
Possibilities of Modern Anti-Relapse Therapy of Urinary Tract Infections in Children: Crutil Trial 1 Year Follow-Up
*Corresponding Author(s):
Dmytro D IvanovProfessor In Nephrology, Head Of Nephrology And RRT Dep, Shupyk National Medical Academy Of Postgraduate Education, Kiev, Ukraine
Tel:+044 2598599,
Email:ivanovdd@i.kiev.ua; mesangium88@gmail.com
Abstract
Purpose
To evaluate the effectiveness of immunoprophylaxis in children with recurrent urinary infections.
Methods
A prospective, open, multicenter, parallel-group randomized CRUTIL study was conducted in 83 children aged 3 to 15 with recurrent Urinary Tract Infections (UTI). Children were randomized into 3 groups: First-UC-consisted of 22 children receiving lysate Urivac therapy, second-UM-consisted of 28 children receiving additional lysate Uro-Vaxom therapy and third-control group-consisted of 33 children receiving standard therapy. The observation period lasted for 24 months. 78 children were available to analyze in 1 year follow-up.
Results
By the end of the study, in the first group, there were 87% (19 children) and in 1-year follow-up were 18 relapse-free patients who has been received hexavalent bacterial lysate (Urivac) vaccine. In the group receiving bacterial monolysate Uro-Vaxom, 72% (20 children) of relapse-free patients were observed (OR=2.5; P>0.05, minimum value of expected event-4.84) and 17 children in 1-year follow-up. Among those patients who did not receive urinary antiseptic at bedtime and bacterial lysates, there were 13 relapse-free children (40%) (?≤0.05, OR=0.26 with the group receiving hexavalent vaccine) and 10 in 1-year follow-up.
Conclusion
Bacterial lysates significantly increase the efficacy of therapy in children with recurrent urinary tract infections. Prescribing of preventive single dose of urinary antiseptic at bedtime and use of hexavalent vaccine Urivac have proved to be the most beneficial in forming of relapse-free progression of the recurring UTIs using 10-days for 6 following months and 10 days each 6 months in 2 years.
Keywords
Follow-up in UTI; Industrially produced bacterial lysates; Recurrent urinary tract infections in children; Urivac; Uro-Vaxom
INTRODUCTION
Recurrent Urinary Tract Infections (UTI) in children present a serious problem in modern pediatric nephrology [1]. UTI may be divided into 2 groups: Recurrent infections in children with anomalies of urinary organs (CAKUT syndrome) that often affect upper urinary tract (pyelonephritis) and infections that are classified as persistent ones [2]. UTIs (second group) that are not characterized by urodynamic disorders are usually classified as unresolved ones and affect lower urinary tract (cystitis) [3]. In unresolved infection, initial therapy is inadequate for elimination of bacterial growth in the urinary tract (inadequate therapy, inadequate antimicrobial urinary concentration, poor renal concentration/gastrointestinal malabsorption) and these infections involve multiple organisms with different antimicrobial susceptibilities and antibiotic resistance [2,4]. Children without CAKUT syndrome excite great clinical interest due to the absence of an objective reason for recurrences, but nevertheless such children often have unresolved infections [5] and asymptomatic progression of the disease [6]. Recurring progression of infections can lead to kidney scarring and a gradual loss of kidney function [7,8].
Frequency of UTI recurrence in children may vary by demographics. In the region where the study below was conducted, frequency of recurrent UTIs is 15% of all UTIs. [9].
European Association of Urology (2012-2019) has been recommending antibiotics and immunoprophylaxis for the treatment of recurrent urinary tract infections. Long-term antibacterial prophylaxis should be considered in cases of high susceptibility to UTI and risk of acquired renal damage: Two recently published prospective randomized trials as well as one meta-analysis demonstrated a significant risk reduction of developing another UTI by using continuous antibiotic prophylaxis [2].
Cochrane Systematic Review states that long-term antibiotics appear to reduce the risk of repeated symptomatic UTI in susceptible children, but the benefit is small-it is lost with antibiotic withdrawal and must be considered together with the increased risk of microbial resistance [10].
Cranberry juice and probiotics may also prevent recurrence of UTI [11]. Immunoprophylaxis is more effective and can be conducted by using industrially produced bacterial lysates, for example, Uro-Vaxom and bacterial autolysates. The latter are more effective, but they are delivered invasively and have a lot of side effects [3].
The findings provided indicate that a universal effective approach that could completely stop recurring of UTI is not determined for today [9].
The objective of this study was a comparative efficacy evaluation of anti-relapse therapy of UTI in children through the use of industrially produced bacterial lysates in the form of vaccines, namely Uro-Vaxom and Urivac.
MATERIALS AND METHODS
A prospective, open, multicenter, parallel-group randomized CRUTIL study (Children’s Recurrent Urinary Tract Infections on bacterial Lysate) was conducted in 83 children aged 3 to 15 (8±2.2) with recurrent UTI in three certified health care facilities of Kyiv (Ukraine) from 2016 to 2018. Diagnosis of UTI was confirmed according to criteria of EAU, 2016-2018 [2]. Study lasted for 18 months+6 months of treatment free follow-up. 1 year after 78 children had been analyzed to prove the results obtained previously.
The duration of the study was dictated by the longest possible follow-up taking in account the possible positive result of immune protection longevity.
Inclusion criteria
Recurrent UTI (2 episodes within 6 months or 3 episodes within a year). Methods for selecting patients: Voluntary participation with parental consent. Randomization relied on random number method; recruitment was based on WHO recommendations [12].
Exclusion criteria
Visual anomalies of urinary organs, carriage of Chlamydia Trachomatis, Ureaplasma Urealiticum, Mycoplasma genitalium, Trichomonas Vaginalis, inflammatory diseases of external genital organs.
Primary endpoint
Number of children with relapse-free progression of UTI.
Secondary endpoint Asymptomatic bacteriuria that is defined as absence of clinical symptoms of UTI, leukocyturia in bacteriuria more than 10.000CFU/mL.
Study protocol
83 children were randomized into 3 groups: First-UC-consisted of 22 children, second-UM-consisted of 28 children and third-control group-consisted of 33 children (Figures 1 and 2). The male to female ratio was 1:6 in the UC group, 1:6 in UM group and 1:5 in control group.
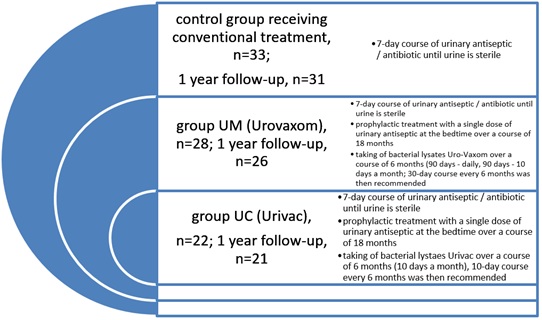 Figure 1: Characteristics of the studied population: Control group (n=33, m:f ratio 1:5, age 3-14 (9±2,1), complicated clinical course in 22%; Urovaxom group (n=28, m:f ratio 1:6, age 5-15 (9±2,2), complicated clinical course in 24%; Urivac group (n=22, m:f ratio 1:6, age 4-15 (9±1,8), complicated clinical course in 25%. Age of the disease 1, 2 years (±0,3).
Figure 1: Characteristics of the studied population: Control group (n=33, m:f ratio 1:5, age 3-14 (9±2,1), complicated clinical course in 22%; Urovaxom group (n=28, m:f ratio 1:6, age 5-15 (9±2,2), complicated clinical course in 24%; Urivac group (n=22, m:f ratio 1:6, age 4-15 (9±1,8), complicated clinical course in 25%. Age of the disease 1, 2 years (±0,3).
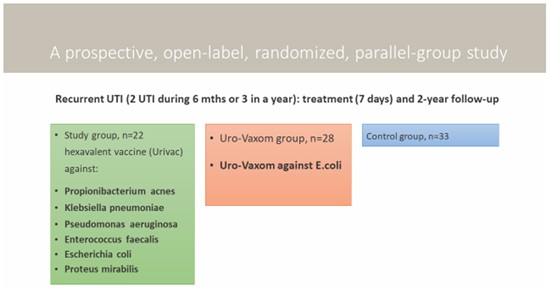 Figure 2: Study design.
Figure 2: Study design.
Uro-Vaxom is bacterial lysates of 18 strains Escherichia coli, 6mg in one capsule; Urivac 5mg consists of 6 lysates: Propionibacterium acnes lysatum cryodessicatum (??? 7083)-1.66mg, Klebsiella pneumoniae lysatum cryodessicatum (??? 7589)-0.67mg, Pseudomonas aeruginosa lysatum cryodessicatum ??? (7590)-0.67mg, Enterococcus faecalis lysatum cryodessicatum (???7591)-0.67mg, Escherichia coli lysatum cryodessicatum (??? 7593)-0.67mg, Proteus mirabilis lysatum cryodessicatum (??? 7592)-0.67mg. ???-Catalog of ?ultures of Microorganisms. According to recommendations of EAU, children of all groups received standard treatment in case of UTI exacerbation [1,2].
UM and UC groups of children of similar age with UTI received bacterial lysates Uro-Vaxom (UM, n=28) over a period of 6 months (taking 1 capsule within 90 days in the morning on an empty stomach and within 90 days more with 10 days a month; then 30-day course once 6 months was recommended) and Urivac (UC, n=22) over a period of 6 months (taking of 1 capsule within 10 days of every month in the morning on an empty stomach; then 10-day course once 6 months). Moreover, patients of these groups also received anti-relapse treatment with a single dose of urinary antiseptic over a course of 18 months. These drugs were officially approved in Ukraine for the treatment of recurrent UTI. These drugs are freely available in pharmacies and a patient can buy them for his or her personal usage according to doctor’s prescription. The dosage will be correctly individualized by doctor.
Control group consisted of 33 children with UTI who did not receive urinary antiseptic and bacterial lysates after the treatment of urinary tract infections (Table 1).
 Table 1: Schedule for 24 months.
Table 1: Schedule for 24 months.
Statistical methods
The data was entered and analyzed by using appropriate statistical measures. Categorical data was presented as number and percentage. Continuous data is presented as mean and Standard Deviation (SD) if normally distributed or median and Interquartile Range (IQR) if not normally distributed. The Student’s t-test is used for the comparison of mean and median deviations, respectively. Multiple logistic regressions are used for estimate the adjusted risk ratio of side effects and cardio-vascular risks. A p-value of less than 0.05 is considered to be significant.
RESULTS
In the first group receiving hexavalent vaccine (Urivac), relapse-free progression was observed in 87% of patients (19 children) by the end of the study. In the group receiving bacterial monolysates Uro-Vaxom, relapse-free progression was observed in 72% (20 children) (OR=2.5; Fisher’s exact test (two-tailed)-0.30622, P>0.05, minimal value of expected event-4.84). Among those patients who did not receive urinary antiseptic at bedtime and bacterial lysates, relapse-free progression was observed in 13 children (40%). 15% increase in efficacy when using 6-valent bacterial lysate was achieved through elimination of Pseudomonas aeruginosa and Enterococcus faecalis (Figure 3)
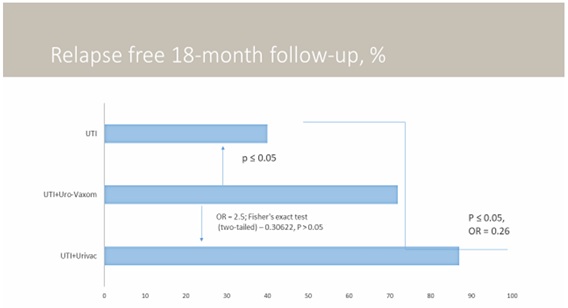 Figure 3: Results of treatment in groups. Characteristics of the studied population: Control group (n=33, m:f ratio 1:5, age 3-14 (9±2,1), complicated clinical course in 22%; Urovaxom group (n=28, m:f ratio 1:6, age 5-15 (9±2,2), complicated clinical course in 24%; Urivac group (n=22, m:f ratio 1:6, age 4-15 (9±1,8), complicated clinical course in 25%. Age of the disease 1, 2 years (±0,3).
Figure 3: Results of treatment in groups. Characteristics of the studied population: Control group (n=33, m:f ratio 1:5, age 3-14 (9±2,1), complicated clinical course in 22%; Urovaxom group (n=28, m:f ratio 1:6, age 5-15 (9±2,2), complicated clinical course in 24%; Urivac group (n=22, m:f ratio 1:6, age 4-15 (9±1,8), complicated clinical course in 25%. Age of the disease 1, 2 years (±0,3).
Six-month follow-up has shown statistical validity in the groups of children receiving bacterial lysates (Figure 4). By the end of the second year, in UM group consisting of 28 children, 11 children had relapse-free progression, but recurrence was observed in 17 children. On the contrary, in UC group consisting of 22 children, 19 children had relapse-free progression, but recurrence was observed in 3 patients. Statistical characteristics are shown in table 2.
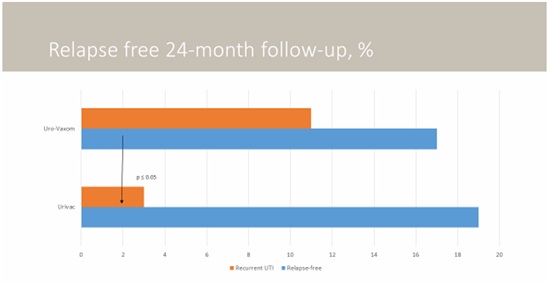 Figure 4: Relapse free 24-months follow-up. Characteristics of the studied population: Control group (n=33, m:f ratio 1:5, age 3-14 (9±2,1), complicated clinical course in 22%; Urovaxom group (n=28, m:f ratio 1:6, age 5-15 (9±2,2), complicated clinical course in 24%; Urivac group (n=22, m:f ratio 1:6, age 4-15 (9±1,8), complicated clinical course in 25%. Age of the disease 1, 2 years (±0,3).
Figure 4: Relapse free 24-months follow-up. Characteristics of the studied population: Control group (n=33, m:f ratio 1:5, age 3-14 (9±2,1), complicated clinical course in 22%; Urovaxom group (n=28, m:f ratio 1:6, age 5-15 (9±2,2), complicated clinical course in 24%; Urivac group (n=22, m:f ratio 1:6, age 4-15 (9±1,8), complicated clinical course in 25%. Age of the disease 1, 2 years (±0,3).
|
|
End of the Trail |
1-Year Follow-up |
|
Absolute risk in Monovaccine group (EER) |
0.864 |
0.654 |
|
Absolute risk in pentavaccine group (CER) |
0.607 |
0.857 |
|
Relative risk (RR) |
1.422 |
0.763 |
|
Standard error of relative risk (S) |
0.174 |
0.168 |
|
The lower limit of 95% CI (CI) |
1.011 |
0.549 |
|
The upper limit of 95% CI (CI) |
2.001 |
1.061 |
|
Relative risk reduction (RRR) |
0.422 |
0.237 |
|
Risk difference (RD) |
0.256 |
0.203 |
|
Number of patients to be treated (NNT) |
3.899 |
4.919 |
|
Sensitivity (Se) |
0.528 |
0.486 |
|
Specificity (Sp) |
0.786 |
0.250 |
Table 2: Monovaccine vs. pentavaccine in 1-year follow-up for recurrent UTI.
1-year follow-up has demonstrated the following results. In the first group receiving hexavalent vaccine (Urivac), relapse-free progression was observed in 86% of patients (18 children from 21) by the end of the study. In the group receiving bacterial monolysates Uro-Vaxom, relapse-free progression was observed in 65% (17 children from 26). Among those patients who did not receive urinary antiseptic at bedtime and bacterial lysates, relapse-free progression was observed in 10 from 31 children (32%). 1-step follow-up has shown slightly increased statistical validation.
DISCUSSION
In our clinic, we have an accepted algorithm that requires exclusion of risk factors probably causing recurrence of UTI; we also evaluate whether parents follow prescriptions properly [13-15]. In case of a repeated episode of UTI, a child consults pediatric gynecologist or urologist to rule out inflammatory diseases of external genital organs (vulvitis, vulvovaginitis, balanoposthitis associated with phimosis); voiding cystourethrogram and ultrasound investigation (MRI if necessary) are performed to rule out the obstructive nature of UTI; one also performs antibody screening to determine such infections as Chlamydia Trachomatis, Ureaplasma Urealiticum, Mycoplasma genitalium and Trichomonas Vaginalis by investigating IgG titers to the given microorganisms that are absent in healthy children. Exclusion of risk factors requires individualization of the treatment approach [9].
Today’s care regimen used for children with recurrent UTI requires achieving of sterile urine through the use of urinary antiseptic (urine culture is done before the prescription of antimicrobial agent and on the 6th day of its administration in order to evaluate eradication of causative organism), prescribing of a preventive dose of urinary antiseptic at bedtime (Furaginum magnesium sulphate, Nifuratelum, trimethoprim/sulfamethoxazole) and conducting of immunoprophylaxis with bacterial lysates [9,16-18].
CRUTIL trial has shown high efficacy of the two lysates used. However, Urivac has proved to have a more lasting effect. Its mode of action aimed at a fast adaptive (acquired) immune response in local inflammation through MALT system activation: Increase of macrophage and T-cell activity, increase in secretory IgG on the surface of urinary tract mucosa and activation of protective adhesion molecules [19]. Clearly, Urivac is more effective because it affects more causative organisms and due to principle of strengthening the response when causative organisms are increased, where the immune protection is directed against a vast list of microorganisms [20].
It is generally known that UTIs are caused by E.Coli, while other gram-negative bacilli, namely Klebsiella, Proteus, Enterobacter, Citrobacter, Serratia, Pseudomonas, gram-positive cocci Enterococci, Staphylococcus epidermidis, Streptococcus faecalis, Streptococcus saprophyticus and Chlamydia trachomatis are more common in complicated and recurrent UTI [21,22]. Expanding of an antigenic spectrum that is a part of immunotherapy produces better results. Indeed, at the time of writing the article, follow-up period lasted for 1 year [23]. A further individualized approach to prescription of lysates has shown low efficiency when lysates were taken daily within a month; but taking of Urivac 10 days a month over a period of 6 months has proved to be more effective. In UC group, no recurrence was observed over the next 6 months. On the contrary, in UM group, 3 children with recurrence were observed (P≤0.05).
It is evident that common treatment approaches that involve the use of probiotics [24,25] and cranberry medicines [26], like an appropriate antibiotics treatment [27], largely depend on regional conditions [28,29].
Experience gained in CRUTIL trial has made it possible to establish treatment regimen for the use of Urivac in different clinical situations:
• Taking 1 capsule on an empty stomach within 10 days – therapy for activation of inflammatory response aimed at arresting inflammation; this therapy is used alongside with antibiotics/urinary antiseptics since Urivac contains inactivated causative organisms. Such a regimen is recommended to be used in acute period.
• Three courses-taking 1 capsule on an empty stomach within 10 days, 20-day pause-this therapy aims at arresting a local inflammation to prevent recurrence in patients who had episodes on uncomplicated UTI.
• Six courses-taking 1 capsule on an empty stomach within 10 days, 20-day pause-this therapy aims at arresting the persistence of the causative organism; it is recommended to be used in patients with chronic recurrent UTI in order to provide anti-relapse treatment.
Significance of CRUTIL trial for patients is that except for the treatment in accordance with international protocols, children received an individual therapy with industrially produced bacterial lysates aimed at ensuring relapse-free progression. Significance for specialists is that one got information on comparative efficacy of anti-relapsing therapy with industrially produced bacterial lysates. Furthermore, weak points of such a therapy were also defined: Duration of the therapy is uncertain; it is also unclear whether the therapy would be more effective due to continuous 1-month course with Uro-Vaxom as compared to Urivac courses lasting 10 days a month within 3 months.
CONCLUSION
- Vaccination is reasonable for recurrent UTI in children.
- Long-term prophylaxis with a single dose of uroseptic at bedtime may be combined with vaccines of bacterial lysates in the morning on an empty stomach.
- Any microbe association with Coli is suitable for hexavalent vaccine against Klebsiella pneumonia, Pseudomonas aeruginosa, Enterococcus faecalis and Proteus mirabilis; 15 % of failed treatments for a reason of non-E. Coli etiology may be successfully treated with hexavalent vaccine.
- 5-2-year management with 6-month follow-up on rUTI shows long-term benefit of Urivac.
- Hexavalent bacterial lysates increase a relapse-free progression of UTI in children up to 87% and are easy in use.
LIMITATIONS
Uro-Vaxom and Urivac instructions do not require a further therapy with bacterial lysates in long-term observation of patients. CRUTIL trial indicates appropriateness of prescribing bacterial lysates to get a response in UTI.
CONFLICT OF INTEREST
Author received honoraria both from Uro-Vaxom and Urivac manufacturers. The authors declare that they have no conflict of interest.
COMPLIANCE WITH ETHICAL STANDARDS
Data was partly presented as oral presentation O-10 possibilities of modern anti-relapse treatment of urinary tract infections in children by Dmytro Ivanov at 51th Annual Scientific Meeting of the ESPN October 4, 2018 in Antalya, Turkey and in national medical journal.
ETHICAL APPROVAL
All procedures involving human participants were performed in accordance with ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Parents and patients provided signed informed consent.
REFERENCES
- Urinary Tract Infection (UTI) in Children. DynaMed, Massachusetts, USA.
- Radmayr C, Bogaert G, Dogan HS, Ko?vara R, Nijman JM, et al. (2019) Paediatric urology. European Association of Urology, Arnhem, Netherlands.
- Chang SJ, Tsai LP, Hsu CK, Yang SS (2015) Elevated postvoid residual urine volume predicting recurrence of urinary tract infections in toilet-trained children. Pediatr Nephrol 30: 1131-1137.
- Koyle MA, Shifrin D (2012) Issues in febrile urinary tract infection management. Pediatr Clin North Am 59: 909-922.
- Craig JC, Williams GJ, Jones M, Codarini M, Macaskill P, et al. (2010) The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: Prospective cohort study of 15 781 febrile illnesses. BMJ 340: 1594.
- Kass EH (1956) Asymptomatic infections of the urinary tract. Trans Assoc Am Physicians 69: 56-64.
- Chang SL, Shortliffe LD (2006) Pediatric urinary tract infections. Pediatr Clin North Am 53: 379-400.
- Karavanaki KA, Soldatou A, Koufadaki AM, Tsentidis C, Haliotis FA, et al. (2017) Delayed treatment of the first febrile urinary tract infection in early childhood increased the risk of renal scarring. Acta Paediatr 106: 149-154.
- Ivanov DD, Korg OM (2014) Nephrology in family doctor’s practice. Zaslavskyi, Ukrainian.
- Williams G, Craig JC (2011) Long?term antibiotics for preventing recurrent urinary tract infection in children. Cochrane Systematic Review-Intervention. Cochrane Library.
- Afshar K, Stothers L, Scott H, MacNeily AE (2012) Cranberry juice for the prevention of pediatric urinary tract infection: A randomized controlled trial. J Urol 188: 1584-1587.
- https://www.who.int/topics/research/en/
- Spencer JD, Bates CM, Mahan JD, Niland ML, Staker SR, et al. (2012) The accuracy and health risks of a voiding cystourethrogram after a febrile urinary tract infection. J Pediatr Urol 8: 72-76.
- Tullus K (2011) Difficulties in diagnosing urinary tract infections in small children. Pediatr Nephrol 26: 1923-1926.
- Tosif S, Baker A, Oakley E, Donath S, Babl FE (2012) Contamination rates of different urine collection methods for the diagnosis of urinary tract infections in young children: An observational cohort study. J Paediatr Child Health 48: 659-664.
- Pennesi M, Travan L, Peratoner L, Bordugo A, Cattaneo A, et al. (2008) Is antibiotic prophylaxis in children with vesicoureteral reflux effective in preventing pyelonephritis and renal scars? A randomized, controlled trial. Pediatrics 121: 1489-1494.
- Tratselas A, Iosifidis E, Ioannidou M, Saoulidis S, Kollios K, et al. (2011) Outcome of urinary tract infections caused by extended spectrum beta-lactamase-producing Enterobacteriaceae in children. Pediatr Infect Dis J 30: 707-710.
- Wang HH, Gbadegesin RA, Foreman JW, Nagaraj SK, Wigfall DR, et al. (2015) Efficacy of antibiotic prophylaxis in children with vesicoureteral reflux: Systematic review and meta-analysis. J Urol 193: 963-969.
- Hanuš M, Matoušková M, Králová V, Hiblbauer J, Szewczyk J, et al. (2015) Immunostimulation with polybaterial lysat in prevention of recurrence infection. Ces Urol 19: 33-43.
- Castro M, Lythe G, Molina-Paris C, Ribero RM (2016) Mathematics in modern immunology. Interface Focus 6: 20150093.
- Shaikh N, Mattoo TK, Keren R, Ivanova A, Cui G, et al. (2016) Early antibiotic treatment for pediatric febrile urinary tract infection and renal scarring. JAMA Pediatr 170: 848-854.
- Craig JC, Simpson JM, Williams GJ, Lowe A, Reynolds GJ, et al. (2009) Antibiotic prophylaxis and recurrent urinary tract infection in children. N Engl J Med 361: 1748-1759.
- Ivanov D (2018) Possibilities of current anti-relapsing treatment of urinary tract infections in children Pediatric Nephrology.
- Lee SJ, Lee JW (2015) Probiotics prophylaxis in infants with primary vesicoureteral reflux. Pediatr Nephrol 30: 609-613.
- Schwenger EM, Tejani AM, Loewen PS (2015) Probiotics for preventing urinary tract infections in adults and children. Cochrane Database Syst Rev 12: 008772.
- Salo J, Uhari M, Helminen M, Korppi M, Nieminen T, et al. (2012) Cranberry juice for the prevention of recurrences of urinary tract infections in children: A randomized placebo-controlled trial. Clin Infect Dis 54: 340-346.
- Salomonsson P, von Linstow ML, Knudsen JD, Heiberg I, Mola G, et al. (2016) Best oral empirical treatment for pyelonephritis in children: Do we need to differentiate between age and gender? Infect Dis (Lond) 48: 721-725.
- Ramos NL, Dzung DT, Stopsack K, Jankó V, Pourshafie MR, et al. (2011) Characterisation of uropathogenic Escherichia coli from children with urinary tract infection in different countries. Eur J Clin Microbiol Infect Dis 30: 1587-1593.
- Ivanov DD, Ivanova TP, Fedorenko OG, Kushnirenko SV, Ivanova MD (2019) Options of modern anti-relapse therapy for urinary tract infections in children: CRUTIL trial. Pochki, 2: 80-87.
SUPPLEMENTARY TABLE
|
|
Urivac Group |
Urovaxom Group |
Control Group |
|
Number of patients |
22 |
28 |
33 |
|
Age, y. o. |
4-15 (9±1,8) |
5-15 (9±2,2) |
3-14 (9±2,1) |
|
m:f ratio |
1:6 (3 boys, 19 girls) |
1:6 (4 boys, 24 girls) |
1:5 (7 boys, 26 girls) |
|
Age of the disease, years |
1,2 years (±0,3) |
1,2 years (±0,3) |
1,2 years (±0,3) |
|
Complicated clinical course, % |
25 |
24 |
22 |
|
Relapse, n cases |
2±1 |
2±1 |
2±1 |
Table S1: Characteristics of the studied population.
Citation: Ivanov DD, Ivanova TP, Fedorenko EG, Kushnirenko SV, Ivanova MD (2020) Possibilities of Modern Anti-Relapse Therapy of Urinary Tract Infections in Children: Crutil Trial 1 Year Follow-Up. J Nephrol Renal Ther 6: 024.
Copyright: © 2020 Dmytro D Ivanov, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

