
Protective Effects of Rubus coreanus Miq. Extracts Against Arsenic Trioxide-Induced Oxidative Stress and Cell Cytotoxicity
*Corresponding Author(s):
Ae-Son OmDepartment Of Food And Nutrition, Hanyang University, Seoul, South Korea
Tel:+82 222201203,
Email:aesonom@hanyang.ac.kr
Abstract
This study investigated the antioxidant activity in vitro and protective effects in murine macrophage cells against arsenic trioxide treatment with Korean bokbunja berry, Rubus coreanus Miquel leave and stem crude extracts (RCMLS). The total phenolic and flavonoid contents in Rubus coreanus Miquel berry, leave and stem were 1.37 ± 0.01 ~ 2.80 ± 0.02 mg GAE/100g and 54.95 ± 1.65 ~ 109.90 ± 3.06 mg QE/100 g, respectively. The DPPH (2,2-diphenyl-1-picrylhydrazyl) and FRAP (ferric Ion reducing antioxidant Potential) values also indicate that RCMLS possess strong antioxidative potentials. Furthermore, at the concentrations of 10, 50, 100 mg/mL, Rubus coreanus Miquel berry crude extracts had no cytotoxicity in Raw 264.7 cells. When murine macrophage cells were challenged with As (Arsenic) to induce oxidative stress, the administered RCMLS had restored cell antioxidative capacities as well as cell viabilities. The protein expression of the oxidative marker enzymes, SOD (superoxide dismutase) 1 and 2, catalase, and Heme-oxygenase was elevated by treating As, and these elevated mode was almost diminished by the addition of RCMLS in macrophage cells. These results suggest that RCMLS have antioxidant and As protective effects, and could be used as a potential antioxidative nutraceutical targeting heavy metal pollution.
Keywords
Antioxidative effect; Arsenic toxicity; Cell cytotoxicity; Rubus coreanus Miq. extracts
Introduction
Rubus coreanus Miquel (R. coreanus), a perennial shrub of the Rosaceae family, is known to possess various phytochemicals in its various parts and thus reported to have antioxidative, anti-inflammatory, anti-hemolytic, anti-cancer effects [1-3]. The unripe fruits of R. coreanus were used in Korean traditional medicinal practice for the treatment of diabetes, spermstorrhea, asthma and allergy-related diseases [4]. It is generally known that the phenolic contents in berries can be influenced by the degree of ripening, growing location or growing practice [5-7], nonetheless, ripe fruits of R.coreanus contain the high amount of anthocyanins mainly due to their dark colors of fruits. Abundant other kinds of phenolic compounds of R. coreanus fruits are ellagic acid, gallic acid, cinnamic acid, protocatechuic acid, sangiin H-4, 23-hydroxytormentic acid, and nigaichgoside F [8-10]. It has been reported that leaves or stems of R. coreanus exert similar kinds of bioactive functions as fruits [11]. Over the past decades the antioxidant hypothesis for the prevention and treatment of non-communicable degenerative diseases has been prevailed targeting how polyphenols or flavonoids would confer their health benefit [12]. After extensive searching the exact mechanism of health preventive measures of these antioxidant natural components, a number of biological activities were found to be important [13]. Besides simple antioxidant function of these natural components, anti-inflammatory activity is emerging as one of the critical functions [14]. Inflammation is a major contributing factor to the development of several degenerative diseases [15-16]. A prolonged pro-inflammatory state so called chronic inflammation may lead to rheumatoid arthritis, atherosclerosis, inflammatory bowel disease and even diabetes [17-18].
As (Arsenic), a ubiquitous environmental toxicant, is a metalloid element and is often detected in lakes or rivers near mines [19]. Currently, EPA (Environmental Protection Agency) classifies As as an endocrine disruptor along with lead, mercury, and cadmium. Arsenic has several chemical forms such as inorganic arsenic (iAs), Monomethylarsonic acid (MMA), Dimethylarsinic acid (DMA), and in drinking water, the known main source of arsenic, where arsenic exists as iAs III and iAs V. Even though the exact mechanism of toxicity is unclear, the increased generation of Reactive Oxygen Species (ROS), such as peroxyl radicals, superoxide radicals, unpaired oxygen, hydroxyl radicals would induce cell cytotoxicity [20]. The inhibition of various cellular enzymes by arsenic occurs through sulfhydryl group binding, lowering their activities [21]. Specially it can interfere glutathione, a potent As detoxifying agent involved in the process of As metabolism [22]. Additionally, arsenic exposure can lead to interfering gene expression of proteins which in turn delineate signal transduction process involved in DNA repair, DNA synthesis, energy production [23].
This study was undertaken to evaluate antioxidant effects of R. coreanus in three different parts namely fruits, leaves and stems, and protective effects against arsenic trioxide-induced oxidative stress in murine macrophage RAW 264.7 cells.
Materials And Methods
Materials, chemicals and reagents
Folin-Ciocalteu’s reagent, 2,2-Diphenyl-1-Picrylhydrazyl (DPPH), 2,4,6-Tripyridyl-Striazine (TPTZ), 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) and 2’7’-dichrorofluorescin diacetate were purchased from Sigma-Aldrich Co. (St. Louis, Mo, USA). Murine macrophage RAW 264.7 cells were from Korean Cell Line Bank (Seoul, Korea). Penicillin-streptomycin solution and 10% fetal bovine serum were from Corning (Tewksbury, MA, USA). RPMI-1640 buffer and phosphate saline buffer were from Welgene (Kyungsang-do, Korea). The primary antibodies were from Snata Cruz Biotechnology Inc (Santa Cruz, CA, USA), the secondary goat or mouse antibodies were from Bio-Rad Lab (Bio-Rad, CA, USA). Goat anti-rabbit antibodies from Young In Frontier (Ab Frontier, Seoul, Korea), and rabbit anti-goat antibodies from Invitrogen Co. (Invitrogen, CA, USA). Rubus coreanus Miquel fruits were obtained from Korean Plant Extraction Bank (Ohchang, Korea). Rubus coreanus Miquel leaves and stems were donated from Korea Bokbunja Research Institute (Gochang, Korea). All the other reagents are analytical grade.
Preparation of Rubus coreanus Miquel leave and stem crude extracts
Rubus coreanus Miquel leaves and stems were dried for 48 h to have a final moisture content of 6%. Dried samples were extracted with hot distilled water (1:100) for 1 h and then this extracted solution was sterilized at 1000C for 20 min, filtered, concentrated for 2 h using Rotary Evaporator and freeze-dried. The resulting powder was further diluted with 95% ethanol to give 5, 10, 15, 30, 50, 75, 100 µg/mL.
The determination of the Total Phenol (TPC) and the Total Flavonoid (TFC) contents
Samples, 1.0 ml (1:9 diluted with distilled water) and 0.2 ml of 2 N Folin-Ciocalteu's reagent were mixed. After 3 minutes, 0.4 ml of 10% Sodium Carbonate solution (Na2CO3) was added and incubated in the dark for one hour at room temperature. Absorbance was measured at 725 nm using an ELISA microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). The TPC in the sample was derived from a standard curve for gallic acid and is expressed as g of gallic acid equivalents per 100 gram (g GAE/100 g). From the total of 1 mL samples (9 extract :1 etOH) 0.5 ml was taken to a test tube and mixed with 0.1 ml of 10% Aluminum Nitrate (Al(NO3)3·9H2O), 0.1 ml of 1 M Potassium Acetate (CH3COOK) and 4.3ml etOH. The resulting solution was reacted for 30 minutes at room temperature in the dark. The absorbance was determined at 415 nm. A standard curve was prepared using quercetin. The TFC in the sample was calculated from the standard curve and is expressed as mg of quercetin equivalents per g (mg QE/g).
DPPH free radical scavenging activity (DPPH assay)
The 100 µL samples of six different concentrations (5, 25, 50, 100, 150, 200 µg/mL) were added to 1.9 ml 0.1 mM DPPH solution and incubated for 30 min at 37°C. Absorbance was measured at 515 nm. Ascorbic acid, α-tocopherol and quercetin were used as positive controls.
Ferric ion Reducing Antioxidant Power (FRAP) assay
The 20 µL samples of three different concentrations (10, 50, 100 µg/mL) were treated with 180 µL FRAP reagent and incubated for 30 min. Absorbance was measured at 593 nm. As positive controls, ascorbic acid was used.
Cell lines and cell viability measurement
Raw 264.7 cells, a mouse leukemic monocyte macrophage cell line (ATCC TIB-71), were cultured in RPMI-1640 media supplemented with 10% inactivated fetal bovine serum and 100 U/mL penicillin, at 37? in a humidified atmosphere of 95% air and 5% CO2. The total of 1×106 of cells were dispersed to each 96-well. After 24 h cultures, and then cells were treated with different concentrations of samples for 4 hours. After adding 100 µl MTT solution (diluted in RPMI 1640 without phenol red) to each well containing samples, and then incubated for 4 hours, the plates were centrifuged for 10 min at 3,000 rpm. Then the MTT solution was removed and added 100 µl DMSO to each well. Finally, the microplate reader (Thermo, Waltham, USA) was used to obtain the absorption values at 540 nm. The cell viability was calculated by the following formulation:
Cell viability (%) = (Ae/Ac)*100%
Ae and Ac mean the absorption value of experiment group (measured under treatment) and control group (without treatment) respectively.
Reactive oxygen species measurement
The cells (1x106) were incubated with 20 µl of three concentrations (0, 50, 100 µg/mL) of extracts for 4 hr. Next, another 4 h incubation was carried out with the addition of 20 µl As2O3 at each well. DCF-DA (2’7’-Dicholorodihydroflorescein diacetate) at the concentration of 25 µM was added to make total of 2 mL each well, and then the microplate was incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2. The chemiluminescence was determined by spectrofluometer at 485 nm excitation and 530 nm emission wavelengths.
Western blotting analysis
The Raw 264.7 cells were washed twice with PBS and lysed with lysis buffer containing RIPA buffer, protease inhibitor, and phosphatase inhibitor. Protein samples were quantified using a PierceTM BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). Next, 20 µl samples were separated on SDS-PAGE gels and electro-transferred onto a methanol-activated PVDF membrane (Roche, Mannheim, Germany). The membranes were blocked with 5% skim milk and probed in primary antibodies overnight. Then the membranes were washed and exposed to horseradish peroxidase–linked secondary anti-rabbit antibody (Cell Signaling Technology, Danvers, MA, USA) or secondary anti-mouse antibody for one hour. The protein bands on the membrane were visualized using ClarityTM Western ECL Substrate (Bio-Rad, Hercules, CA, USA) and imaged with ChemiDoc (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Data were expressed as Mean ± Standard Deviation (SD) of three similar and independent experiments. Difference between the groups were analyzed by using T-test using SPSS for Windows Ver. 25.0 (SPSS Inc., Chicago, ILL, USA)
Results
Total Phenolic and Flavonoid Content (TPC and TFC)
The data regarding TPC and TFC of ethanol extracts of R. coreanus fruits, leaves and stems are shown in figures 1 & 2. As gallic acid equivalent, the total phenol contents of R. Coreanus Fruits (FRE), Leaves and Stems (LSRE), and Fruit, Leaves and Stems (FLSRE) were 1.37 0.01, 2.80 0.02, 2.09 0.01 gGA per 100 g ethanol extracts respectively. The total estimated flavonoid contents of R. Coreanus Fruits (FRE), Leaves and Stems (LSRE), and Fruit, Leaves and Stems (FLSRE) were 54.95 1.65, 109.90 3.06, 105.68 3.61 mg QE (Quercetin equivalent) per 100 g ethanol extracts respectively.
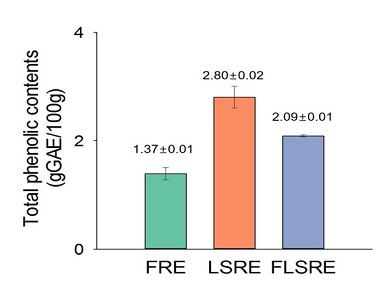 Figure 1: Total phenolic content of ethanol extracts from Rubus coreanus Miq.
Figure 1: Total phenolic content of ethanol extracts from Rubus coreanus Miq.
Values are expressed as mean of triplicate measurements. FRE=fruit of Rubus coreanus Miq. Ethanol extract; LSRE=leaf and stem of Rubus coreanus Miq. Ethanol extract; FLSRE=fruit, leaf and stem of Rubus coreanus Miq. Ethanol extract.
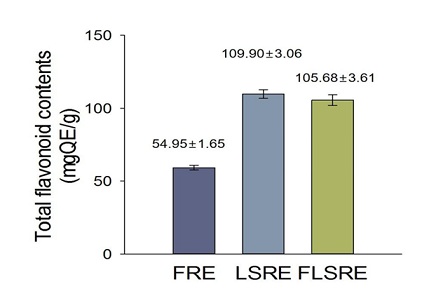 Figure 2: Total flavonoid content of ethanol extracts from Rubus coreanus Miq.
Figure 2: Total flavonoid content of ethanol extracts from Rubus coreanus Miq.
Values are expressed as mean of triplicate measurements. FRE=fruit of Rubus coreanus Miq. Ethanol extract; LSRE=leaf and stem of Rubus coreanus Miq. Ethanol extract; FLSRE=fruit, leaf and stem of Rubus coreanus Miq. Ethanol extract.
Antioxidant properties
To determine the antioxidant effects, 0, 5, 25, 50, 100, 150 and 200 mg/mL FRE, LSRE and FLSRE were tested applying DPPH assay. The results are shown in figure 3, FRE, LSRE and FLSRE had 70.0%, 84.4% and 68.0% of DPPH free radical scavenging activity, respectively. The FRAP values 10, 50, and 100 µg/mL of FRE, LSRE and FLSRE were shown in figure 4 and leaves and stems (LSRE) exhibited the highest FRAP values.
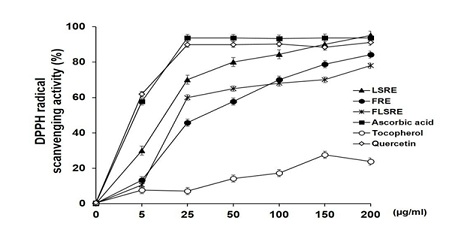 Figure 3: DPPH radical scavenging activity of ethanol extracts from Rubus coreanus Miq. and several antioxidants.
Figure 3: DPPH radical scavenging activity of ethanol extracts from Rubus coreanus Miq. and several antioxidants.
Values are expressed as mean of triplicate measurements. FRE=fruit of Rubus coreanus Miq. Ethanol extract; LSRE=leaf and stem of Rubus coreanus Miq. Ethanol extract; FLSRE=fruit, leaf and stem of Rubus coreanus Miq. Ethanol extract.
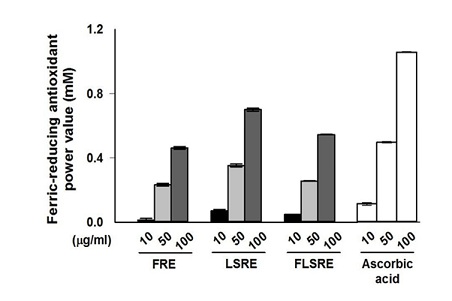 Figure 4: Ferric-reducing antioxidant power value of ethanol extracts from Rubus coreanus Miq. and ascorbic acid.
Figure 4: Ferric-reducing antioxidant power value of ethanol extracts from Rubus coreanus Miq. and ascorbic acid.
Values are expressed as mean of triplicate measurements. FRE=fruit of Rubus coreanus Miq. Ethanol extract; LSRE=leaf and stem of Rubus coreanus Miq. Ethanol extract; FLSRE=fruit, leaf and stem of Rubus coreanus Miq. Ethanol extract.
Effect of Rubus coreanus Miquel extracts on cell cytotoxicity of RAW 264.7 cells
The cytotoxicity of various concentrations of R. coreanus extracts (10, 50, 100 µg/mL) to 264.7 cells was tested. After treating macrophage cells with R. coreanus extracts for 24 hours, an MTT assay was performed. At 10, 50, 100 µg/mL concentrations of R. coreanus extracts (FRE, LSRE and FLSRE) showed over 100% survival rates compared to the negative control (Figure 5). Therefore, in further experiments, concentrations up to 100 µg/mL were used because they did not significantly affect cell proliferation and survival of RAW 264.7 cells.
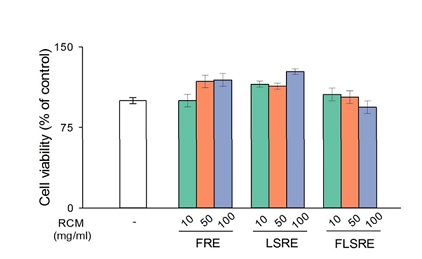 Figure 5: Effect of ethanol extracts from Rubus coreanus Miq. on cell viability in RAW 264.7 cells.
Figure 5: Effect of ethanol extracts from Rubus coreanus Miq. on cell viability in RAW 264.7 cells.
Cells were treated with FRE, LSRE and FLSRE (A : 100, B : 50, C : 10 µg/ml) for 24 h then percentage of cell viability were measured by MTT assay. Values are expressed as mean of triplicate measurements. FRE=fruit of Rubus coreanus Miq. Ethanol extract; LSRE=leaf and stem of Rubus coreanus Miq. Ethanol extract; FLSRE=fruit, leaf and stem of Rubus coreanus Miq. Ethanol extract.
Restoration of cell viability suppressed by As with R. coreanus extracts
First, to determine the dose-dependent cytotoxic effect of As on RAW 264.7 cells, cells were treated with 0, 1.25, 2.5 and 5.0 µM Arsenic trioxide for 24 hours, MTT assay was performed (Figure 6). Cell viability was decreased by As to 60% with 2.5 µM and to 40% with 5.0 µM As respectively. Next, the restoration capacity of R. coreanus extracts on cytotoxicity caused by the addition of As compound was tested, and at the concentration of 2.5 µM As, all of three kinds of extracts (FRE, LSRE and FLSRE) exhibited over 80% recovery of damaged cell cytotoxicity (60%) depending on the concentrations of supplemented R. coreanus extracts. As for higher concentration of as (5.0 µM) this recovery was significantly elevated even though the magnitude of recovery was lower than 2.5 µM As (Figure 6).
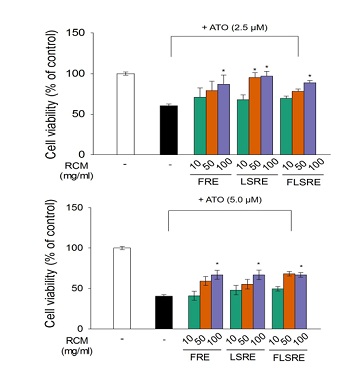 Figure 6: Protective effect of ethanol extracts from Rubus coreanus Miq. on RAW 264.7 cells from As2O3-induced cell death.
Figure 6: Protective effect of ethanol extracts from Rubus coreanus Miq. on RAW 264.7 cells from As2O3-induced cell death.
RCM inhibition of As2O3-induced cell death by MTT assay. Cells were treated with 2.5M(A) and 5.0M(B), of As2O3 respectively in the presence of LSRE (50 and 100 µg/ml) treatment or 24 h, and then percentage of cell viability were measured by MTT assay. Values are expressed as mean of triplicate measurements. *p < 0.01 indicated a significant difference in As2O3 treated groups compared with untreated group. LSRE=leaf and stem of Rubus coreanus Miq. Ethanol extract; FLSRE=fruit, leaf and stem of Rubus coreanus Miq. Ethanol extract; ATO=Arsenic Trixide (As2O3).
Quenching of ROS (reactive oxygen species) induced by as by treating cells with coreanus extracts
The increased the level of ROS by the addition of As was found to be lowered by the treatment with 50 µg/mL R. coreanus extracts to the level of 56-78% ROS and up to 54-80% with 100 µg/mL concentrations (Figure 7). Specially the leaves and stems extracts showed the highest ROS quenching effect at the both concentrations of 50 and 100 µM.
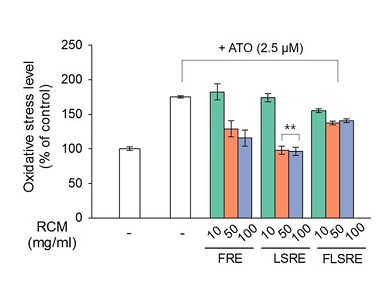 Figure 7: Effect of ethanol extracts from Rubus coreanus Miq. on RAW 264.7 cells from As2O3-induced reactive oxygen species (ROS) production.
Figure 7: Effect of ethanol extracts from Rubus coreanus Miq. on RAW 264.7 cells from As2O3-induced reactive oxygen species (ROS) production.
RAW 264.7 cells were treated with 2.5 M, of As2O3, respectively in the presence of FRE, LSRE and FLSRE (10, 50 and 100 µg/ml) treatment or 24 h, and then percentage of oxidative stress level were measured by DCF-DA assay. Values are expressed as mean of triplicate measurements. *p < 0.01 indicated a significant difference in As2O3 treated groups compared with untreated group. FRE=fruit of Rubus coreanus Miq. Ethanol extract; LSRE=leaf and stem of Rubus coreanus Miq. Ethanol extract; FLSRE=fruit, leaf and stem of Rubus coreanus Miq. Ethanol extract; ATO=Arsenic Trioxide (As2O3). however, the addition of R. coreanus extracts decreased the expression of these enzymes. Specially leaves and stems showed the highest reduction in all the enzyme protein expression.
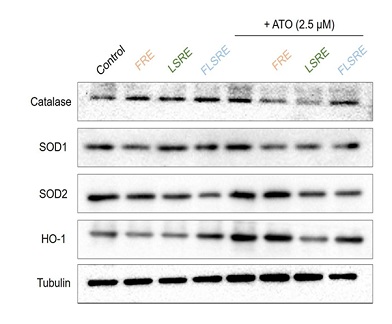 Figure 8: Protein expression of HO-1, catalase, SOD1 (Cu/Zn SOD) and SOD2 (Mn-SOD) in RAW 264.7 cells treated with ethanol extracts from Rubus coreanus Miq. and As2O3.
Figure 8: Protein expression of HO-1, catalase, SOD1 (Cu/Zn SOD) and SOD2 (Mn-SOD) in RAW 264.7 cells treated with ethanol extracts from Rubus coreanus Miq. and As2O3.
RAW 264.7 cells were treated with RCM (FRE, LSRE and FLSRE) 100 µg/ml or combine-treated with RCM plus As2O3 for 24 h. Cells were then harvested and the protein expression was detected by western blot analysis. Proteins (10 µg) of the total cell lysates were loaded in 12% SDS-PAGE. The expression level of tubulin proteins was examined and served as a loading control. FRE=fruit of Rubus coreanus Miq. ethanol extract; LSRE=leaf and stem of Rubus coreanus Miq. Ethanol extract; FLSRE=fruit, leaf and stem of Rubus coreanus Miq. Ethanol extract.
Discussion
A large number of populations are exposed to undesirable amount of arsenic through drinking water and food. High concentrations of arsenic in drinking water and food, or from particular environmental conditions, can bring detrimental toxicities [24]. Those who are exposed to high concentrations may develop acute, subacute, or chronic consequences of toxic symptoms. Inorganic along with organic arsenicals are known to bind strongly to thiol groups, which together with seleno-enzymes, and they are vulnerable targets of toxic activities of As [25]. As is a well-known carcinogen influencing various organs, specially, lung, urinary tract, skin cancers are reported at levels above 50 µg/L drinking water [26]. As low as 10 µg/L could induce bladder cancers according to the recent summary report of epidemiological studies [27]. The prolonged exposure is known to be linked to peripheral neuropathy, cardiovascular disease [28]. One research even suggests that exposure to environmental arsenic should include a risk factor for the development of children autism spectrum disorder [29]. There are claims that arsenic exposure may precipitate type 2 diabetes in susceptible individuals [30] and furthermore, increased oxidative stress caused by arsenic leading to aggravated atherosclerosis and lowered endothelial functions [31].
R. coreanus Miquel (bokbunja) Korean black raspberry, is native to Korea and other Northeast Asia countries known to exert health-promoting effects including diminishing oxidative stress from various origins, dampening inflammation and further ameliorating liver steatosis, obesity and cancer [32-38]. This study has two fold aims: i.e. it is to compare the total phenolic and flavonoid contents, free radical scavenging effects and the protective effects against oxidative stress induced by arsenic trioxide of fruits, leaves and stems. The total polyphenol contents of the ultrasonication extracted R. coreanus samples ranged from 1.37 to 2.80 gGA per 100g freeze-dried powder. The highest polyphenol concentration was found with combinations of fruits, leaves, stems. The higher flavonoid contents were obtained with leaves and stems, when they were expressed in milligrams of quercetin equivalent per 100 G extracts. Similar results of antioxidative activities were obtained for leaves and stems, where they showed the higher values for the DPPH radical scavenging assay and FRAP (ferric-reducing antioxidant power) values. Generally, research on berries has traditionally focused on their antioxidant properties and has been shown in vitro system to carry antioxidant capabilities [39]. This study extended to evaluate the ROS scavenging effects of this berry and its parts in arsenic –induced stress in murine macrophage 264.7 cells. The evident anti-cytoxicity effects were observed with the addition of R. coreanus ethanol extracts in As treated macrophage cells. Furthermore, the antioxidative and anti-inflammatory capacities of RCM powder were shown in macrophage cultured treated with arsenic. The downregulation of anti-inflammatory enzymes such as SOD1(Cu/Zn superoxide dismutase), SOD2 (Mn superoxide dismutase), catalase and heme oxygenase-1 protein by the addition of RCM fruits, leaves and stems in arsenic-treated macrophage cells demonstrates that the altered balance between oxidative stress conditions caused by arsenic trioxide and antioxidant defense system may have restored by R. coreanus fruit, leave and stem extracts.
Conclusion
Freeze-dried powder of R. coreanus Miquel fruits, leaves and stems exhibited considerable protective effect against arsenic-induced toxic condition as shown by the regulation of antioxidant activities and controlling some of antioxidant enzymes. This finding provide evidence for the beneficial capacities of R. coreanus Miquel leaves and stems for health promotion in the presence of arsenic contamination.
Conflict of Interest
No known conflicts of interest
References
- Jang TS, Yang JC, Lim SY, Kim B (2014) Antioxidant and antihemolytic activity of ethanol extracts of Rubus coreanus Miquel. Journal of the Korean Applied Science and Technology 31: 130-135.
- Baek EY, Lee SM, Lee JE, Park E, Kim Y, et al. (2013) Effect of Rubus coreanus Miquel on prostate tumour growth. Journal of Functional Foods 5: 1478-1486.
- Kim SK, Kim H, Kim SA, Park HK, Kim W (2013) Anti-inflammatory and anti-superbacterial activity of polyphenols isolated from black raspberry. The Korean journal of physiology & pharmacology: official journal of the Korean Physiological Society and the Korean Society of Pharmacology 17: 73-79.
- Om AS, Song YN, Noh G, Kim H, Choe J (2016) Nutrition composition and single, 14-day and 13-week repeated oral dose toxicity studies of the leaves and stems of Rubus coreanus Miquel. Molecules 21: 65.
- Zoratti L, Jaakola L, Ha?ggman H, Giongo L (2015) Anthocyanin profile in berries of wild and cultivated Vaccinium spp. along altitudinal gradients in the Alps. Journal of Agricultural and Food Chemistry 63: 8641-8650.
- Uleberg E, Rohloff J, Jaakola L, Tro?st K, Junttila O, et al. (2012) Effects of temperature and photoperiod on yield and chemical composition of northern and southern clones of bilberry (Vaccinium myrtillus L.). Journal of Agricultural and Food Chemistry 60: 10406-10414.
- Howard LR, Clark JR, Brownmiller C (2003) Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. Journal of the Science of Food and Agriculture 83: 1238-1247.
- Kim YH, Choi JH, Rim HK, Kang HJ, Chang SG, et al. (2011) 23-Hydroxytormentic acid and niga-ichgoside F1 isolated from Rubus coreanus attenuate cisplatin-induced cytotoxicity by reducing oxidative stress in renal epithelial LLC-PK1 cells. Biological and Pharmaceutical Bulletin 34: 906-911.
- Lee J, Dossett M, Finn CE (2012) Rubus fruit phenolic research: The good, the bad, and the confusing. Food chemistry 130: 785-796.
- Kim HS, Park SJ, Hyun SH, Yang SO, Lee J, et al. (2011) Biochemical monitoring of black raspberry (Rubus coreanus Miquel) fruits according to maturation stage by 1H NMR using multiple solvent systems. Food Research International 44: 1977-1987.
- Lee MJ, Lee SJ, Choi HR, Lee JH, Kwon JW, et al. (2014) Improvement of cholesterol and blood pressure in fruit, leaf and stem extracts from black raspberry in vitro. Korean Journal of Medicinal Crop Science 22: 177-187.
- Fraga CG, Croft KD, Kennedy DO, Barberán FAT (2019) The effects of polyphenols and other bioactives on human health. Food & function 10: 514-528.
- Prenzler PD, Ryan D, Robards K (2021) Introduction to basic principles of antioxidant activity 1-62.
- Lu CC, Yen GC (2015) Antioxidative and anti-inflammatory activity of functional foods. Current Opinion in Food Science 2: 1-8.
- Islam M, Alam F, Solayman M, Khalil M, Kamal MA, et al. (2016) Dietary phytochemicals: natural swords combating inflammation and oxidation-mediated degenerative diseases. Oxidative medicine and cellular longevity 2016: 5137431.
- Lüscher TF (2015) Ageing, inflammation, and oxidative stress: final common pathways of cardiovascular disease. European heart journal 36: 3381-3383.
- Libby P, Ridker PM, Hansson GK, Leducq Transatlantic Network on Atherothrombosis (2009) Inflammation in atherosclerosis: from pathophysiology to practice. Journal of the American college of cardiology 54: 2129-2138.
- Fan F, Galvin A, Fang L, White DA, Moore XL, et al. (2014) Comparison of inflammation, arterial stiffness and traditional cardiovascular risk factors between rheumatoid arthritis and inflammatory bowel disease. Journal of Inflammation 11: 1-9.
- Thornton I, Farago M (1997) The geochemistry of arsenic. Arsenic: exposure and health effects 1-16.
- Hu Y, Li J, Lou B, Wu R, Wang G, et al. (2020) The role of reactive oxygen species in arsenic toxicity. Biomolecules 10: 240.
- Shen S, Li XF, Cullen WR, Weinfeld M, Le XC (2013) Arsenic binding to proteins. Chemical reviews 113: 7769-7792.
- Hall MN, Niedzwiecki M, Liu X, Harper KN, Alam S, et al. (2013) Chronic arsenic exposure and blood glutathione and glutathione disulfide concentrations in Bangladeshi adults. Environmental health perspectives 121: 1068-1074.
- Sage AP, Minatel BC, Ng KW, Stewart GL, Dummer TJ, et al. (2017) Oncogenomic disruptions in arsenic-induced carcinogenesis. Oncotarget 8: 25736.
- Andrew AS, Jewell DA, Mason RA, Whitfield ML, Moore JH, et al. (2008) Drinking-water arsenic exposure modulates gene expression in human lymphocytes from a US population. Environmental health perspectives 116: 524-531.
- Nurchi VM, Buha Djordjevic A, Crisponi G, Alexander J, Bjørklund G, et al. (2020) Arsenic toxicity: molecular targets and therapeutic agents. Biomolecules 10: 235.
- Yu HS, Liao WT, Chai CY (2006) Arsenic carcinogenesis in the skin. Journal of biomedical science 13: 657-666.
- Mink PJ, Alexander DD, Barraj LM, Kelsh MA, Tsuji JS (2008) Low-level arsenic exposure in drinking water and bladder cancer: a review and meta-analysis. Regulatory Toxicology and Pharmacology 52: 299-310.
- Bjørklund G, Tippairote T, Rahaman MS, Aaseth J (2020) Developmental toxicity of arsenic: a drift from the classical dose–response relationship. Archives of Toxicology 94: 67-75.
- Skalny AV, Simashkova NV, Klyushnik TP, Grabeklis AR, Bjørklund G, et al. (2017) Hair toxic and essential trace elements in children with autism spectrum disorder. Metabolic brain disease 32: 195-202.
- Maull EA, Ahsan H, Edwards J, Longnecker MP, Acien AN, et al. (2012) Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environmental health perspectives 120: 1658-1670.
- Kumagai Y, Pi J (2004) Molecular basis for arsenic-induced alteration in nitric oxide production and oxidative stress: implication of endothelial dysfunction. Toxicology and applied pharmacology 198: 450-457.
- Park JH, Oh SM, Lim SS, Lee YS, Shin HK, et al. (2006) Induction of heme oxygenase-1 mediates the anti-inflammatory effects of the ethanol extract of Rubus coreanus in murine macrophages. Biochemical and Biophysical Research Communications 351: 146-152.
- Lee JE, Cho SM, Park E, Lee SM, Kim Y, et al. (2014) Anti-inflammatory effects of Rubus coreanus Miquel through inhibition of NF-κB and MAP Kinase. Nutrition Research and Practice 8: 501-508.
- Bhandary B, Lee GH, Marahatta A, Lee HY, Kim SY, et al. (2012) Water extracts of immature Rubus coreanus regulate lipid metabolism in liver cells. Biological and Pharmaceutical Bulletin 35: 1907-1913.
- Lee KH, Jeong ES, Jang G, Na JR, Park S, et al. (2020) Unripe Rubus coreanus Miquel extract containing ellagic acid regulates AMPK, SREBP-2, HMGCR, and INSIG-1 signaling and cholesterol metabolism in vitro and in vivo. Nutrients 12: 610.
- Do SH, Lee JW, Jeong WI, Chung JY, Park SJ, et al. (2008) Bone-protecting effect of Rubus coreanus by dual regulation of osteoblasts and osteoclasts. Menopause 15: 676-683.
- Kim KJ, Jeong ES, Lee KH, Na JR, Park S, et al. (2020) Unripe Rubus coreanus miquel extract containing ellagic acid promotes lipolysis and thermogenesis in vitro and in vivo. Molecules 25: 5954.
- Kim Y, Lee SM, Kim JH (2014) Unripe Rubus coreanus Miquel suppresses migration and invasion of human prostate cancer cells by reducing matrix metalloproteinase expression. Bioscience, Biotechnology, and Biochemistry 78: 1402-1411.
- Manganaris GA, Goulas V, Vicente AR, Terry LA (2014) Berry antioxidants: small fruits providing large benefits. Journal of the Science of Food and Agriculture 94: 825-833.
Citation: Song Y-N, Chung W-K, Kim JH, Om A-S (2023) Protective Effects of Rubus coreanus Miq. Extracts Against Arsenic Trioxide-Induced Oxidative Stress and Cell Cytotoxicity. J Altern Complement Integr Med 9: 325.
Copyright: © 2023 You-Na Song, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

