
Prognostic Value of Sleep Disturbances in Covid-19 Patients
*Corresponding Author(s):
Renzo RozziniGeriatric Department, Fondazione Poliambulanza Istituto Ospedaliero, Via Bissolati 57 - 25124 Brescia, Italy
Email:renzo.rozzini@poliambulanza.it
Abstract
Study objectives: To evaluate sleep disorders in hospitalized patients with respiratory failure due to COVID-19 and assess their potential association with prognosis.
Methods: The association between sleep disturbances and in hospital mortality in 1337 COVID-19 patients was assessed.
Results: Sleep disturbances were higher in older patients and in severe infections compared with non-severe infections. Sleep disturbances were associated with in-hospital mortality, and in a Cox regression analysis maintain their independent association also after controlling for potential confounders (SARS-CoV-2 infection severity, sex, age and age-related diseases, i.e., coronary heart diseases and Chronic Kidney Disease (CKD).
Conclusion: Sleep disturbances should be considered a poor prognostic marker in COVID-19 patients.
Keywords
Aging; Comorbidity; COVID-19; Sleep disturbances
Abbreviations
ACE2: Angiotensin-Converting Enzyme 2
ARDS: Acute Respiratory Distress Syndrome
CKD: Chronic Kidney Disease
COVID-19: Corona Virus Disease 19
CRP: C-reactive Protein
IL-6: Interleukin-6
PaO2/FiO2: Arterial Oxygen Partial Pressure (PaO2)/ Fractional Inspired Oxygen (FiO2)
SIAARTI: Italian Society of Anesthesiology, Analgesia, Resuscitation and Intensive Care
SIMIT: Italian Society for Infectious and Tropical Diseases
TNF: Tumor Necrosis Factor
Introduction
The major complications of COVID-19 are respiratory (interstitial pneumonia, pulmonary embolism), renal and cardiac. Neurological symptoms are also described, and among them agitation and confusion (which are common in delirium syndrome) are frequently reported [1-3]. Previous reports indicate that about 50% of patients infected with Sars-Cov2 developed sleep disorders [4]. Despite a large body of literature, the association between sleep duration and mortality risk is not clear demonstrated [5]. Aim of this study is to evaluate sleep disorders in a large group of hospitalized patients with respiratory failure due to COVID-19 and assess their potential association with prognosis.
Methods
Our hospital (Fondazione Poliambulanza Istituto Ospedaliero) is located in Brescia, in the region of Lombardy, east of Milan, the area with the highest rate of SARS-CoV-2 infection and death in Italy. During the period between March 1 and April 30, 2020, 1337patients were admitted to hospital affected by COVID-19 [6]. Information collected were age, sex, PaO2/FiO2, chest X Ray or computed tomography, comorbidities (ischemic heart disease, hypertension, diabetes, malignancies, neurodegenerative diseases), laboratory tests and treatments performed (antibiotics, antivirals, corticosteroids, tocilizumab and oxygen therapy: i.e., from nasal cannula to high flow cannula oxygen therapy to invasive ventilation).
Severity of SARS-CoV-2 infection was assessed by Italian Society of Anesthesiology, Analgesia, Resuscitation and Intensive Care (SIAARTI) criteria: pneumonia Stage III (PaO2/FiO2 ratio >300); pneumonia Stage IV- mild Acute Respiratory Distress Syndrome (ARDS) (200<PaO2/FiO2<300), Stage IV-moderate ARDS (100<PaO2/FiO2<200) and Stage IV-severe ARDS (PaO2/FiO2 <100mmHg) [7]. All patients were treated according to the Italian Society for Infectious and Tropical Diseases (SIMIT) Guideline [8].
Sleep difficulties were reported by nurses on the electronic records of the ward; on the basis of the severity of sleep disturbances physicians prescribed appropriate therapy (i.e., benzodiazepines), using a standard protocol. For the purposes of this study, we reported the presence of sleep disturbances when they required drug treatment.
Results
1337 consecutively admitted patients have been studied (mean age: 68.6±13.7, males: 885; patients in Stage III: n= 313, in Stage IV (mild ARDS): n=544; Stage IV (moderate ARDS): n=211; in Stage IV (severe ARDS): n=125. In-hospital mortality was 25.0% (n=334). 20.4% of them had sleep disturbances. Sleep disturbances were higher in older patients (9,5%, 20,1%, 25,7%, 27,1% respectively in < 60, 61-70, 71-80, 81+ years old) (chi square test: p < 0.001), and in severe infections compared with non-severe infections (15%, 20.6%, 25.6% and 23.2%) respectively in Stage III, Stage IV-mild, Stage IV-moderate and in Stage IV-severe (chi square test: p < .006).
In hospital survival according to sleep disturbances is shown in figure 1.
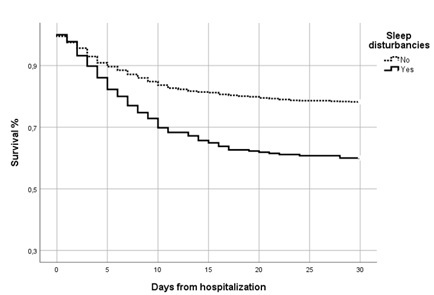 Figure 1: Survival according to sleep disturbances in hospitalized COVID-19 patients.
Figure 1: Survival according to sleep disturbances in hospitalized COVID-19 patients.
P < 0.0001 for different survival in patients without or with sleep disturbances on log-rank test.
In bivariate analysis the presence of sleep disturbances was associated with greater in-hospital mortality (RR: 2.0, CI 95% 1.6-2.5). In a Cox regression analysis sleep disturbances maintain their independent association (HR: 2.1, CI 95% 1.6-2.7) with in-hospital mortality also after controlling for potential confounders: pneumonia severity-PaO2/FiO2 mmHg: HR 0.994, CI95% 0.992-0.995, per unit increase; being over 65: HR: 3.5, CI 95% 2.3-5.3; being male: HR: 1.0, CI 95% 0.8-1.0; chronic heart disease: HR: 1.6, CI 95% 1.2-2.1, and CKD: HR: 2.6, CI 95% 2.0-3.4 (Table 1).
|
|
HR |
95% CI |
p |
|
Sleep disturbances |
2.1 |
1.6-2.7 |
.001 |
|
PaO2/FiO2 mmHg (for unit increase) |
0.994 |
0.992-0.995 |
.001 |
|
Chronic Heart Disease |
1.6 |
1.2-2.1 |
.001 |
|
Chronic Kidney Disease |
2.6 |
2.0-3.4 |
.001 |
|
Age (65+) |
3.5 |
2.3-5.3 |
.001 |
|
Gender (males) |
1.0 |
0.8-1.0 |
.076 |
Table 1: Sleep disturbances and in-hospital mortality risk in 1337 hospitalized COVID-19 patients.
RR: Relative Risk. C.I.: Confidence Interval
Cox regression was used to identify predictors of in-hospital mortality. Variables significantly associated to mortality in bivariate analysis: age (65+), gender (males), hypertension, Chronic Heart Disease, previous cancer, diabetes mellitus, chronic kidney disease, obesity, respiratory failure, and sleep disturbances were included as potential confounders in a final model with in-hospital death as the outcome.
Discussion
Information about the quantity and quality of sleep in patients in general hospital wards is lacking and contradictory due to different assessment methods and setting, ranging between 30% to 80% [5]. Although there is a paucity of literature regarding the effects of sleep loss in hospitalized patients, recent systematic reviews of the literature show that sleep duration, with both long and short sleep, is associated with mortality in a U-shaped fashion, with the lowest risk found in those who report sleep durations around 7 hours [9]. Typically, short sleep duration is thought to increase mortality risk through adverse endocrinologic, immunologic or metabolic effects, thus inducing chronic, low grade inflammation, increasing cortisol secretion or altering growth hormone metabolism [10].
Among the factors that are likely to disrupt sleep are environmental factors (e.g., noise, lights), medical care related factors (e.g., early morning wake up, drips, diagnostic procedures), patient factors (e.g., pain, constipation, urinary retention, dyspnoea, medications) and metabolic or neurochemical abnormalities in conditions such as infections. Specific symptoms or diseases, such as anxiety, depression, dementia, delirium, Parkinson disease may also induce sleep alterations.
Our study has been conducted in patients with COVID-19 characterized by severe respiratory failure, kidney and heart damage and an excessive inflammation. In this condition sleep disturbances are found in almost 20% of hospitalized patients and were significantly more common in elderly compared with younger patients and severely infected compared with non-severely infected. The causes of sleep disturbances in COVID-19 patient may be environmental and biological: patients are in a non-comfortable condition due to noise, to the presence of the technological apparatus, to the precarious relations with doctors and nurses, to the stress induced by the imposed separation from their relatives, and due to the acute disease, manly respiratory difficulties. The sum of these condition predisposes patients also to delirium, as it has been reported [11].
Sleep reduction could exert an effect on life duration through different mechanisms. A direct role on inflammation in short sleepers is biological plausible, as sleep loss has been previously associated with increased blood pressure and heart rate, altered metabolic and endocrine, as well as catecholamine signaling, all inducing an increased sympathetic activation, and a pro-inflammatory effect [12]. In fact, markers of inflammation were significantly associated with reported sleep duration. Interleukin-6 (IL-6) and Tumor Necrosis Factor (TNF) alfa levels were higher in patients with reported sleep durations <6 or >8 h per night. A similar but no significant trend was found for also C-reactive Protein (CRP) [13].
A different possible mechanism may be ascribed to Angiotensin-Converting Enzyme 2 (ACE2), identified as the functional receptor for SARS-CoV-2 present in multiple human organs, including nervous system and skeletal muscles. The expression and distribution of ACE2 suggests that the SARS-CoV-2 may cause some neurologic manifestations [14]. The data reported in this study could support this interpretation, since the effect of sleep disturbances is independent form other factors increasing mortality by them self, through a direct biological effect of SARS-CoV-2 on brain function. Further studies are needed to clarify the association between sleep disorders and mortality in COVID19 patients, in any case, sleep disturbances should be counted among the signs of poor prognosis.
Contribution
RR and AB, designed the study, contributed to and revised critically. All authors read and approved the final manuscript.
Acknowledgment
This study was approved by the Poliambulanza Scientific Advisory Committee and covered by its ethics approval.
Consent for Publication
Not applicable.
Competing Interests
All authors declare that they have no competing interests. All authors contribute and approved the manuscript. All authors declare no financial support and no conflicts of interests.
References
- Pleasure SJ, Green AJ, Josephson SA (2020) The spectrum of neurologic disease in the Severe Acute Respiratory Syndrome Coronavirus 2 pandemic infection: Neurologists move to the frontlines. JAMA Neurol 77: 679-680.
- Spudich S, Nath A (2022) Nervous system consequences of COVID-19. Science 375: 267-269.
- Mao L, Jin H, Wang M, Hu Y, Chen S, et al. (2020) Neurologic Manifestations of hospitalized patients with Coronavirus disease 2019 in Wuhan, China. JAMA Neurol 77: 683-690.
- Jahrami HA, Alhaj OA, Humood AM, Alenezi AF, Fekih-Romdhane F, et al. (2022) Sleep disturbances during the COVID-19 pandemic: A systematic review, meta-analysis, and meta-regression. Sleep Med Rev 62: 101591.
- Kurina LM, McClintock MK, Chen JH, Waite LJ, Thisted RA, et al. (2013) Sleep duration and all-cause mortality: A critical review of measurement and associations. Ann Epidemiol 23: 361-370.
- Rozzini R, Bianchetti A (2020) COVID Towers: low- and medium-intensity care for patients not in the ICU. CMAJ 192: 463-464.
- SIAARTI (2020) Percorso assistenziale per il paziente affetto da COVID-19: Sezione 2. SIAARTI, Rome, Italy.
- SIMIT (2020) Società Italiana di Malattie Infettive e Tropicali- Sezione Regione Lombardia Vademecum per la cura delle persone con malattia da COVI-19. SIMIT.
- Rod NH, Kumari M, Lange T, Kivimäki M, Shipley M, et al. (2014) The joint effect of sleep duration and disturbed sleep on cause-specific mortality: Results from the Whitehall II cohort study. PLoS One 9: 91965.
- Hall MH, Smagula SF, Boudreau RM, Ayonayon HN, Goldman SE, et al. (2015) Association between sleep duration and mortality is mediated by markers of inflammation and health in older adults: The health, aging and body composition study. Sleep 38: 189-195.
- Watson PL, Ceriana P, Fanfulla F (2012) Delirium: Is sleep important? Best Pract Res Clin Anaesthesiol 26: 355-366.
- Zambrelli E, Canevini M, Gambini O, D'Agostino A (2020) Delirium and sleep disturbances in COVID-19: A possible role for melatonin in hospitalized patients? Sleep Med 70: 111.
- Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, et al. (2009) Sleep duration and biomarkers of inflammation. Sleep 32: 200-204.
- Pleasure SJ, Green AJ, Josephson SA (2020) The Spectrum of Neurologic Disease in the Severe Acute Respiratory Syndrome Coronavirus 2 Pandemic Infection: Neurologists Move to the Frontlines. JAMA Neurol 77: 679-680.
Citation: Rozzini R, Bianchetti A (2022) Prognostic Value of Sleep Disturbances in Covid-19 Patients. J Gerontol Geriatr Med 8: 146.
Copyright: © 2022 Renzo Rozzini, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

