Primary Intrahepatic Mixed Neuroendocrine–Non neuroendocrine Neoplasm (MiNEN)-Rare Solid Cystic Neoplasm of Liver
*Corresponding Author(s):
Priya NathaniConsultant Radiologist, Yashoda Superspeciality Hospital, Secunderabad , Telangana, India
Tel:040 23244440,
Email:drpriyanathani76@gmail.com
Abstract
Aim: Mixed Neuroendocrine –Non-neuroendocrine neoplasm (MiNEN)) is a rare tumour of the gastrointestinal tract and hepato-pancreatic biliary system having both neuroendocrine and non-neuroendocrine components. Most commonly these tumours are present in gastrointestinal tract -appendix, colon, stomach, and pancreas. We present a case of primary intrahepatic MiNEN, so far second case in our knowledge,manifesting as predominantly solid and solid- cystic masses with few scattered areas of calcifications in both lobes of liver.
Keywords
MiNEN; Primary liver tumour; Solid cystic mass with calcifications
Introduction
Common solid- cystic tumors of liver in adults include primary hepatic tumors such as embryonal sarcoma, biliary cystadenoma and adenocarcinoma, Hepatocellular carcinoma post-locoregional therapy or secondary to spontaneous necrosis and secondaries from a known or unknown primary such as from neuroendocrine tumors, gastrointestinal stromal tumors, sarcoma, melanoma, few subtypes of lung and breast carcinoma, colon and ovarian cancers [1].
Case Report
A 54yr-old female with complaints of nausea & vomiting presented in liver outpatient department. On general examination she was thin built and moderately nourished with a large abdominal mass in the right hypochondrium extending into right lumbar region. The other systemic examinations were normal.
On biochemistry: Her liver function tests were deranged with SGPT - 219 IU/L, SGOT–445IU/L & total bilirubin -2.4 mg/dl. Other parameters such as Amylase 325 U/L, Lipase 1170 U/L&LDH 784U/L were raised.
Her Viral markers (HBV,HCV,HIV) were negative. The tumour marker panel showed elevated Ca19-9 105000 IU/ML, CEA -71500IU/ML, Chromogranin 1728ng/ml
Rest of blood parameters were within normal limits,
On imaging
Ultrasound (USG) examination of the abdomen revealed hepatomegaly and a large hyperechoic solid mass in right lobe with internal irregular anechoic areas and peripheral foci of calcifications. Another focal solid -cystic lesion with predominant cystic component was seen in segment IV of left lobe (Figure 1). Colour Doppler revealed increased vascularity in the solid component of the masses. Rest of the liver showed homogenous echotexture with smooth contour and margins.
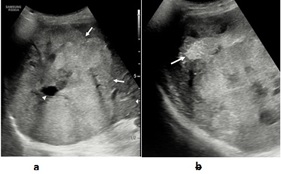 Figure 1: a- Ultrasound shows large heterogeneous, predominantly hyperechoic mass (white arrows) with internal necrotic areas (arrow head). b- Ultrasound of the same mass in different plane shows focal calcification (white arrow).
Figure 1: a- Ultrasound shows large heterogeneous, predominantly hyperechoic mass (white arrows) with internal necrotic areas (arrow head). b- Ultrasound of the same mass in different plane shows focal calcification (white arrow).
Multidetector computerized tomography (MDCT)
A pre and post contrast computed tomographic scan of the patient was performed and the images acquired in arterial, portal venous and delayed phase. Non-contrast scan showed heterogeneous, iso to hypodense lesions in both lobes with foci of calcification in both the lesions (Figure 2).
Post contrast scan revealed the largest lesion measuring 12×11.4×11 cm in the right lobe, involving segment VIII, VII, and VI. The solid portions showed enhancement on arterial phase (Figure 2) with washout in the portal-venous phase (Figure 2) and an enhancing wall. Central areas of necrosis were seen in the lesion. The lesion was causing capsular bulge & mass effect over the hepatic veins and compression of the right portal venous branches. No thrombosis or intravascular extension was noted.
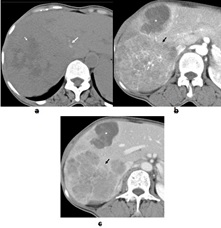 Figure 2: a- MDCT unenhanced axial image showing solid lesion in right lobe with central hypodense areas (short white arrow) and peripheral areas of calcification (long white arrow). b- MDCT post contrast arterial phase axial image shows criss-cross linear areas of hypervascular enhancement in the right lobe lesion (black arrow). Another lesion in segment 4 lesion shows cystic /necrotic areas with peripheral nodular enhancement (white star). c- MDCT post contrast porto-venous phase axial image shows washout in the right lobe lesion with peripheral wall enhancement. Necrotic lesion in segment 4 (white star).
Figure 2: a- MDCT unenhanced axial image showing solid lesion in right lobe with central hypodense areas (short white arrow) and peripheral areas of calcification (long white arrow). b- MDCT post contrast arterial phase axial image shows criss-cross linear areas of hypervascular enhancement in the right lobe lesion (black arrow). Another lesion in segment 4 lesion shows cystic /necrotic areas with peripheral nodular enhancement (white star). c- MDCT post contrast porto-venous phase axial image shows washout in the right lobe lesion with peripheral wall enhancement. Necrotic lesion in segment 4 (white star).
Another predominantly necrotic/cystic lesion measuring 5×3.9 cm was seen in the left lobe segment IVa. It showed eccentric solid enhancing component and enhancing walls with perilesional, subcapsular enhancement on arterial phase iso attenuating to surrounding liver on portal-venous& delayed phase. No other focal area of abnormality was seen in rest of the abdomen.
Magnetic resonance imaging (MRI)
MRI was also performed on 3 Tesla. On T1 W scan (Figure 3) the lesions appeared iso to hypointense with lobulated margins in both right and left lobes. T2 W images (Figure 3) showed heterogeneous intermediate to hyperintense signal of solid component with multiple areas of necrosis in right lobe lesion. The lesion in left lobe showed more cystic appearance with eccentric intermediate signal intensity solid component. No evidence of dilated intra and extrahepatic biliary radicles. Diffusion weighted imaging (b-800) (Figure 3) showed hyperintense signal in the solid component with corresponding areas of restriction on ADC in both the lesions (Figure 3). The contrast dynamics of the lesions in both lesions were similar to CT scan that is arterial phase enhancement of solid component with wash out in portal venous phase and delayed phase and non-enhancing necrotic / cystic part (Figure 3).Presence of peripheral enhancing rim and solid components were better seen on delayed phase on MRI as compared to CT scan.
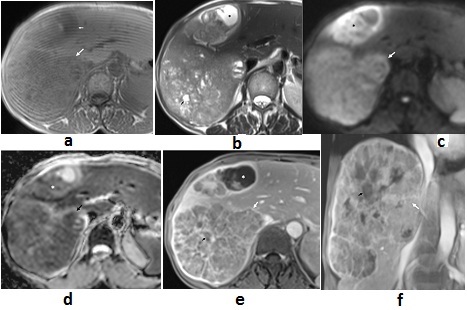 Figure 3: a- T1 W axial MRI shows, a large mass with hypointense to isointense signal in the right lobe (long white arrow). Similar lesion is seen in segment IV (small white arrow). b- T2 W Fat MRI axial image, shows a large right lobe heterogeneous hyper to intermediate intensity lesion with areas of necrosis (black arrow). Segment 4 lesion demonstrates eccentric necrotic area (black star). c- Diffusion weighted axial image in the right lobe lesion shows hyperintense signal (white arrow). The solid component in the segment 4 lesion shows hyperintense signal (black star). d- ADC axial image shows signal drop corresponding to the hyperintense signal in Fig. 3 b. e- T1 W fat suppressed post contrast hepatic venous phase axial image shows heterogeneousenhancement and peripheral enhancing rim (white arrow) in the right lobe mass. Black small arrow is necrosis. Segment 4 lesion shows enhancement with eccentric necrotic area (white star). f- T1 W fat suppressed post contrast hepatic venous phase coronal image shows the cranio-caudal extent of the right lobe lesion (white arrow).
Figure 3: a- T1 W axial MRI shows, a large mass with hypointense to isointense signal in the right lobe (long white arrow). Similar lesion is seen in segment IV (small white arrow). b- T2 W Fat MRI axial image, shows a large right lobe heterogeneous hyper to intermediate intensity lesion with areas of necrosis (black arrow). Segment 4 lesion demonstrates eccentric necrotic area (black star). c- Diffusion weighted axial image in the right lobe lesion shows hyperintense signal (white arrow). The solid component in the segment 4 lesion shows hyperintense signal (black star). d- ADC axial image shows signal drop corresponding to the hyperintense signal in Fig. 3 b. e- T1 W fat suppressed post contrast hepatic venous phase axial image shows heterogeneousenhancement and peripheral enhancing rim (white arrow) in the right lobe mass. Black small arrow is necrosis. Segment 4 lesion shows enhancement with eccentric necrotic area (white star). f- T1 W fat suppressed post contrast hepatic venous phase coronal image shows the cranio-caudal extent of the right lobe lesion (white arrow).
On the basis of above imaging findings, differential diagnosis of Neuroendocrine tumours of liver (Primary, Secondary) & multifocal biliary cystadenocarcinomas were thus put forward.
A 68 Ga DOTA-NOC PET CT was performed and showed mild peripheral uptake in the tumour with central necrotic area. (SUV max 6.7) (Figure 4). No other area of uptake was observed in rest of the body.
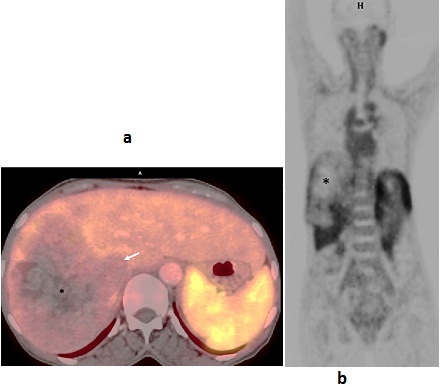 Figure 4: a. 68 Ga DOTA-NOC PET CT axial image shows peripheral uptake in the tumor (white arrow). Central necrotic area does not show uptake (black star). b. 68 Ga DOTA-NOC PET CT coronal image, shows similar findings as figure a.
Figure 4: a. 68 Ga DOTA-NOC PET CT axial image shows peripheral uptake in the tumor (white arrow). Central necrotic area does not show uptake (black star). b. 68 Ga DOTA-NOC PET CT coronal image, shows similar findings as figure a.
Diagnostic USG guided biopsy was performed. Histopathology slides showed low grade tumour with amphophilic granular cytoplasm with mucin. On immunohistochemistry -tumour cells co-expressed neuroendocrine markers like chromogranin, INSM -1 with markers of biliary differentiation like CK7, CK19and MUC 1, Hepato- cellular markers Hepar 1 and Arginase were negative. Tumour showed low proliferative Index: Mib 1 Activity 3% Suggesting Primary Intrahepatic Mixed neuroendocrine – non neuroendocrine neoplasm (MiNEN)-Amphicrine Carcinoma (Probably Biliary origin) (Figure 5).
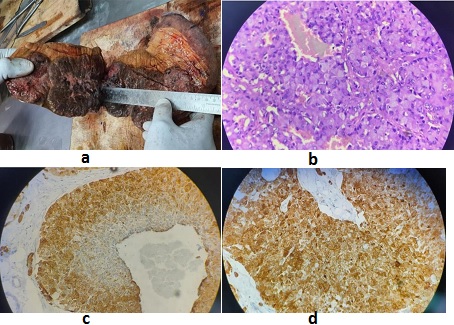 Figure 5: a. Gross findings respected specimen shows gray brown nodular masses with high vascularity. b. Microscopic findings show low nuclear grade monomorphic tumour showing salt and pepper type chromatin with intracytoplasmic mucin. All the nodules have similar tumour morphology. c. Immunohistochemistry shows tumour cells co expresses biliary markers (CK7, CK 19 and MUC 1). d. Immunohistochemistry shows tumour cells co express Neuroendocrine markers (INSM-1, Chromogranin and Synaptophysin).
Figure 5: a. Gross findings respected specimen shows gray brown nodular masses with high vascularity. b. Microscopic findings show low nuclear grade monomorphic tumour showing salt and pepper type chromatin with intracytoplasmic mucin. All the nodules have similar tumour morphology. c. Immunohistochemistry shows tumour cells co expresses biliary markers (CK7, CK 19 and MUC 1). d. Immunohistochemistry shows tumour cells co express Neuroendocrine markers (INSM-1, Chromogranin and Synaptophysin).
Management and follow up: As 68 Ga DOTA-NOC PET CT revealed no other area of uptake; she was taken up hepatic resection. Laparotomy revealed a large mass attached to the right lobe and segment 4 of liver, it was followed by an extended right hepatectomy. Gross specimen cut section of the lesions showed haemorrhagic grey brown variegated tumour with mucoid glistening white patches and central necrosis (Figure 5). Parenchymal cut margin was 0.2 to 0.3cms away; no thrombus was seen in vessels. Representative sections were evaluated for histopathology and immunohistochemistry.
Patient was discharged in ambulatory condition, came for follow once with normal clinical, laboratory and imaging findings and thereafter lost to follow-up.
Discussion and Review of Literature
Imaging features of Common solid cystic tumors (Complex cysts) of liverare fluid density/intensity lesions with any of the following additional findings such as enhancing walls/septations/mural nodules/solid components/, calcification/haemorrhagic or proteinaceous contents [2].
Neoplastic complex cystic masses in liver in adults includeprimary hepatic tumors such as embryonal sarcoma, hepatocellular carcinoma post- locoregional therapy or secondary to spontaneous necrosis, biliary cystadenoma and adenocarcinoma and secondaries from a known or unknown primary such as from neuroendocrine tumours, gastrointestinal stromal tumours, sarcoma, melanoma, few subtypes of lung and breast carcinoma, colon and ovarian cancers.
Undifferentiated embryonal sarcoma of the liver are rare and highly malignant childhood tumours, with few cases reported in adults, Cross-sectional imaging with multiphase contrast CT or magnetic resonance imaging (MRI) usually reveals a large hypodense mass, often well circumscribed with a pseudo capsule, multiple hyperdense septations of various thickness, and predominantly water attenuation with rare calcifications. Delayed contrast enhanced imaging may show mild, heterogeneous, and peripheral enhancement secondary to the extensive central necrosis and/or cystic change [3,4].
Hepatocellular carcinoma (HCC): The commonest primary malignant neoplasm of the liver typically occurs within a cirrhotic liver or patients with high risk factors such as chronic Hepatitis B infection. Imaging shows morphological features of volume redistribution with coarse echotexture/heterogenous attenuation/signal intensity, surface nodularity of liver and signs of portal hypertension. Typical solid HCC is seen as hypo-, iso- or hyperechoic solid lesion in relation to the adjacent parenchyma .Colour Doppler shows internal high-velocity signals due to arterioportal shunting .On Unenhanced CT these are ill-defined low attenuation lesions ,post contrast triphasic CT shows non rim nodular enhancement during the arterial phase, as it is a hyper vascular tumour supplied via the hepatic artery and becoming hypoattenuating to the liver parenchyma during the portal phase ,delayed phase (wash out phenomenon) with surrounding enhancing capsule. Post-locoregional therapy, the lesion undergoes coagulative necrosis and then appears as a solid cystic mass. An internal hypodense area higher than simple fluid attenuation on unenhanced CT, high signal intensity on T1-weighted images is visualised which persists for several months post ablation. Post contrast study may show a viable enhancing solid component and a non-enhancing necrotic area suggesting partial response or no response to treatment [5,6]. Associated features of Cirrhosis and history of radiological intervention helps in reaching the correct diagnosis.
Biliary cystadenomas are rare tumours occur predominantly in middle-aged female patients and may potentially transform into cystadenocarcinomas. At CT and MR imaging, a typical biliary cystadenoma or cystadenocarcinoma appears as a large, solitary, unilocular or multilocular cystic lesion with well-circumscribed smooth margins and internal septa, Solid components of the tumour demonstrates early heterogeneous enhancement which is often minimal in intensity, distinguishing these tumours from the extensive enhancement of HCC which remains is odense / intense to surrounding parenchyma on portal -venous and delayed phases. Calcification in the wall and the septa may be seen in few cases. An overlap of findings may be seen in cystadenomas /cystadenocarcinoma but solid mural nodule, or coarse calcification along the wall or thick septa is more suggestive cystadenocarcinoma [5].
Liver is a common site for metastases from many primary cancers. Common solid-cystic metastasesfrom a known or unknown primary to liver are from neuroendocrine tumours, gastrointestinal stromal tumours, sarcoma, melanoma, and lung and breast carcinoma. Such appearance is because of spontaneous intra-tumoral necrosis /haemorrhage or abundant mucin production by acinar structures and glandular tissues from mucinous adenocarcinoma as in Carcinoma of colon, ovary [5]. Metastases from neuroendocrine tumor show, transient intense arterial enhancement becoming iso/hypoattenuating to liver during the portal phase unlike other causes of metastases. Central necrosis, rim enhancement and calcification can also be demonstrated. These can demonstrate multiplicity and a variation in size and the background liver shows homogenous attenuation /signal intensity with smooth margins. In the absence of high-risk factors for cirrhosis, above features differentiate them from hepatocellular carcinoma.
Case discussion
The case in point showed typical features of complex cyst with focal lesions showing enhancing solid component, necrotic areas and calcific foci in the background of morphologically normal liver with no signs of portal hypertension and absent clinical history of high-risk factors of cirrhosis. The solid portion showed arterial phase enhancement with washout in portal venous and delayed phase, multiple tortuous arteries within the tumor so it was considered a hyper vascular tumour -Neuroendocrine origin. Possibility of multifocalbiliary cystadenocarcinomas was also suggested (as calcifications were seen and CA19-9 was elevated). The preoperative and post hepatectomy specimen biopsy suggested Mixed Non neuroendocrine and neuroendocrine neoplasm based on histopathological and Immunohistochemistry examination. Histology played a vital role reaching the diagnosis [7].
MiNEN who classification, treatment strategy of MiNEN, Mixed neuroendocrine non-neuroendocrine neoplasms, as per the 2017–2019 WHO definition represent an extremely rare diagnosis [8]. Surgical resection is considered the first treatment of choice for resectable liver lesions [9]. For unresectable lesions the use of platinum, gemcitabine or S-1 based chemotherapy and TAE [10] has been reported [11]. Multidisciplinary approach is required for planning the treatment strategies based upon the most aggressive and/or predominant tumour type on the histology [8]. Neoadjuvant regimens may down stage adenocarcinoma-dominant tumors [10]. Palliative chemotherapy offered to the patients with advanced disease,as upfront treatment, or after palliative surgery [8].
Conclusion
MiNEN presents as focal lesion with nonspecific imaging findings or combined imaging features as in this case and is diagnosed on tumour marker levels, histopathology and immunochemistry. Identification of the combined histology is of prognostic significance and management requires multi-disciplinary team approach.
References
- Mortelé KJ, Ros PR (2001) Cystic Focal Liver Lesions in the Adult: Differential CT and MR Imaging Features 21: 895-910.
- Vachha B, Sun MRM, Siewert B, Eisenberg RL (2011) Cystic lesions of the Liver. Am J Roentgenol 196: W355-W366.
- Buetow PC, Buck JL, Pantongrag-Brown L, Marshall WH, Ros PR, et al. (1997) Undifferentiated (embryonal) sarcoma of the liver: pathologic basis of imaging findings in 28 cases. Radiology 203:779-783.
- Zhang H, Lei L, Zuppan CW, Raza AS (2016) Undifferentiated embryonal sarcoma of the liver with an unusual presentation: case report and review of the literature. J Gastrointest Oncol 7: S100-S106.
- Qian LJ, Zhu J, Zhuang ZG, Xia Q, Liu Q, et al. (2013) Spectrum of Multilocular Cystic Hepatic Lesions: CT and MR Imaging Findings with Pathologic Correlation. Radiographics 33.
- Yaghmai V, Besa C, Kim E, Gatlin JL, Siddiqui NA, et al. (2013) Imaging assessment of hepatocellular Carcinoma Response to Locoregional and Systemic Therapy. Am J Roentgenol 201: 80-96.
- Das P, Sharma P, Nakra T, Ghosh S, Yadav R, et al. (2017) Spectrum of hepatobiliarycystic lesions: A 7-year experience at a tertiary care referral centre in North India and review of literature. Indian J Pathol Microbiol 60: 487-500.
- Frizziero M, Chakrabarty B, Negy B, Lamarca A, Hubner RA, et al.(2020) Mixed Neuroendocrine Non-Neuroendocrine Neoplasms: A Systematic Review of a Controversial and Underestimated Diagnosis. J Clin Med 9:273.
- Endocrine and Neuroendocrine Cancers (2019) Clinical Practice Guidelines for Gastroenteropancreatic Neuroendocrine Neoplasms (GEP-NEN), 2nd edn 2019.
- Zhao ZM, Wang J, Ugwuowo UC, Wang L, Townsend JP (2018) Primary hepatic neuroendocrine carcinoma: report of two cases and literature review. BMC Clin Pathol 18: 3.
- de Mestier L, Cros J, Neuzillet C, Hentic O, Egal A, et al. (2019) Digestive system mixed neuroendocrine-non-neuroendocrine neoplasms (MiNEN). Ann Endocrinol 80: 172-173.
Citation: Nathani P, Maheshwari S, Shah M, Kapoor D, Gopal K, et al. (2021) Primary Intrahepatic Mixed Neuroendocrine–Nonneuroendocrine Neoplasm (MiNEN)-Rare Solid Cystic Neoplasm of Liver. J Gastroenterol Hepatology Res 6: 035.
Copyright: © 2021 Priya Nathani, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.