obscure entity. PIG is closely associated with immune diseases, which may be a transient phenomenon caused by immune disorders.
Keywords
Glomerular basement membrane; Immune disorders; Nephritis; Nephrotic syndrome; Podocyte infolding glomerulopathy; Renal biopsy
Abbreviations
PIG: Podocyte Infolding Glomerulopathy
GBM: Glomerular Basement Membrane
NS: Nephrotic Syndrome
EM: Electron Microscopy
Introduction
Podocytic Infolding Glomerulopathy (PIG) is a peculiar glomerular abnormality characterized the thickened Glomerular Basement Membrane (GBM) on account of podocyte infolding and invagination into the glomerular basement membranes widespread. It was first reported in 2007 and soon proposed as a new disease entity by Joh et al. [1]. Since the first report of this peculiar EM finding in 2002, it was thought to be a variant of Membranous Nephropathy (MN) exhibiting annular subepithelial deposits [2]. It is only in the past few years that PIG has begun to be recognized as a possible new and distinct disease entity. According to a nationwide survey of 25 cases in Japan, PIG is heterogeneous both clinically and morphologically. Light microscopic features of PIG may include membranous nephropathy-like GBM thickening, Focal Segmental Glomerulosclerosis (FSGS), or minimal glomerular alteration; immune-type deposits may or may not be present; and it may be associated with or without collagen diseases. PIG has been reported in Japan and Indian and Korean. However, genetic or regional differences have not been reported. We report a case from China fulfilling the diagnostic criteria of PIG in a patient presenting with Systemic Lupus Erythematosus. Because there have been only a few reports of this entity in the literature confined geographically to Indian [3] and Korean [4] and Japan [5-11]. There is a need to share information about this entity from all parts of the world to characterize the spectrum of this glomerulopathy.
Report Of Cases
In case report 1, a 17-year-old Chinese female was hospitalized for more than 4 years with edema in both lower limbs. Four years ago, she was admitted to the hospital for soy-stained urine and systemic lupus erythematosus, and was diagnosed as type III lupus nephritis by renal biopsy (no specific examination, examination and renal biopsy report was found, and no electron microscopy examination was performed). Later, she was treated with prednisone acetate and leflunomide according to the 2016 treatment standard for lupus nephritis. In September 2017, the patient went to the local hospital for reexamination of anti-double-stranded DNA (a-dsDNA) immunobloting 2+, and was treated with Mortimexophenate (MMF) and methylprednisolone plus hydroxychloroquine for control of the disease. During the disease period, the patient took medication irregularly, the patient's compliance was poor, and the treatment plan was changed several times (the specific medication situation and examination results were unknown). In January 2020, the patient went to the local hospital for reexamination, and the anti-double-chain DNA (a-dsDNA) immunoblot showed positive results of 2+. According to the treatment standard of lupus nephritis in 2020, the local doctor gave prednisone acetate tablets, tacrolimus and hydroxychloroquine plus MMF. During this period, the patient reported joint soreness of both lower extremities. On July 28, 2020, the patient came to our hospital for reexamination and was given additional treatment with prednisone acetate tablets, and admitted to our department for examination. According to the relevant examination, the SLE-DAI score was 15, and the active stage of lupus nephritis was considered.The patient's 24-hour urinary protein quantitation was 458mg/24h and albumin 29.8g/L. Due to the
unchanged protein intake and normal liver function, unexplained hypoproteinemia occurred clinically, and the family members required repeated renal biopsy.
Additional serologic tests revealed positive antinuclear antibodies (antinuclear antibody was detected: 1/3200 homogeneous/cytoplasm). The patient was treated with Prednisone Acetate Tablets, tacrolimus, hydroxychloroquine and mycophenolate mofetil for three months, after which proteinuria decreased to the normal range and albumin increased. On physical examination, the patient had pallor and bilateral pitting edema. There are scattered rashes on bilateral cheeks and lower limbs. Blood pressure was recorded as 108/70 mm Hg. Auxiliary examination as shown in table 1.
|
|
Case 1 |
Case 2 |
|
Age/Sex |
17/F |
64/M |
|
Blood pressure (mmHg) |
108/70 |
138/56 |
|
Combined diseases
|
SLE |
Peripheral vascular disease, Chronic Gastritis |
|
Urinalysis |
||
|
Urinary protein (g/day) |
0.46 |
8.4 |
|
Occult blood |
(-) |
1+ |
|
Blood chemistry |
|
|
|
BUN (mg/dl) |
86.4 |
82.8 |
|
Cr (mg/dl) |
0.53 |
7.4 |
|
Total protein (g/l) |
7.31 |
4.19 |
|
Albumin (g/l) |
2.98 |
2.7 |
|
T-cholesterol (mg/dl) |
86.4 |
112.3 |
|
Serology |
||
|
Antinuclear antibody |
1/3200 |
(-) |
|
C3 (mg/dl) |
44.5 |
60.2 |
|
C4 (mg/dl) |
9.3 |
22.1 |
|
Serum Cys (mg/dl) |
9.4 |
12.5 |
|
RF(IU/mL) |
46.5 |
<2 |
|
Antistreptococcal hemolysin O (IU/mL) |
<25.0 |
<25.0 |
|
CRP (mg/dl) |
9.3 |
22.1 |
|
HBsAg |
(-) |
(-) |
|
HCV Ab |
(-) |
(-) |
|
TPHA |
(-) |
(-) |
Table 1: Clinical and laboratory fifindings of the patients.
Note: BUN blood urea nitrogen, Cr serum creatinine, T-Cholesterol total cholesterol,C3 Complement 3, C4 Complement 4, RF rheumatoid factors, CRP C-reactive protein, HBsAg hep atitis B surface antigen, HCVAb antibody to the hepatitis C virus,TPHA Treponema pallidum hemagglutination test.
Kidney biopsy revealed a total of 24 glomeruli, 2 of which showed focal segmental mesangial proliferation with mild capillary wall thickening and focal segmental sclerosis (Figures 1A and 1B). Immunofluorescence (IF) showed positivity for immunoglobulin A (IgA) and M (IgM) (trace) deposition in the mesangium and capillary loops (Figures 1C and 1D). Immunofluorescent staining for PLA2R (trace) showed positivity. EM showed irregular thickening of GBMs with peculiar subepithelial clusters of microspherules. Diffuse podocyte foot-process effacement associated with microvillous transformation and podocyte cytoplasmic vacuoles indicative of podocyte injury were noted. Most of the structures were located at intramembranously without a contact to endothelial cell or podocyte, but some were close to or seemed to connect to these cells. Additionally, there was global infolding of podocyte cell processes into the underlying thickened GBMs, remarkable for its diffuse involvement and proximity of the invaginations to the microspherular clusters (Figures 1E and 1F). There were discrete electron-dense deposits in the GBM and the mesangium. A final diagnosis of Lupus nephritis with PIG was rendered based on these findings.
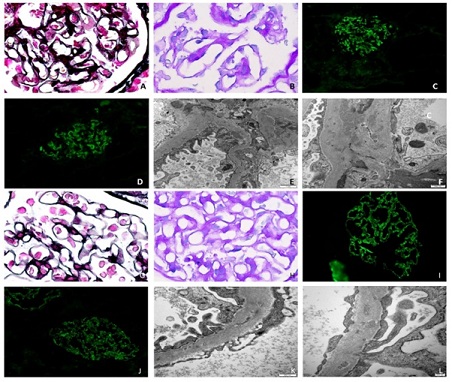 Figure 1: A. Light microscopy findings of renal biopsies under (PAMS) staining, glomerular capillary walls werethickened with a diffuse bubbling appearance (Figure a) (PAMS, 1000×); B. Light microscopy findings of renalbiopsies glomerular capillary walls were thickened with a diffuse bubbling appearance (Figure b) (PAS, 1000×). C. Immunofluorescence microscopy findings of renal biopsies. Trace amounts of IgA (Figure C)and IgM (Figure D) deposition in the mesangium (immunofluorescence 400×). E. Thickened glomerular basement membrane (GBM) with abundant subepithelial microparticular aggregates (arrow) characteristic of podocyte infolding glomerulopathy (PIG), deposition in the mesangium and the GBM. (transmission EM; original magnification, 2,000×). F. Microparticular aggregates in the GBM surrounding a degenerated tip of invaginated podocyte foot process. Electron densities are present in the GBM and the mesangium with microtubules (transmission EM; original magnification, 100,000×). G. Light microscopy findings of renal biopsies under (PAMS) staining, glomerular capillary walls were thickened with a diffuse bubbling appearance (Figure a) (PAMS, 1000×); H. Light microscopy findings of renal biopsies glomerular capillary walls were thickened with a diffuse bubbling appearance (Figure b) (PAS, 1000 × ). I. Immuno fluorescence microscopy findings of renal biopsies. Trace amounts of IgG (Figure I) and IgG3 (Figure J) deposition in the GBM (immunofluorescence 400×). K. Thickened glomerular basement membrane (GBM) with abundant subepithelial microparticular aggregates (arrow) characteristic of podocyte infolding glomerulopathy (PIG), deposition in the GBM. (transmission EM; original magni fi cation, 100,000 × ). L. Microparticular aggregates in the GBM surrounding a degenerated tip of invaginated podocyte foot process. Electron densities are present in the GBM and the mesangium with microtubules (transmission EM; original magnification, 500,000×).
Figure 1: A. Light microscopy findings of renal biopsies under (PAMS) staining, glomerular capillary walls werethickened with a diffuse bubbling appearance (Figure a) (PAMS, 1000×); B. Light microscopy findings of renalbiopsies glomerular capillary walls were thickened with a diffuse bubbling appearance (Figure b) (PAS, 1000×). C. Immunofluorescence microscopy findings of renal biopsies. Trace amounts of IgA (Figure C)and IgM (Figure D) deposition in the mesangium (immunofluorescence 400×). E. Thickened glomerular basement membrane (GBM) with abundant subepithelial microparticular aggregates (arrow) characteristic of podocyte infolding glomerulopathy (PIG), deposition in the mesangium and the GBM. (transmission EM; original magnification, 2,000×). F. Microparticular aggregates in the GBM surrounding a degenerated tip of invaginated podocyte foot process. Electron densities are present in the GBM and the mesangium with microtubules (transmission EM; original magnification, 100,000×). G. Light microscopy findings of renal biopsies under (PAMS) staining, glomerular capillary walls were thickened with a diffuse bubbling appearance (Figure a) (PAMS, 1000×); H. Light microscopy findings of renal biopsies glomerular capillary walls were thickened with a diffuse bubbling appearance (Figure b) (PAS, 1000 × ). I. Immuno fluorescence microscopy findings of renal biopsies. Trace amounts of IgG (Figure I) and IgG3 (Figure J) deposition in the GBM (immunofluorescence 400×). K. Thickened glomerular basement membrane (GBM) with abundant subepithelial microparticular aggregates (arrow) characteristic of podocyte infolding glomerulopathy (PIG), deposition in the GBM. (transmission EM; original magni fi cation, 100,000 × ). L. Microparticular aggregates in the GBM surrounding a degenerated tip of invaginated podocyte foot process. Electron densities are present in the GBM and the mesangium with microtubules (transmission EM; original magnification, 500,000×).
The patient was treated with high-dose prednisolone and mycophenolate mofetil for 6 weeks, and a slow tapering schedule of steroids was planned. In view of aggressive disease, she was also given a single dose of 1 g of rituximab. On follow-up after 7 months, there was marked improvement in kidney function (serum creatinine, 0.98mg/dL) and slow resolution of proteinuria (urine protein excretion of 1g/d; urinary protein -creatinine ratio, 2.16).
In case report 2, a 64-year-old China man was admitted to the hospital because he found foam urine for more than 2 months and edema of both lower limbs for 2 weeks. The patient suffered from chronic gastritis for 30 years and was treated by regular oral administration of omeprazole 1 tablet QD with blood pressure of 138/56mmHg. Physical examination revealed edema in both lower extremities. Auxiliary examination (Table 1 case 2).
The kidney biopsy showed: Immunofluorescence results showed that IgG and IgG3 were positive,IgG1, IgG2, IgG4, IgA, IgM, CLQ, C3, C4, TG, K chain, λ chain, HBsAg, HBcAg and PLA2R were negative (Table 2).Congo red staining was negative.The light microscopy diagnosis revealed a membranous lesion. Renal biopsy pathology showed 11 glomerular, glomerular mesangial cell and mesangial matrix mild hyperplasia, open good capillary loops, segmental thickening of basement membrane, a few balls balloon adhesions renal small tube cavity see protein tube and a little red tube, interstitial inflammat ory cell infiltration, no obvious fibrosis, small artery wall thickening (Table 2). Electron microscopic examination showed glomerular mesangial cell and mesangial matrix segmental mild hyperplasia, glomerular capillary loops is opening good, the podocyte foot process fusion (>80%), no obvious endothelial cell hyperplasia, segmental in basement membrane and epithelial next see a small number of electron dense deposit, part of the epithelium of electron density inhomogeneity bright areas around the dense, The segments of the basement membrane were irregularly thickened and insect-etched. Microspheres of different sizes and microtubular membranous structures were observed in the basement membrane (Table 2). There was no obvious proliferation of epithelial cells in the parietal layer of renal cyst, and no crescent body was observed. Vacuolar degeneration of renal tubular epithelial cells. There was no special lesion in renal interstitium. The diagnosis revealed a membranous lesion. A final diagnosis of Membrane nephropathy with PIG was rendered based on these findings.
Patient was discharged with a diagnosis of membranous nephropathy with PIG; chronic kidney disease stage 1; pulmonary infection; week Perivascular disease. According to KDGO guidelines, treatment with (moxifloxacin hydrochloride injection) Biflox 400mg, 1 bottle a day, methylprednisolone sodium succinate: methylprednisolone 40mg once a day, with Enox Heparin sodium injection (Kexai) 2000AXaIU, 0.5 vials, once a day; Tolvaptan tablets (Su Maca) 1/2 tablet per day; 1 capsule of calcitriol per day.
Discussion
Although it is worthwhile to discuss the salient features of this case to shed more light on our understanding of this entity, it is uncertain whether PIG is a new disease entity or a transient morphologic phenomenon. The biopsy specimen of lightmicroscopic examination in this case showed mild thickening of capillary walls with focal segmental sclerosis. The presence of microparticles in the GBM was first described in 1973 [12]. The microparticles were regarded as sequelae of GBM injury and repair related to immune deposition, since they appeared focally and mostly in cases of membranous nephropathy. However, EM revealed subepithelial clusters of microspheres measuring 50 to 70nm, some having distinct double membranes (Figures 1F and 1L).We reviewed 32 PIG patients (Table 2 ), age 17 to 70, mean age 43.4±23.3, female 24 (75%), male 8 (25%), hypertension 13 (40.6%), and 62.5% of patients who were treated with glucocorticoid alone in remission. In case1, systemic lupus erythematosus was associated with a large amount of protein renal biopsy, so the treatment effect was poor. Four years later, repeated renal biopsy was performed PIG by electron microscope. Microstructures in the GBM are typically not found in classic MN, aside from occasional granular and membranous debris in stage III MN and in rare cases of hepatitis B virus-associated glomerulonephritis [6]. In 2008, Japan reported 12 out of 25 pigs with SLE and only 1 PIG alone, and three different folding forms of podocyte were described [13]. Immune EM analysis of the glomerular lesions of PIG has demonstrated vimentin and complement C5b-C9 in the extracellular organized structures in the GBM, indicative of either podocyte or endothelial cell origin of these particles [14]. Heretofore, one PIG with undifferentiated connective tissue disease was reported in India in 2017 [15]. A 45-year-old Indian woman with a diagnosis of undifferentiated connective tissue disease was prescribed methotrexate and hydroxychloroquine for symmetric non deforming inflflammatory polyarthritis of 1 year’s duration. Four months later, she presented with bilateral pedal edema and generalized malaise of 2 months’ duration. There was no relevant past or family history of autoim mune diseases. Another PIG with paraganglioma was reported in South Korea in 2016 [16]. It was a 44-year-old female was admitted to our hospital for the evaluation of proteinuria, which was detected in a regular checkup. She had a history of surgical excision of a 1.5-cm-sized paraganglioma in the left neck three years ago. We report two cases of PIG associated with Systemic Lupus Erythematosus in a 17-year-old female China who presented with Edema in disputea and a 64-year-old China man who found nephrotic syndrome. Ultrastructural analysis of the kidney biopsy specimen revealed unusual subepithelial aggregates of microspherules admixed with few microtubules alongside extensive infolding of podocyte foot processes into the underlying GBMs. Broadly speaking, nephrotic syndrome was clinically diagnosed in 25% (8/32) of PIG patients, and was diagnosed by electron microscopy as podocyte microvesicles encapsulated in the basement membrane. Most 16 (69.6%) patients were effectively relieved by glucocorticoid therapy. PIG was first found in membranous nephropathy [6], and its pathogenesis may be similar to that of membranous nephropathy.The authors hypothesized that the basement membrane may be responsible for the obstacle of immune recognition of podocytes, and then the basement membrane gradually envelops the podocytes to achieve the purpose of phagocytic repair. The whole pathogenesis is similar to that of membranous nephropathy in which the subepithelial immune complex is engulfed by the basement membrane, but the specific pathogenesis needs further experimental confirmation.
|
Characteristic |
Value |
||
|
Agea(year) |
43.4±23.3 |
||
|
Sex |
Female |
24(75%) |
|
|
Male |
8(25%) |
||
|
NS |
8(25%) |
||
|
Blood pressure(mmHg) |
Hypertension |
13(40.6%) |
|
|
Normol |
19(63.3%) |
||
|
Hematuria b(HPF) |
<10 |
25(78.1%) |
|
|
>10 |
7(21.9%) |
||
|
Proteinuria Initial(g/day) |
2.7±5.6 |
||
|
Renal function (mg/dl) |
Cr ≤1.2 |
25(78.1%) |
|
|
Cr>1.2 |
7(21.9%) |
||
|
Treatment |
PLS |
16(69.6%) |
Remission:10 62.5% |
|
PLS+MMF |
4(17.4%) |
Remission:3 75% |
|
|
ARB |
2(8.7%) |
||
|
Diuretics |
2(8.7%) |
||
|
Remission |
Yes |
19(59.4%) |
|
|
No |
13(40.6%) |
||
|
Concomitant diseases |
Yes |
28(87.5%) |
|
|
No |
4(12.5%) |
||
|
Concomitant diseases |
SLE |
18(56.3%) |
|
|
MCTD |
1(3.1%) |
||
|
RA |
1(3.1%) |
||
|
HBVsAg |
1(3.1%) |
||
|
Ovarian mature teratoma |
1(3.1%) |
||
|
VUR with bilateral hydronephrosis |
1(3.1%) |
||
|
Sjo¨gren’s synd |
2(6.2%) |
||
|
Peripheral vascular disease |
1(3.1%) |
||
|
Tumor lysis syndrome |
1(3.1%) |
||
|
Hypothyroidism, chronic thyroiditis |
1(3.1%) |
||
|
Np |
4(12.5%) |
||
Table 2: Pathological profifile of PIG (32 cases).
Note: NS nephrotic syndrome, HPF high-power field, Cr serum creatinine, PSL prednisolone, MMF mycophenolate mofetil, CyA Cyclosporine A, ARB angiotensin II receptor antagonist, ND not done, np not particular, SLE systemic lupus erythematosus, RA rheumatoid arthritis, MCTD mixed connective- tissue disease, VUR vesicoureteral reflux.
In conclusion, the extent and severity of PIG disease raises doubts about deficiencies in immune recognition and repair mechanisms. More case reports and experimental information are needed to better understand the clinical course and pathogenesis of this disease.
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Funding from the Medical Innovation Team Foundation is gratefully acknowledged.
References
- Joh K, Taguchi T, Shigematsu H, Kobayashi Y, Sato H, et al. (2008) Proposal of podocytic infolding glomerulopathy as a new disease entity: A review of 25 cases from nationwide research in Japan. Clin Exp Nephrol 12: 421-431.
- Debiec H, Guigonis V, Mougenot B, Decobert F, Haymann JP, et al. (2002) Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med 346: 2053-2060.
- Matthai SM, Mohapatra A, Mathew AJ, Roy S, Varughese S, et al. (2017) Podocyte Infolding Glomerulopathy (PIG) in a patient with undifferentiated connective tissue disease: A case report. Am J Kidney Dis 72: 149-153.
- Kye WK, Hyeon JJ, Jang HL (2016) Podocytic infolding glomerulopathy: A case report. Ultrastruct Pathol 40: 374-377.
- Mii A, Shimizu A, Masuda Y (2008) A case of lupus nephritis with diffuse podocytic infolding into the glomerular basement membrane. Clin Exp Nephrol 12: 479-484.
- Masuda Y, Mii A, Shimizu A, Fujita E, Aki K, et al. (2008) Invagination and infolding of podocytes in glomerular basement membrane in the cases of primary membranous nephropathy. Clin Exp Nephrol 12: 440-449.
- Koike K, Utsunomiya Y, Ito Y, Tokudome S, Miyazaki Y, et al. (2008) A case of Glomerulopathy showing podocytic infolding in association with Sjogren’s syndrome and primary biliary cirrhosis. Clin Exp Nephrol 12: 489-493.
- Kitazawa K, Joh K, Akizawa T (2008) A case of lupus nephritis coexisting with podocytic infolding associated with Takayasu’s arteritis. Clin Exp Nephrol 12: 462-466.
- Yoshimura K, Joh K, Kitamura H, Takahashi Y, Yokote S, et al. (2008) A case report of glomerulopathy-associated podocytic infolding in a patient with tumor lysis syndrome. Clin Exp Nephrol 12: 522-526.
- Sugiyama H, Maruyama M, Morinaga H, Inoue T, Takiue KI, et al. (2008) Unique microstructures and podocytic infolding in glomerular basement membrane associated with collagen diseases: A report of three cases. Clin Exp Nephrol 12: 450-454.
- Harada M, Kamijo Y, Ehara T, Shimojo H, Shigematsu H, et al. (2014) A case of podocytic infolding glomerulopathy with multiple myeloma. BMC Nephrol 15: 32.
- Burkholder PM, Hyman LR, Barber TA (1973) Extracellular cluster of spherical microparticle in glomeruli in human renal glomerular disease. Lab Invest 28: 415-425.
- Yukinari M, Akiko M, Akira S, Fujita E, Aki K, et al. (2008) Invagination and infolding of podocytes in glomerular basement membrane in the cases of primary membranous nephropathy. Clin Exp Nephrol 12: 440-449.
- Fujigaki Y, Muranaka Y, Sakakima M, Ohta I, Sakao Y, et al. (2008) Analysis of intra-GBM microstructures in a SLE case with Glomerulopathy associated with podocytic infolding. Clin Exp Nephrol 12: 432-439.
- Smita MM, Anjali M, Ashish JM, Roy S, Varughese S, et al. (2017) Podocyte Infolding Glomerulopathy (PIG) in a patient with undifferentiated connective tissue disease: A case report. Am J Kidney Dis 72: 149-153.
- Kye WK, Hyeon JJ, Jang HL (2016) Podocytic infolding glomerulopathy: A case report. Ultrastruct Pathol 40: 374-377.
Citation: Yan Z, Qian J, Sun B, Zhu J (2022) Podocyte Infolding Glomerulopathy (PIG): Two Case Reports and Review of the Literature. J Nephrol Renal Ther 8: 070.
Copyright: © 2022 Zhao Yan, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

