
Protective Effect of 17β Estradiol against Kidney Injury in a Mouse Model of Unilateral Renal Ischemia
*Corresponding Author(s):
Carolyn M EcelbargerCenter For The Study Of Sex Differences In Health And Disease Georgetown University, Washington DC, United States
Tel:+1 2026870653,
Fax:+1 2026872040
Email:ecelbarc@georgetown.edu
Abstract
Renal ischemia can lead to irreversible damage to the kidney including atrophy and fibrosis. Young females generally experience attenuated pathology due to the protective actions of ovarian steroids, in particular 17β-Estradiol (E2); however, the mechanisms of action remain murky. We evaluated the effects of E2 on pathology associated with renal ischemic injury in atherosclerosis-prone apolipoprotein E knockout mice. Seven groups of mice (n=6/group) were studied for 60 days: 1) Ovariectomized (OVX) female with Left Renal Artery incomplete ligation (RAL) and subcutaneously implanted placebo pellets; 2) OVX-RAL females with E2 pellets; 3) intact females with RAL; 4) intact males with RAL; 5) intact females with sham-RAL surgeries; 6) intact males with sham surgeries; and 7) OVX females with sham surgeries. E2 replacement substantially reduced renal atrophy of the ligated kidney (by over 50%). Western blotting revealed that E2 attenuated the reduction in Aquaporin-2 (AQP2), the bumetanide-sensitive Na-K-2Cl cotransporter (NKCC2), and the sodium phosphate cotransporter (NaPi-2) in the ischemic kidney; and further increased AQP2 and NaPi-2 in the contralateral kidney. E2 also increased transforming-growth-factor β1 (TGFβ1) in both kidneys, and reduced the degree of fibrosis. Assessment of metabolic parameters revealed a strong trend for reduction in circulating insulin (18%) and triglycerides (46%) with E2 replacement; however, plasma and renal interleukin-6 were increased. Overall E2 replacement attenuated atrophy and fibrosis associated with renal ischemia and preserved transporter/channel profile, despite increased TGF-β1 and IL-6. These findings are relevant to menopause or in other states of low estrogen coupled to renal ischemia.
Keywords
Apolipoprotein E; Cytokines; Inflammation; Ovariectomy; Renal artery ligation
INTRODUCTION
Acute or chronic renal ischemia (impaired blood flow) accompanies a number of conditions or events including: surgery, trauma, atherosclerosis, hypertension, idiopathic Renal Artery Stenosis (RAS), and general aging. RAS is the most common cause of End-Stage Renal Disease (ESRD) in elderly patients. It has been reported at an incidence of 18% in subjects 64-74 years of age, on autopsy, and 42% in those above the age of 75 [1]. Atherosclerosis accounts for 90% of the lesions that obstruct blood flow to the renal arteries resulting in stenosis. Moreover, renal artery stenosis increases the risk for adverse cardiovascular outcomes nearly 2-fold [2]. Specific polymorphisms in Apolipoprotein E (ApoE) have been associated with increased prevalence of RAS in human subjects [3]. RAS, as well as other forms of renal ischemia, can result in irreversible renal damage and sometimes ESRD.
It has been known for some time that 17β-estradiol (E2) provides protection in young females against progression and severity of a number of vascular disorders including ischemia-associated renal pathology [4]. However, with the advent of menopause, circulating levels of ovarian steroids rapidly decline. In parallel, the rate of Cardiovascular Disease (CVD) increases to the point where it is the leading cause of death in women over 50 [5]. While simple replacement of these missing steroids would seem a plausible answer, large, randomized prospective trials conducted over 2 decades ago, such as the Women’s Health Initiative (WHI) have shown that Hormone Replacement Therapy (HRT) using common estrogenic formulations were not only “non-protective”, but actually increased the risk for CVD [6,7]. As a result of the short-comings that surfaced from these early studies, it became apparent that the actions and metabolism of ovarian steroids over the lifespan are quite complex and in imminent need of further study.
Therefore, in spite of intense research on mechanisms underlying renal-ischemia- associated pathology, treatments remain unsatisfactory. Acutely, arterial blockage can often be reversed by balloon angioplasty and/or stent implantation; however, these procedures often result in vessel restenosis [8]. The interplay of growth factors, lipids, and oxidative factors are implicated in both atherosclerosis and restenosis. E2 has the potential to be protective against pathology associated with these disorders, but further study is warranted.
For example, while several studies suggest that E2 is anti-inflammatory; other studies find activation of growth-related pathways including TGFb1 [9]. E2 also has an unclear relationship with interleukin-6 (IL-6), a major marker of vascular inflammation. In one study, oral conjugated estrogen (Premarin) led to a 48% increase in circulating IL-6 [10]. On the other hand, in another study, serum E2 and IL-6 were negatively correlated in women during the menopausal transition [11]. Spleen and plasma IL-6 concentrations were higher in BALB-/c female mice (versus male mice), reduced by ovariectomy, and associated with the severity of hepatitis [12]. It is likely that some of these differential effects of E2 on interleukin-6 are dose and host dependent.
E2 may provide protection through 3 known receptors: ERα, ERβ, and G-Protein-Coupled Estrogen Receptor (GPR30). ERα and ERβ are nuclear receptors derived from two different genes and both are localized to kidney, although precise tubular/vascular localizations are unclear. Studies by Lane associates [13], suggest ERα may have a role in mediating compensatory growth of the kidney during diabetes. In addition to the nuclear receptors, evidence for rapidly-acting, membrane-associated estrogen receptors in kidney have been demonstrated [14]. Both ERα and GPR30 have been localized to membranes and may mediate some of the rapid, non-genomic actions of estradiol in kidney. E2 has been shown to have vasodilator actions, and produce nitric oxide via the GPR30 [15], which may be one means by which it affords protection.
In this study, we utilize a novel mouse model of unilateral renal ischemia associated with systemic inflammation (ApoE knockout mice with partial left renal artery ligation, RAL). We test whether E2 replacement in RAL provides protection against atrophy, fibrosis, and dedifferentiation of the ischemic kidney. Furthermore, we evaluate potential mechanisms underlying differences observed including assessing metabolic parameters, growth factors, and inflammatory markers.
METHODS
Experimental animals
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Georgetown University prior to initiating studies. Apolipoprotein E (ApoE) KO (Apoetm1Unc, 007069 stock number) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) at 10 weeks of age. Mice were equilibrated for 2 weeks then divided into 7 treatments (n=6/treatment): 1) Intact Male (M); 2) Intact Male with Renal Artery Ligation (M-RAL); 3) Intact Female (F); 4) Intact Female with Ligation (F-RAL); 5) Female with Ovariectomy (F-OVX); 6) Female with Ligation and OVX (F-RAL-OVX); and 7) Female with Ligation, OVX, and 17β-estradiol replacement (F-RAL-OVX+E2). For RAL, mice were anesthetized with a ketamine/xylazine cocktail, then an abdominal incision was made, and the left renal artery exposed. A tapered 32-gauge needle was positioned on top of the artery, and a silk suture (6-0) tied around both the artery and the needle. Then the needle was removed leaving a loosely ligated artery [16]. Stouffer et al. [16], have demonstrated significantly reduced renal blood flow by laser Doppler in this model. For OVX, both ovaries were located, ligated and removed. Sham surgeries were performed on intact (females only) and non-RAL mice (male and female) consisting of a modest manipulation of the ovaries or renal artery, respectively, before surgical closure. OVX was performed under the same anesthesia as the RAL (or sham manipulation). Placebo (groups 1-6) or E2-releasing (group 7) pellets (Innovative Research, Sarasota, FL) were implanted subcutaneously into all mice near the end of the anesthesia period. E2 pellets were predicted to release approximately 2μg/day for 60 days. Urine was collected in mouse metabolic cages (24 hour), and semi-fasting (5-hour) blood glucose was measured in the final week. After 60 days, animals were deeply anesthetized and euthanized by exsanguination following cardiac puncture. Blood and the right and left kidneys were obtained for analysis.
Histology
A section of the right and left kidneys were fixed (4% paraformaldehyde) and mounted on slides for histochemical analyses [17]. Masson’s trichrome staining was conducted in the Georgetown University Histology Core. Images were taken at 400X magnification on an Olympus SZX7 microscope equipped with an XC50 digital camera. Images (9/kidney/mouse) were examined for the degree of tubulointerstitial fibrosis using a qualitative assessment scoring method as described previously [18-20]. The scoring is based on presence of blue color (collagen), its location (apical or basolateral), interstitial cell proliferation, and tubule dilatation. Scoring was done on sections in a blinded fashion.
Immunohistochemistry
Paraffin-embedded sections of kidney were rehydrated, treated with citric acid buffer, for antigen retrieval, blocked, and incubated overnight with primary antibodies against CD68 (rabbit polyclonal, Abcam ab125212). The second day, after application of the secondary anti-rabbit antibody, the slides were developed with a Horseradish Peroxidase (HRP) and DAB chromogen detection system (Vector Laboratories). This was followed by a second night incubation with primary antibody against FOX3P (mouse monoclonal, Abcam, ab36607) and M.O.M. (mouse-on-mouse) blocking reagent. After treatment with anti-mouse secondary, slides were developed with HRP and TMB (chromagen). After cover slipping, slides were imaged on an Olympus BX43 microscope with a 40X objective and 10X ocular.
ELISAs, colorimetric assays, and blood glucose
Semi-fasting blood glucose (5-hour fast) was measured in the final week of the study with the use of a handheld glucometer (FreeStyle Lite, Abbott). Plasma levels of triglycerides (Biovision Incorporated, SKU: K622), insulin (EMD Millipore EZRMI-13K), interleukin-6 (Mouse IL-6 ELISA MAX™, BioLegend), and 17β-estradiol (Abcam, ab108667) were measured by colorimetric or ELISA kits. IL-6 kidney right and left kidney concentrations were also assayed in selected groups.
Western blotting
Renal cortex samples were obtained from both the left (ischemic) and right (contralateral) kidneys and processed for western blotting. Western blotting was performed as previously described [21]. Briefly, samples were prepared by homogenizing the tissues in a buffer containing protease inhibitors. After determining the protein concentrations, the homogenates were solubilized in Laemmli sample buffer. Quality of tissue sample preparation was assessed by staining loading gels with Coomassie-blue (Gelcode Blue, Pierce Endogen, Rockford, IL), and then examining the sharpness of the bands. To assess the alterations in the protein abundances, semi-quantitative immunoblotting was performed. For immunoblotting, 10-30µg of protein from each sample was loaded into individual lanes of minigels of 7, 10 or 12% polyacrylamide (precast, Bio-Rad). Blots were probed with our own rabbit peptide-derived polyclonal antibodies against aquaporin-2 (aqp2, AQP2) [17], the sodium-potassium-2-chloride cotransporter (slc12a1, NKCC2) [17] and the sodium phosphate cotransporter (slc34a1, NaPi-2a) [17]. Peptide sequences used to generate antibodies have been previously described [22-24]. Commercial antibodies were used to probe for transforming growth factor β1 (TGF-β1, Novus Biologicals, NB100-91995), vascular endothelial growth factor (VEGF, Novus Biologicals, NB100-664). Loading accuracy was evaluated by probing the lower portion of the nitrocellulose membranes (or stripping and reprobing) with β-actin monoclonal antibody (SigmaChemical Co., St. Louis, MO).
Statistical analysis
Quantitative data are expressed as mean±SEM. Data were analyzed by one-way or two-way Analysis of Variance (ANOVA). Significant differences between pairs of means were determined by a multiple comparisons test following a significant (p<0.05) one-way ANOVA (SigmaPlot 10.0, Chicago, IL). P-values representing significant differences in the post-hoc testing were adjusted for “multiple comparisons”. Two-way ANOVA was used to determine the effect of kidney (right or left) and estradiol (presence or absence) on western blots and Masson’s trichrome staining.
RESULTS
Body and kidney weights
Final body and kidney weights are shown in figure 1. Male mice were significantly heavier than females (panel A). There were modest differences in body weight between female groups; however, none were significant (as assessed by multiple comparisons testing following one-way ANOVA). There was a strong trend for the F (sham females) to be weigh less than OVX females (non-adjusted p-value=0.00609, critical p-level<0.005). RAL had no effect on final body weight. Final wet weights of the contralateral (right) and ligated (or sham, left) kidneys are shown in figure 1B. M-RAL mice had significantly heavier right kidneys than F (intact sham females) mice (multiple comparisons test following a significant, p=0.001, one-way ANOVA). There were no other significant differences in the weights of the right kidney, although most RAL groups showed some evidence of compensatory enlargement (hatched bars) M mice (intact sham males) had significantly heavier left kidneys than F-RAL and F-RAL-OVX groups (one-way ANOVA p-value=0.002). E2 replacement increased mean left kidney weight in RAL females (black bar) so that it was not significantly different from the other groups.
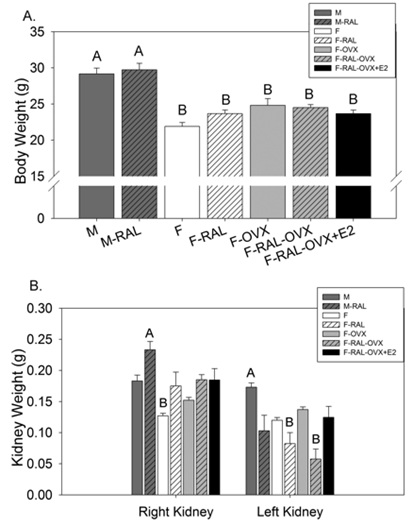 Figure 1: Body and kidney weights- A: Final body weights; B: final kidney weights. “A” is assigned to the highest mean and significantly greater than “B”. Bars without assigned letters indicated that that mean was not significantly different from any other mean (multiple comparisons test following a significant one-way ANOVA); n=6 mice/group; M- Male, RAL- Renal Artery Ligation, F- Female, OVX- Ovariectomy, E2- 17β-estradiol.
Figure 1: Body and kidney weights- A: Final body weights; B: final kidney weights. “A” is assigned to the highest mean and significantly greater than “B”. Bars without assigned letters indicated that that mean was not significantly different from any other mean (multiple comparisons test following a significant one-way ANOVA); n=6 mice/group; M- Male, RAL- Renal Artery Ligation, F- Female, OVX- Ovariectomy, E2- 17β-estradiol.
Urine electrolytes
Urine electrolytes (concentrations and absolute excretion- 24 hours) were measured primarily as an index of food intake, but also to determine whether treatments affected excretion of major electrolytes (Table 1). In general, males had slightly greater urine excretion of Na+, K+, and Cl- than most female groups; however, these differences were not significant by one-way ANOVA. Similarly, we did not observe that ovariectomy, RAL, or E2 replacement significantly altered these parameters.
|
Group |
Urine Volume (ml/d) |
Urine Sodium (mM) |
Urine Potassium (mM) |
Urine Chloride (mM) |
Urine Sodium (mmol/d) |
Urine Potassium (mmol/d) |
Urine Chloride (mmol/d) |
|
M |
2.3±0.2 |
132±6 |
240±20 |
186±14 |
298±26 |
538±45 |
417±34 |
|
M-RAL |
2.4±0.3 |
126±7 |
214±13 |
172±10 |
299±36 |
503±58 |
404±46 |
|
F |
1.3±0.2 |
137±9 |
244±14 |
188±11 |
191±39 |
341±67 |
263±54 |
|
F-RAL |
1.6±0.3 |
121±11 |
238±25 |
183±19 |
197±42 |
367±56 |
286±50 |
|
F-OVX |
1.6±0.3 |
150±11 |
261±14 |
200±9 |
231±27 |
408±54 |
315±44 |
|
F-RAL-OVX |
2.0±0.3 |
123±14 |
231±22 |
182±21 |
231±22 |
439±50 |
342±42 |
|
F-RAL-OVX+E2 |
2.0±0.3 |
119±12 |
239±25 |
172±23 |
218±17 |
436±32 |
309±21 |
|
ANOVA (p-value) |
0.17 |
0.37 |
0.79 |
0.90 |
0.11 |
0.16 |
0.14 |
Table 1: Urine Electrolyte Concentration and Absolute Excretion*.
Note: *mean ±sem; Data were analyzed by one-way ANOVA (p-values indicating significance were adjusted for multiple comparisons); no significant differences were found.
Metabolic profile
Metabolic parameters including E2 concentrations in plasma are shown in figure 2. Plasma E2 levels were a little over 2-fold higher (on average) in OVX + E2 replaced mice relative to all other groups, and significantly higher than F-RAL and F-OVX groups (Figure 2A). Semi-fasting blood glucose (panel B), plasma triglycerides (panel C), and plasma insulin (panel D) concentrations were not different amongst groups (one-way ANOVA); however, there was a strong trend for these values to be reduced in intact females and in OVX+E2 females.
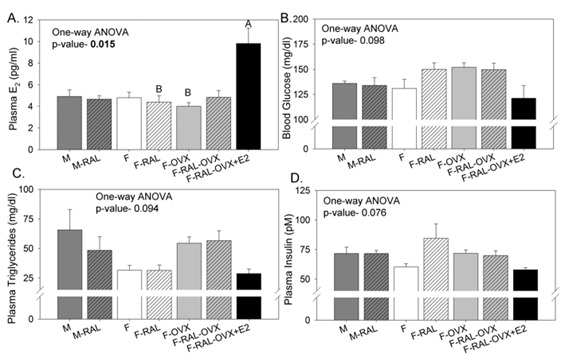 Figure 2: Metabolic factors and hormone concentrations- A: Plasma E2; B: Blood glucose; C: Plasma triglycerides; D: plasma insulin. “A” is assigned to the highest mean and significantly greater than “B”. All means without letters were not different from any of the other means (multiple comparisons test following a significant one-way ANOVA); n=6 Mice/group; M- Male, RAL- Renal Artery Ligation, F- Female, OVX- Ovariectomy, E2- 17b-estradiol.
Figure 2: Metabolic factors and hormone concentrations- A: Plasma E2; B: Blood glucose; C: Plasma triglycerides; D: plasma insulin. “A” is assigned to the highest mean and significantly greater than “B”. All means without letters were not different from any of the other means (multiple comparisons test following a significant one-way ANOVA); n=6 Mice/group; M- Male, RAL- Renal Artery Ligation, F- Female, OVX- Ovariectomy, E2- 17b-estradiol.
Interleukin-6 (IL-6) in kidneys and plasma
The cytokine, interleukin-6, which has both inflammatory and anti-inflammatory actions, was evaluated in plasma (Figure 3A). Overall RAL had a tendency to increase plasma IL-6 by about 10% (hatched bars) except in E2-replaced mice, where the mean increase was much greater. M-RAL had significantly higher plasma IL-6 concentrations than F-OVX mice. E2-replaced females had the highest mean level of plasma IL-6 although due to high variability it wasn’t significantly different from other groups (by multiple comparisons testing). Kidney (right and left) concentrations of IL-6 were evaluated in selected groups (Figure 3B). Intact Females (F) had higher IL-6 concentrations in the contralateral right kidney than did F-RAL-OVX. In the left (RAL) kidney, E2 replacement in RAL females increased IL-6 concentrations to a level comparable to the right kidney in intact and replaced groups.
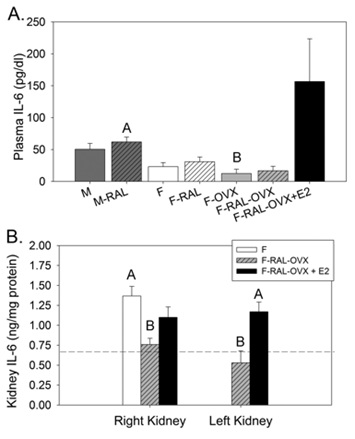 Figure 3: Interleukin-6- A: plasma concentrations; B: kidney concentrations in selected groups (as indicated). “A” is assigned to the highest mean and significantly greater than “B”. All means without letters were not different from any of the other means (multiple comparisons test following a significant one-way ANOVA); n=6 mice/group; M- Male, RAL- Renal Artery Ligation, F- Female, OVX- Ovariectomy, E2- 17β-estradiol.
Figure 3: Interleukin-6- A: plasma concentrations; B: kidney concentrations in selected groups (as indicated). “A” is assigned to the highest mean and significantly greater than “B”. All means without letters were not different from any of the other means (multiple comparisons test following a significant one-way ANOVA); n=6 mice/group; M- Male, RAL- Renal Artery Ligation, F- Female, OVX- Ovariectomy, E2- 17β-estradiol.
Histology
To determine the effect of E2 therapy on collagen deposition in the right and left kidneys of the female OVX RAL mice, we conducted Masson’s trichrome staining (Figure 4). Using a customized scoring system [20,25,26], we assessed Interstitial Fibrosis Index (TIFI). In this scoring system, scores range from 0 (no evidence of fibrosis) to 4 (highest incidence). As expected, median levels of fibrosis were significantly higher in the left (ligated) versus the right (contralateral) kidney. Furthermore, E2 replacement led to a significantly lower TIFI score (p=0.025). Mouse individual TIFI are shown in panel D. Mean TIFI scores and 2-way ANOVA statistics are shown in panel E.
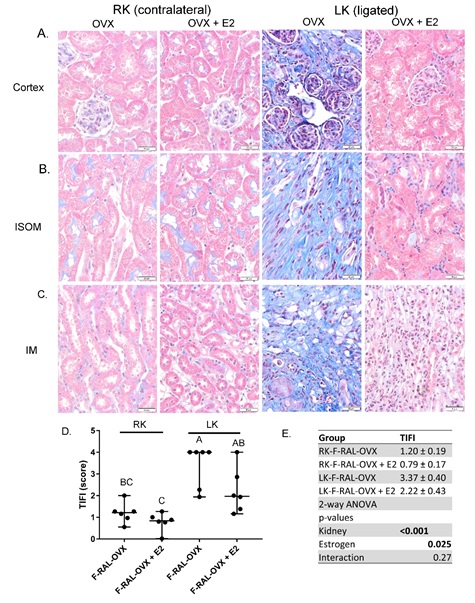 Figure 4: Masson’s trichrome staining of kidney- Representative images from A: cortex; B: inner stripe of the outer medulla (ISOM); and C: inner medulla (IM) in female, ovariectomized (OVX) mice with placebo or E2 replacement. Collagen stains blue. D. Graph indicating individual TIFI (tubular interstitial fibrosis index) scores calculated in each mouse. Median score and 95% confidence intervals are indicated with horizontal lines. “A” is assigned to the highest mean and significantly greater than “BC” or “C”, but not “AB”. “AB” is significantly greater than “C”, but not “BC” (multiple comparisons test following a significant one-way ANOVA, n=6/group). E. Mean TIFI scores in each group and 2-way ANOVA p-values.
Figure 4: Masson’s trichrome staining of kidney- Representative images from A: cortex; B: inner stripe of the outer medulla (ISOM); and C: inner medulla (IM) in female, ovariectomized (OVX) mice with placebo or E2 replacement. Collagen stains blue. D. Graph indicating individual TIFI (tubular interstitial fibrosis index) scores calculated in each mouse. Median score and 95% confidence intervals are indicated with horizontal lines. “A” is assigned to the highest mean and significantly greater than “BC” or “C”, but not “AB”. “AB” is significantly greater than “C”, but not “BC” (multiple comparisons test following a significant one-way ANOVA, n=6/group). E. Mean TIFI scores in each group and 2-way ANOVA p-values.
Immunohistochemistry
To evaluate the degree and type of inflammatory infiltration in the ligated and contralateral kidneys, we conducted immunohistochemistry for CD68 (macrophage marker) and Foxp3 (T-regulatory cell marker, Figure 5). Qualitatively, we found a greater degree of staining for macrophages in the ischemic kidney, as we predicted, in response to the localized injury (green arrows). However, even the contralateral kidney in both E2 and placebo-treated mice showed some presence of macrophages (top panels). Fox3p is a transcription factor found nearly exclusively in T-regulatory (Treg) cells, thus can be used as a marker to some extent. We found apparent increased nuclear localization of Fox3p (blue arrows) in the placebo treated mice.
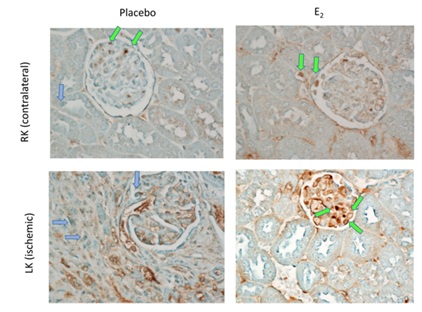 Figure 5: CD68 and FOXP3 immunohistochemistry- Representative Immunohistochemical staining for CD68 (cluster of differentiation 68, macrophage marker, brown stain and green arrows) and FOXP3 (forkhead box P3 transcription factor and marker for T-regulatory cells, blue stain and blue arrows) of kidney cortex of F-RAL-OVX (Placebo) and F-RAL-OVX+E2 (E2). Qualitatively FOXP3 staining appeared to have greater nuclear localization in the placebo-treated mice, while macrophages showed greater infiltration of glomerulus in E2-treated mice. F- Female; RAL- Renal Artery Ligation; E2- 17β-estradiol.
Figure 5: CD68 and FOXP3 immunohistochemistry- Representative Immunohistochemical staining for CD68 (cluster of differentiation 68, macrophage marker, brown stain and green arrows) and FOXP3 (forkhead box P3 transcription factor and marker for T-regulatory cells, blue stain and blue arrows) of kidney cortex of F-RAL-OVX (Placebo) and F-RAL-OVX+E2 (E2). Qualitatively FOXP3 staining appeared to have greater nuclear localization in the placebo-treated mice, while macrophages showed greater infiltration of glomerulus in E2-treated mice. F- Female; RAL- Renal Artery Ligation; E2- 17β-estradiol.
Maintenance of transporter/channel levels
To further evaluate the impact of E2 replacement after RAL on epithelial structure and differentiation, we evaluated the protein levels of 3 transporters/channels in major tubule reabsorptive cell types: 1) proximal tubule (sodium phosphate cotransporter, NaPi-2), 2) thick ascending limb (sodium potassium 2-chloride cotransporter, NKCC2), and 3) connecting tubule and collecting duct (aquaporin 2, AQP2) in the F-RAL-OVX and F-RAL-OVX+E2 groups. Representative western blots for these transporters and channel are shown in figure 5, 6. The densitometry summary (data normalized to β-actin) is shown in panel B. In general, the expression of transporters was reduced in the ischemic left kidney (relative to the right kidney), but there was high variability. E2 replacement tended to increase the expression of all 3 proteins, even in the absence of RAL. The two bands apparent for NKCC2 are likely the dimer and monomeric forms of this protein, as we’ve previously described [27]. Greater degrees of the dimer were found in the left kidney, perhaps due to high levels of fibrotic tissue and cross-linking. AQP2 also ran as two specific bands with the upper band representing a glycosylated form of the protein as previously described [22]. For both proteins, for densitometry the two bands were summed (area X density).
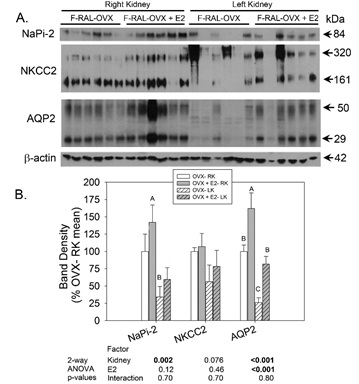 Figure 6: Western blotting of transporters/channels- A: Representative western blots probed with antibodies against NaPi-2, NKCC2, AQP2, and β-actin (for loading correction). Each lane is loaded with a sample from a different mouse right and left kidney whole-cell homogenates (n=6 mice/group). B: Summary showing mean band densities (±standard error) for each group right and left kidneys. Bands densities were expressed relative to the right kidney for the OVX-RAL group (set to 100%). “A” is significantly greater than “B”, and “B” is significantly greater than “C” (multiple comparisons test following a significant one-way ANOVA, n=6/group). Two-way ANOVA (kidney X treatment) p-values are provided below graph.
Figure 6: Western blotting of transporters/channels- A: Representative western blots probed with antibodies against NaPi-2, NKCC2, AQP2, and β-actin (for loading correction). Each lane is loaded with a sample from a different mouse right and left kidney whole-cell homogenates (n=6 mice/group). B: Summary showing mean band densities (±standard error) for each group right and left kidneys. Bands densities were expressed relative to the right kidney for the OVX-RAL group (set to 100%). “A” is significantly greater than “B”, and “B” is significantly greater than “C” (multiple comparisons test following a significant one-way ANOVA, n=6/group). Two-way ANOVA (kidney X treatment) p-values are provided below graph.
Growth-related proteins
Proteins that facilitate compensatory enlargement and tissue remodeling are shown in figure 7. TGF-β1 protein levels were about 50% lower in the ischemic, left kidney and increased by E2 in both kidneys. Vascular Endothelial Growth Factor (VEGF) was increased to the greatest extent in the atrophied (left) kidney of the non-replaced group. In contrast, in the contralateral kidney, E2 increased VEGF expression. All bands were summed for VEGF.
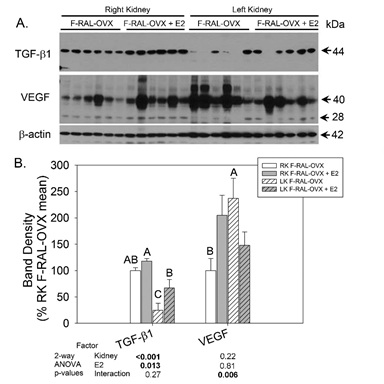 Figure 7: Western blotting of growth factors- A: Representative blots probed with antibodies against TGF-β, VEGF, and β-actin (for loading correction). Each lane is loaded with a sample from a different mouse right and left kidney whole-cell homogenates (n=6 mice/group). B: Summary showing mean band densities (±standard error) for each group right and left kidneys. Bands densities were expressed relative to the right kidney for the OVX-RAL group (set to 100%). “A” is significantly greater than “B”, and “B” is significantly greater than “C” “AB” is different from “C”, but not “A” or “B” (multiple comparisons test following a significant one-way ANOVA, n=6/group). Two-way ANOVA (kidney X treatment) p-values are provided below graph.
Figure 7: Western blotting of growth factors- A: Representative blots probed with antibodies against TGF-β, VEGF, and β-actin (for loading correction). Each lane is loaded with a sample from a different mouse right and left kidney whole-cell homogenates (n=6 mice/group). B: Summary showing mean band densities (±standard error) for each group right and left kidneys. Bands densities were expressed relative to the right kidney for the OVX-RAL group (set to 100%). “A” is significantly greater than “B”, and “B” is significantly greater than “C” “AB” is different from “C”, but not “A” or “B” (multiple comparisons test following a significant one-way ANOVA, n=6/group). Two-way ANOVA (kidney X treatment) p-values are provided below graph.
Correlations of kidney weight with proteins
We next correlated the weight of the kidney(s) with the western blot band density to determine whether expression of evaluated proteins correlated with changes in wet weight (Figure 7, 8). We found significant correlations of 4 out of 5 proteins with kidney weight (panel F). AQP2 (panel C) and TGF-β (panel D) were highly positively correlated with kidney weight. VEGF was significantly negatively correlated with kidney weight (panel E).
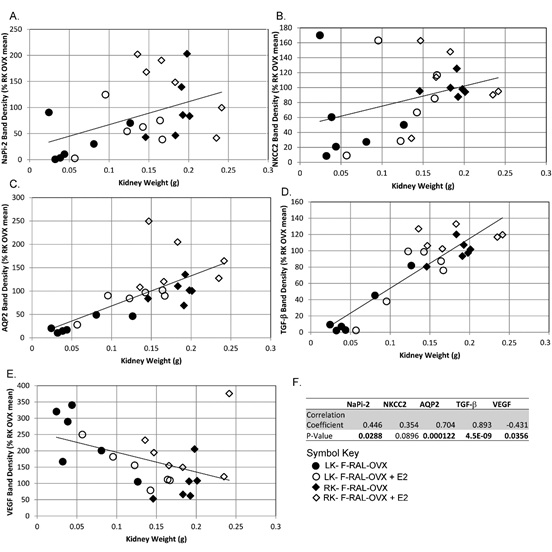 Figure 8: Correlations of proteins with kidney weight- Correlation of normalized protein band densities with kidney weight for A. Napi-2; B. NKCC2, C. AQP2, D. TGF-β, and E. VEGF; filled circles- left kidney F-RAL-OVX, open circles- left kidney F-RAL-OVX+E2, filled diamonds- right kidney F-RAL-OVX, open diamonds- right kidney F-RAL-OVX+E2. F. R and p-values for the correlations. Bolded p-values indicate a significant correlation.
Figure 8: Correlations of proteins with kidney weight- Correlation of normalized protein band densities with kidney weight for A. Napi-2; B. NKCC2, C. AQP2, D. TGF-β, and E. VEGF; filled circles- left kidney F-RAL-OVX, open circles- left kidney F-RAL-OVX+E2, filled diamonds- right kidney F-RAL-OVX, open diamonds- right kidney F-RAL-OVX+E2. F. R and p-values for the correlations. Bolded p-values indicate a significant correlation.
DISCUSSION
Clinically pre-menopausal women have been demonstrated to experience less severe renal injury in response to both acute and chronic renal ischemia as compared to men [28,29]. However, this distinct female sex advantage appears to disappear with menopause [25]. Ischemic events in the kidney can occur acutely, e.g., in surgery or trauma, or chronically, e.g., due to genetic or environmental factors leading to renal artery stenosis. Mechanisms protective of young women are unclear, but likely involve ovarian steroids and their impact on inflammation, oxidative stress, blood pressure, and cellular apoptosis/regeneration [28]. Therefore restoring ovarian steroids (or ovarian-steroid-like mimics) in post-menopausal women (and other women of low-estrogen status) could potentially short-circuit pathways of renal damage and postpone or eliminate the progression to ESRD. Nonetheless, the side-effects of replacement are still in need of additional scrutiny. In this study, we evaluated the effects of E2 replacement on chronic partial Renal Artery Ligation (RAL) in young Ovariectomized (OVX) female ApoE KO (atherosclerosis-prone) mice. We found that, E2 replacement (60 days) significantly attenuated atrophy and fibrosis of the ligated kidney, and the loss of major epithelial transporters. These observations will be discussed in greater detail below.
Our animal model was adapted from that of Stouffer et al. [16], who had demonstrated that unilateral RAL causes a short-term vascular inflammatory reaction in ApoE KO mice. They showed RAL in mice led to the development of atheroma in the abdominal and carotid arteries, which was associated with macrophage infiltration, as well as, dedifferentiation of smooth muscle cells [16]. Their study did not report changes in the kidneys. In our current study, we found partial RAL in the ApoE KO mouse reduced the wet weight of the ligated kidney by around 50%; however, this reduction was significantly attenuated in the female ovariectomized mice with replacement of E2. This is not entirely surprising, as 17β-estradiol is a known genomic stimulator of growth through regulation of transcription via the estrogen response element (ERE). In fact, VEGF is one known growth factor in which an ERE has been reported [30]. In addition, E2 can bind to non-genomic receptors that mediate crosstalk with other growth factors and G-protein-coupled signaling pathways including Mitogen-Activated-Protein Kinase (MAPK) and Phosphatidylinositol-3-Kinase (PI3K/AKT) [31].
Furthermore, in the E2 -treated mice, we found attenuation in the reduction (in the ligated kidney) of the protein levels for 3 transport/channel proteins that we used as markers of epithelial integrity, i.e., Aquaporin-2 (AQP2), marker for collecting duct principal cells, the Sodium Phosphate Cotransporter (NaPi-2), marker for proximal tubule, and the Sodium-Potassium-2-Chloride Cotransporter (NKCC2), marker for the thick ascending limb. Of the 3, the expression of AQP2 was most highly correlated (r=0.70, p=0.00012) with the weight of the kidney. This suggested that the attenuation in atrophy with E2 was directly associated with the maintenance of epithelial cell differentiation, and this relationship appeared somewhat more critical in the collecting duct. It is unclear whether AQP2, NKCC2, or NaPi-2 genes have estrogen response elements in their 5’ flanking regions; however, all 3 of these genes have been reported to have sex differential regulation of one form or another. We previously found that E2 replacement to ovariectomized rats reduced NKCC2 and NaPi-2 [32]. Similarly, in another study, we found reduced AQP2 and NaPi-2 in kidneys from female versus male mice [17]. Therefore, the directional change in these transporters/channels with E2 replacement did not support a direct effect of E2 to influence gene expression, but rather a generalized pattern of maintenance of the epithelial phenotype. Thus, we found evidence for preservation of the major reabsorptive cell types of the renal tubule, i.e., proximal tubule cells, thick ascending limb cells, and collecting duct cells in the E2-treated RAL females. Whether E2-therapy was as beneficial to endothelial cells of the renal blood supply is less certain. VEGF (primarily expressed in the circulatory cells of the kidney) was increased by E2 in the right (contralateral kidney), but suppressed in the left (ligated) kidney. It is possible that this the result, rather than a cause, for relatively less ischemia in the E2-treated mice.
The attenuated atrophy and improvement in transporter/channel expression, was associated with a reduction in the Tubular Interstitial Fibrotic Index (TIFI) in the kidneys from E2-treated mice. The TIFI indicated significantly more fibrosis in the left (ligated) kidneys in both treatment groups; however, 4 out of 6 mice in the non- E2 replaced group had severe fibrosis and the maximum score, while this score was received by only one of the E2 replaced mice. It is important to note that TIFI was variable animal-to-animal. It is likely that when fibrosis and epithelial-to-mesenchymal transition initiates, it rapidly progresses.
E2 potentially could reduce renal pathology in this model in a number of potential ways. One might be via direct anti-inflammatory actions. We measured interleukin-6 (IL-6) in plasma and kidneys as an indicator of inflammation. Surprisingly, IL-6 levels were increased by E2 in both plasma and in kidney. The relationship between E2 and IL-6 is complex. In one study, macrophage-derived IL-6 levels after burn injury were found initially suppressed in female mice, but then increased in the later course of injury [33]. The rise in IL-6 after injury is thought to result in immunosuppression [16]. Physiological levels of E2 are reported to stimulate the immune system, while higher, i.e., pregnancy levels, of E2 are reported to suppress the immune system [34]. Thus, the E2 dose provided our mice (or the fact that it was constantly infused rather than cyclical) could be interpreted as producing a phenotype with regard to IL-6 more similar to a “pregnancy” state, and might be associated with reduced immune responses.
It is important to note, however, that IL-6 has inflammatory, anti-inflammatory, as well as, regenerative actions, dependent on the ratio of expression of membrane bound to soluble IL-6R [35]. Activation of membrane bound IL-6R (in combination with gp130) has been demonstrated to activate STAT3 (signal transducer and activator of transcription 3) leading to epithelial cell proliferation and inhibition of cell apoptosis (at least in intestinal cells) [36]. Furthermore, IL-6R KO mice were shown to develop glucose intolerance and insulin resistance [37]. Thus, activation of IL-6 production by E2 may have a role in both regeneration of the kidney, as well as, marginal improvement in metabolic parameters, i.e., plasma insulin and triglycerides and blood glucose. Additional studies would be needed to test a causative role of IL-6 in these actions. STAT3 is also phosphorylated (activated) directly by estrogen, and has postulated to protect against damage in the brain due to cerebral ischemia via this mechanism [38].
We found evidence of modest macrophage infiltration into both ischemic and contralateral kidneys of mice that had undergone renal artery ligation. This was not unexpected and supported a systemic response, primarily affecting the kidney with greater ischemia. In general, E2 enhanced macrophage infiltration particularly into the glomerulus. Estrogen receptors activate macrophages and can be involved in differential polarization [39]. Additional study into this area is warranted.
E2 replacement also increased the expression of transforming growth factor beta (TGF-β1) in both the ischemic and contralateral kidney. TGF-β1 has been implicated in the Epithelial-to-Mesenchymal Transition (EMT) characteristic of renal disease-associated fibrosis by signaling through the SMAD and SMAD-independent pathways. A recent study by Tam et al. [9], similarly showed an increase in lung TGF-β1 with E2 replacement to ovariectomized mice after exposure to smoke. In this case, the increase was associated with stenosis and fibrosis of pulmonary airways. Therefore E2 may have a role in fibrotic repair pathways. In our study, we saw significant, but not total attenuation of fibrosis in the ligated kidney with variability in how animals responded; however, it was clear that E2 attenuated loss of kidney mass with ischemia, which was highly correlated with TGF-β1 expression. It is likely these factors are related. In addition to roles in proliferation and differentiation of cells, TGF-β1 plays an important role in control of the immune system. Furthermore, estrogen has been demonstrated to regulate nuclear factor kappa-B (NF-kB) [40], an important transcriptional determinant of cell survival, proliferation, inflammation, and immune regulation.
Another means whereby E2 may have been protective against renal atrophy is via a reduction in blood pressure. We did not have the opportunity to collect blood pressure data on our mice; however, Stouffer et al. [16], have demonstrated a modest rise in blood pressure in the partial RAL model. Numerous investigations by our group [41] and others [42-44], have demonstrated that E2 replacement to OVX rodents reduces blood pressure and/or ameliorates over-activity of the Renin-Angiotensin-System (RAS). Thus, it is very plausible that reduced activity of the intrarenal and perhaps systemic RAS plays some role in the protection afforded by E2 in our study. Moreover, E2 via activation endothelial Nitric Oxide Synthase (eNOS) and nitric oxide synthesis could improve ischemia-mediated reduced renal blood flow and impart renal benefit via this means [45]. Estrogen supplementation has been shown to improve endothelium-related dilation through increasing level of nitrite/nitrate in aged women, but not in men [46].
Overall, our study demonstrates that E2 replacement to ovariectomized female ApoE KO mice not only attenuates atrophy and fibrosis in the ischemic kidney, but also assists in the maintenance of epithelial transporter/channel profile. These findings are particularly relevant for post-menopausal women, as well as, young women with low E2 levels, either due to surgery or other means, and are susceptible to or experiencing renal ischemia.
ACKNOWLEDGMENT
The authors thank Shehar-Bano Awan, B.S., summer volunteer, for technical assistance with western blotting and subsequent densitometric analysis.
GRANTS
This research was supported by a Marriott Foundation Fellowship (LJ) and NIH R01 DK082507 and Georgetown University Department of Medicine (CE). CH was supported through the Short-Term Research Experience for Under-Represented Persons (STEP-UP) via the American Physiological Society during a summer internship.
DISCLOSURE
Parts of this work have been presented in preliminary form at the 46th Annual Meeting of the American Society of Nephrology, Atlanta, GA, at the Organization for the Study of Sex Differences (OSSD) annual meeting in Minneapolis, MN, and at the Experimental Biology meeting (2014) in Diego, CA. These data appear in printed abstracts accompanying these meetings.
AUTHOR’S CONTRIBUTION
Dr. Li designed the experiments, conducted animal surgeries, cared for animals, conducted western blotting, ELISA, and immunohistochemistry. She also was responsible for some statistical analyses, data interpretation, and drafting and approving the final version of the manuscript. Ms. Holloway conducted western blotting, data analysis, and data interpretation. She also drafted small portions of the Results and Methods, and approved the final version of the manuscript. Rajni Sharma and Swasti Tiwari conducted and scored Masson’s trichrome images. Dr. Tiwari assisted with writing of Discussion, and both approved final version of the manuscript. Mr. Lee conducted imaging and approved the final version of the manuscript. Dr. Ecelbarger assisted in design of the experiments, supervised work, analyzed the data statistically, drafted figures, and wrote large portions of the Discussion, Results, and Introduction. She also approved the final version of the manuscript.
REFERENCES
- Khatami MR (2013) Ischemic nephropathy: More than a simple renal artery narrowing. Iran J Kidney Dis 7: 82-100.
- Edwards MS, Hansen KJ, Craven TE, Bleyer AJ, Burke GL, et al. (2004) Associations between renovascular disease and prevalent cardiovascular disease in the elderly: A population-based study. Vasc Endovascular Surg 38: 25-35.
- Heidari MM, Foruzannia SK, Khatami M, Hadadzadeh M, Meybodi ME (2013) Apolipoprotein e gene polymorphism in Iranian coronary atherosclerosis patients candidate for coronary artery bypass graft. Iran J Basic Med Sci 16: 841-844.
- L'hermite M, Simoncini T, Fuller S, Genazzani AR (2008) Could transdermal estradiol + progesterone be a safer postmenopausal HRT? A review. Maturitas 60: 185-201.
- Rosano GM, Fini M (2002) Postmenopausal women and cardiovascular risk: Impact of hormone replacement therapy. Cardiol Rev 10: 51-60.
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, et al. (2004) Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women's Health Initiative randomized controlled trial. JAMA 291: 1701-1712.
- Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, et al. (2006) Conjugated equine estrogens and coronary heart disease: The women's health initiative. Arch Intern Med 166: 357-365.
- von Birgelen C, Hartmann M (2004) Intravascular ultrasound assessment of coronary atherosclerosis and percutaneous interventions. Minerva Cardioangiol 52: 391-406.
- Tam A, Churg A, Wright JL, Zhou S, Kirby M, et al. (2016) Sex differences in airway remodelling in a mouse model of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 193: 825-834.
- Herrington DM, Brosnihan KB, Pusser BE, Seely EW, Ridker PM, et al. (2001) Differential effects of E and droloxifene on C-reactive protein and other markers of inflammation in healthy postmenopausal women. J Clin Endocrinol Metab 86: 4216-4222.
- Yasui T, Maegawa M, Tomita J, Miyatani Y, Yamada M, et al. (2007) Changes in serum cytokine concentrations during the menopausal transition. Maturitas 56: 396-403.
- Cho J, Kim L, Li Z, Rose NR, Talor MV, et al. (2013) Sex bias in experimental immune-mediated, drug-induced liver injury in BALB/c mice: suggested roles for Tregs, estrogen, and IL-6. PloS One 8: 61186.
- Lane PH (2008) Estrogen receptors in the kidney: Lessons from genetically altered mice. Gend Med 5: 11-18.
- Bjornstrom L, Sjoberg M (2005) Mechanisms of estrogen receptor signaling: Convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19: 833-842.
- Seok YM, Jang EJ, Reiser O, Hager M, Kim IK (2012) 17beta-Estradiol induces vasorelaxation in a G-protein-coupled receptor 30-independent manner. Naunyn Schmiedebergs Arch Pharmacol 385: 945-948.
- Stouffer GA, Pathak A, Rojas M (2010) Unilateral renal artery stenosis causes a chronic vascular inflammatory response in ApoE-/- mice. Trans Am Clin Climatol Assoc 121: 252-266.
- Sharma N, Li L, Ecelbarger CM (2015) Sex differences in renal and metabolic responses to a high-fructose diet in mice. Am J Physiol Renal Physiol 308: 400-410.
- Maric C, Sandberg K, Hinojosa-Laborde C (2004) Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17beta-estradiol in the aging Dahl salt sensitive rat. J Am Soc Nephro 15: 1546-1556.
- Mohan A, Singh RS, Kumari M, Garg D, Upadhyay A, et al. (2016) Urinary Exosomal microRNA-451-5p Is a potential early biomarker of diabetic nephropathy in rats. PloS One 11: 0154055.
- Singh RS, Chaudhary DK, Mohan A, Kumar P, Chaturvedi CP, et al. (2016) Greater efficacy of atorvastatin versus a non-statin lipid-lowering agent against renal injury: Potential role as a histone deacetylase inhibitor. Sci Rep 6: 38034.
- Song J, Hu X, Riazi S, Tiwari S, Wade JB, et al. (2006) Regulation of blood pressure, the epithelial sodium channel (ENaC), and other key renal sodium transporters by chronic insulin infusion in rats. Am J Physiol Renal Physiol 290: 1055-1064.
- DiGiovanni SR, Nielsen S, Christensen EI, Knepper MA (1994) Regulation of collecting duct water channel expression by vasopressin in Brattleboro rat. Proc Natl Acad Sci USA 91: 8984-8988.
- Kim GH, Ecelbarger CA, Mitchell C, Packer RK, Wade JB, et al. (1999) Vasopressin increases Na-K-2Cl cotransporter expression in thick ascending limb of Henle's loop. Am J Physiol 276: 96-103.
- Kim GH, Martin SW, Fernandez-Llama P, Masilamani S, Packer RK, et al. (2000) Long-term regulation of renal Na-dependent cotransporters and ENaC: response to altered acid-base intake. Am J Physiol Renal Physiol 279: 459-467.
- Maric C, Xu Q, Sandberg K, Hinojosa-Laborde C (2008) Age-related renal disease in female Dahl salt-sensitive rats is attenuated with 17 beta-estradiol supplementation by modulating nitric oxide synthase expression. Gend Med 5: 147-159.
- Mohan A, Singh RS, Kumari M, Garg D, Upadayay A, et al. (2016) Urinary exosomal microRNA-451-5p is a potential early biomarker of diabetic nephropathy in rats. PLoS One 11: 0154055.
- Ecelbarger CA, Terris J, Hoyer JR, Nielsen S, Wade JB, et al. (1996) Localization and regulation of the rat renal Na(+)-K(+)-2Cl- cotransporter, BSC-1. Am J Physiol 271: 619-628.
- Hutchens MP, Dunlap J, Hurn PD, Jarnberg PO (2008) Renal ischemia: Does sex matter? Anesth Analg 107: 239-249.
- Kher A, Meldrum KK, Wang M, Tsai BM, Pitcher JM, et al. (2005) Cellular and molecular mechanisms of sex differences in renal ischemia-reperfusion injury. Cardiovasc Res 67: 594-603.
- Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, et al. (2000) Regulation of Vascular Endothelial Growth Factor (VEGF) gene transcription by estrogen receptors alpha and beta. Proc Natl Acad Sci USA 97: 10972-10977.
- Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, et al. (2004) Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res 10: 331-336.
- Riazi S, Maric C, Ecelbarger CA (2006) 17-beta Estradiol attenuates streptozotocin-induced diabetes and regulates the expression of renal sodium transporters. Kidney Int 69: 471-480.
- Gregory MS, Faunce DE, Duffner LA, Kovacs EJ (2000) Gender difference in cell-mediated immunity after thermal injury is mediated, in part, by elevated levels of interleukin-6. J Leukoc Biol 67: 319-326.
- Kovacs EJ, Messingham KA, Gregory MS (2002) Estrogen regulation of immune responses after injury. Mol Cell Endocrinol 193: 129-135.
- Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S (2011) The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813: 878-888.
- Tilg H, Moschen AR, Kaser A, Pines A, Dotan I (2008) Gut, inflammation and osteoporosis: Basic and clinical concepts. Gut 57: 684-694.
- Matthews VB, Allen TL, Risis S, Chan MH, Henstridge DC, et al. (2010) Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia 53: 2431-2441.
- Dziennis S, Jia T, Ronnekleiv OK, Hurn PD, Alkayed NJ (2007) Role of signal transducer and activator of transcription-3 in estradiol-mediated neuroprotection. The Journal of neuroscience: J Neurosci 27: 7268-7274.
- Villa A, Rizzi N, Vegeto E, Ciana P, Maggi A (2015) Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci Rep 5: 15224.
- Mercantepe T, Unal D, Selli J, Mercantepe F, Unal B, et al. (2016) Protective effects of estrogen and bortezomib in kidney tissue of post-menopausal rats: An ultrastructural study. Ren Fail 38: 1129-1135.
- Ji H, Zheng W, Wu X, Liu J, Ecelbarger CM, et al. (2010) Sex chromosome effects unmasked in angiotensin II-induced hypertension. Hypertension 55: 1275-1282.
- Gupte M, Thatcher SE, Boustany-Kari CM, Shoemaker R, Yiannikouris F, et al. (2012) Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler Thromb Vasc Biol 32: 1392-1399.
- Reckelhoff JF, Zhang H, Srivastava K (2000) Gender differences in development of hypertension in spontaneously hypertensive rats: Role of the renin-angiotensin system. Hypertension 35: 480-483.
- Xue B, Pamidimukkala J, Hay M (2005) Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol 288: 2177-2184.
- Satake A, Takaoka M, Nishikawa M, Yuba M, Shibata Y, et al. (2008) Protective effect of 17beta-estradiol on ischemic acute renal failure through the PI3K/Akt/eNOS pathway. Kidney Int 73: 308-317.
- Kawano H, Motoyama T, Kugiyama K, Hirashima O, Ohgushi M, et al. (1997) Gender difference in improvement of endothelium-dependent vasodilation after estrogen supplementation. J Am Coll Cardiol 30: 914-919.
Citation: Li L, Holloway C, Lee H, Sharma R, Tiwari S, et al. (2021) Protective Effect of 17β Estradiol against Kidney Injury in a Mouse Model of Unilateral Renal Ischemia. J Nephrol Renal Ther 7: 046.
Copyright: © 2021 Lijun Li, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

