
Primary Adrenal Insufficiency Due to Tuberculous Adrenalitis in a Patient without Active Pulmonary Tuberculosis
*Corresponding Author(s):
Ali A AchiraDepartment Of Endocrinology And Metabolism, Wayne State University, Michigan, United States
Tel:+1 3137451464,
Email:aachira@med.wayne.edu
Abstract
Our objective is to identify diagnostic clues indicative of primary adrenal insufficiency secondary to Tuberculosis (TB) in a patient with negative Mycobacterium TB PCR analysis.
Methods
We described a case report, and we performed all the possible investigations to confirm the diagnosis.
Results
A 38 year old man was admitted with signs and symptoms of adrenal insufficiency. Morning cortisol level was low, with very high Adrenocorticotropic Hormone (ACTH). He failed the cosyntropin stimulation test. Tuberculin skin test (Purified Protein Derivate-PPD) and QuantiFERON test were positive. CT scans of the abdomen and pelvis showed diffuse, enlarged adrenal glands with a 5.8 cm mass in the left adrenal gland, the biopsy of which showed chronic inflammation with Langerhans giant cells. However, Mycobacterial stains, cultures, and Polymerase Chain Reaction (PCR) results were negative. Primary adrenal insufficiency secondary to TB was the presumptive diagnosis, and the patient was started on adrenal replacement therapy and anti-tubercular therapy.
Conclusion
This case highlights the fact that negative Mycobacterium culture and TB PCR results may not rule out tuberculous adrenalitis. A presumptive clinical diagnosis based on physical, radiographic, and histopathological findings is sufficient for initiating therapy for adrenal TB.
Keywords
Mycobacterium; Tuberculin skin
ABBREVIATIONS
TB - Tuberculosis
ACTH - Adrenocorticotropic Hormone
CT - Computed Tomography
PCR - Polymerase Chain Reaction
INTRODUCTION
CASE REPORT
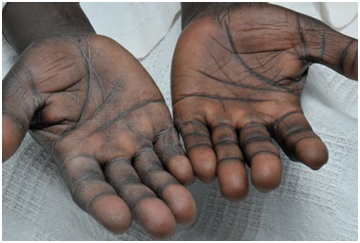
Laboratory tests indicated low serum sodium (125 mEq/L [normal, 136-142 mEq/L]), high serum potassium (6.2 mEq/L [normal, 3.5-5.0 mEq/L]), high serum creatinine (1.5 mg/dL [normal, 0.6-1.2 mg/dL]), and low serum 8 AM cortisol (2 µg/dL [normal, 5-25 µg/dL]). Results of the cosyntropin stimulation test were consistent with primary adrenal insufficiency, because random cortisol level was 1.8 µg/dL (normal, 5-25 µg/dL) before the test, and 1.9 µg/dL 60 minutes after the test (normal, post 60 minutecortisol level >18 µg/dL). His ACTH level at 8 AM was 655 pg/mL (normal,
Further investigation of adrenal insufficiency revealed a positive PPD test (22 × 28 mm induration), and the result of the TB QuantiFERON Gold test was high (>10 IU/mL [FDA cut point for a positive result is >0.34 IU/mL]).
Lab tests summarized in the table below:
|
Patient’s results |
Normal range |
Comment |
|
|
Serum sodium |
125 mEq/L |
136-142 mEq/L |
Low |
|
Serum potassium |
6.2 mEq/L |
3.5-5.0 mEq/L |
High |
|
Serum creatinine |
1.5 mg/dL |
0.6-1.2 mg/dL |
High |
|
Serum 8 AM cortisol |
2 µg/dL |
5-25 µg/dL |
Low |
|
Cosyntropin stimulation test |
|||
|
Random cortisol level before cosyntropin |
1.8 µg/dL |
Normal, 5-25 µg/dL |
Low |
|
Serum cortisol 60 min after cosyntropin |
1.9 µg/dL |
Normal, post 60 minute >18 µg/dL |
Low |
|
ACTH |
655 pg/mL |
High |
|
|
PPD test |
22 × 28 mm induration |
Positive |
|
|
TB QuantiFERON Gold |
>10 IU/mL |
High |
FDA cut point for a positive result is >0.34 IU/mL |
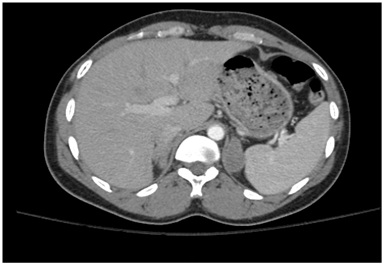
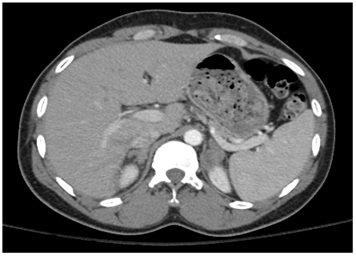 Figure 2B: CT scan of the abdomen showing asymmetric, bilateral enlargement of the adrenal glands.
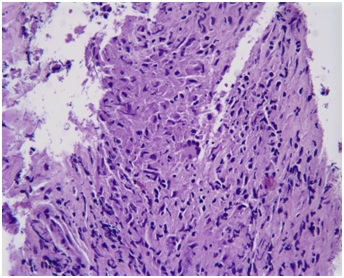
Figure 2B: CT scan of the abdomen showing asymmetric, bilateral enlargement of the adrenal glands.Biopsy of the mass showed chronic inflammation with Langerhans giant cells (Figure 3) and necrotic tissue. These results were histologically consistent with a granulomatous process (Tuberculous adrenalitis). No normal adrenal tissue could be identified. There was no evidence of malignancy. Fungal cultures and stains of the tissue biopsy were negative. Bacterial cultures and gram stains were negative. Mycobacterial cultures and stains for acid-fast bacilli were negative after >8 weeks. Mycobacterium TB PCR analysis of tissue biopsy was negative. Urine cultures for Mycobacterium were negative.
Additional evaluation to determine alternative causes of adrenal insufficiency was conducted, including tests for: HIV, syphilis, fungal antibodies against Aspergillus, Blastomyces, Coccidiodes, Candida, and Histoplasma and fungal cultures of the adrenal gland and adrenal antibodies. All of these tests were negative and did not implicate an alternate cause. The patient was started on isoniazid, rifampin, ethambutol and pyrazinamide, in addition to adrenal insufficiency treatment.
Treatment for TB Adrenalitis is same duration as for pulmonary TB with 4 drug therapy (Isoniazid, rifampin, ethambutol and pyrazinamide) for 8 weeks (Intensive phase) followed by 2 drug therapy (Isoniazid and rifampin) for 18 weeks (Continuation phase).
Acute symptoms improved after starting hormone replacement therapy. However it is difficult to ascertain if the patient has responded to TB treatment or hormone replacement therapy as it could be the result of both. It is difficult to tease out which resulted in improvement and is likely due to the treatment of both the hormonal abnormalities as well as the infection. The immediate improvement seen was likely from the steroid replacement and less from the TB medications.
Monitoring plan is to get repeat CXR to monitor the lung findings at 2 months of treatment and at the end of treatment visit. Plan also to repeat imaging of the abdomen at 2 months and the end of the treatment to assess for changes in the enlarged adrenal gland.
DISCUSSION
This patient had extra-pulmonary TB involving the adrenal glands, resulting in adrenal insufficiency. However, it is unknown when he was first exposed to TB. TB infection frequently begins in the lungs and may disseminate via the haematogenous route to extra-pulmonary sites, especially to the organs with high blood flow, such as the spleen, liver, bone marrow, kidneys, and adrenal glands [6]. Dissemination of M. tuberculosis may occur at the time of primary pulmonary infection or later, due to a re-infection or reactivation of a previous infection [6]. Characteristic granulomas may result from acute lymphohematogenous dissemination (soft or exudative granuloma, frequently having acid-fast bacilli) or discharge of bacilli into the microscopic blood vessels within the caseous lesions (Hard granuloma, frequently having no acid-fast bacilli) [7]. In the present case, no acid-fast bacilli were detected in granulomas from the adrenal biopsy. Enlargement of both adrenal glands can occur in most (90 %) patients with tuberculous adrenal insufficiency [8-10]. Imaging findings may vary with the stage and activity of the inflammatory process. In an early tuberculous adrenalitis, bilateral adrenal enlargement is typical, as in the present case, which can include a central necrotic area of low attenuation and a peripheral enhancing rim. At the late or healing stage, an enlargement of tuberculous adrenal glands may partially or completely resolve, with or without calcification or atrophy [11-13]. No adrenal calcification was observed in the present patient.
TB cannot be excluded in some patients if Acid Fast Bacilli (AFB) smear results are negative. Thus, a clinician’s judgment should be used regarding the initiation of empiric TB therapy, while awaiting culture results [14-16].
In the USA, approximately 17 % of reported new cases of pulmonary TB have negative cultures. Failure to isolate M. tuberculosis from appropriately collected patient specimens suspected of pulmonary TB (on clinical or radiographic grounds) does not exclude a diagnosis of TB. It is unclear whether anti tuberculous therapy can rescue the adrenal function in these patients. Some studies show that the recovery of adrenal function can occur in patients treated for TB [14-16], but a lack of adrenal recovery 2-5 years after therapy has also been observed [17]. In our patient, TB treatment was initiated, in addition to hydrocortisone and fludrocortisone. Adrenal function will be monitored during the course of therapy. However, recovery is less likely, as the biopsy failed to identify any normal adrenal tissue, indicating that >90 % of adrenal tissue had been destroyed by the infection. TB is not expected to spread as it has been treated. No other damage to other organs anticipated following treatment.
CONCLUSION
ACKNOWLEDGMENT
REFERENCES
- Oelkers W (1996) Adrenal insufficiency. N Engl J Med 335: 1206-1212.
- Kele?timur F, Ozbakir O, Sa?lam A, Oztürk F, Yücesoy M (1993) Acute adrenocortical failure due to tuberculosis. J Endocrinol Invest 16: 281-284.
- Vita JA, Silverberg SJ, Goland RS, Austin JH, Knowlton AI (1985) Clinical clues to the cause of Addison’s disease. Am J Med 78: 461-456.
- Llewelyn M, Adler M, Steer K, Pasvol G (1999) Acute adrenal insufficiency precipitated by isolated involvement of the adrenal gland by tuberculosis. J Infect 39: 244-245.
- Lam KY, Lo CY (2001) A critical examination of adrenal tuberculosis and a 28-year autopsy experience of active tuberculosis. Clin Endocrinol (Oxf) 54: 633-639.
- Guttman PH (1930) Addison’s disease: A staristical analysis of 566 cases and a study of the pathology. Arch Pathol 10: 742-935.
- Sharma SK, Mohan A (2004) Extrapulmonary tuberculosis. Indian J Med Res 120: 316-353.
- Doppman JL, Gill JR, Nienhuis AW, Earll JM, Long JA (1982) CT findings in Addison’s disease. J Comput Assist Tomogr 6: 757-761.
- Guo YK, Yang ZG, Li Y, Ma ES, Deng YP, et al. (2007) Addison’s disease due to adrenal tuberculosis: Contrast-enhanced CT features and clinical duration correlation. Eur J Radiol 62: 126-131.
- Wang YX, Chen CR, He GX, Tang AR (1998) CT findings of adrenal glands in patients with tuberculous Addison’s disease. J Belge Radiol 81: 226-228.
- Kawashima A, Sandler CM, Fishman EK, Charnsangavej C, Yasumori K, et al. (1998) Spectrum of CT findings in nonmalignant disease of the adrenal gland. Radiographics 18: 393-412.
- Yang ZG, Guo YK, Li Y, Min PQ, Yu JQ, et al. (2006) Differentiation between tuberculosis and primary tumors in the adrenal gland: evaluation with contrast-enhanced CT. Eur Radiol 16: 2031-2036.
- Guo YK, Yang ZG, Li Y, Deng YP, Ma ES, et al. (2007) Uncommon adrenal masses: CT and MRI features with histopathologic correlation. Eur J Radiol 62: 359-370.
- Sharma SK, Tandan SM, Saha PK, Gupta N, Kochupillai N, et al. (2005) Reversal of subclinical adrenal insufficiency through antituberculosis treatment in TB patients: a longitudinal follow up. Indian J Med Res 122: 127-131.
- Bhatia E, Jain SK, Gupta RK, Pandey R (1998) Tuberculous Addison's disease: lack of normalization of adrenocortical function after anti-tuberculous chemotherapy. Clin Endocrinol (Oxf) 48: 355-359.
- Penrice J, Nussey SS (1992) Recovery of adrenocortical function following treatment of tuberculous Addison's disease. Postgrad Med J 68: 204-205.
- Keven K, Uysal AR, Erdogan G (1998) Adrenal function during tuberculous infection and effects of antituberculosis treatment on endogenous and exogenous steroids. Int J Tuberc Lung Dis 2: 419-424.
Citation: Achira AA, Abushanab D, Elbadawi H, Dhar S and Taha W (2019) Primary Adrenal Insufficiency Due to Tuberculous Adrenalitis in a Patient without Active Pulmonary Tuberculosis. J Hum Endocrinol 4: 014.
Copyright: © 2019 Ali A Achira, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

