
Quality of Life and Health Related Quality of Life Among End-Stage Kidney Disease Patients: Methodology Assessment
*Corresponding Author(s):
Issa Al SalmiThe Renal Medicine, The Royal Hospital, Muscat, Oman
Tel:96892709000,
Email:isa@ausdoctors.net
Abstract
Introduction
End-Stage Kidney Disease (ESKD) is a serious and irreversible condition. Understanding the impact of ESKD and its treatment on an individual's Quality of Life (QoL) is important.
Methods
We presents the framework that was used to guide this study, study design, sampling, and procedures for data collection including the measurement used as well as clinical setting. The data analysis plan of the three phases of this study is also described. Finally, data management, and the plan of risk assessment that was placed to anticipate potential risks to the project are discussed.
Results
A three-phase, cross-sectional, correlational study was conducted to explore the meaning of QoL and to determine factors affecting QoL and HRQoL in patients with ESKD. A targeted sample of around 450 patients undergoing HD at outpatient dialysis units was used. Eight measures, in total, were administered to patients undergoing regular HD sessions. Data analyses included descriptive statistics and exploratory and confirmatory factor analysis, as well as various sequential multiple-regression models, to determine the influence of study-predictor variables on physical-component summary, mental component summary, role-functioning component summary and QoL index dialysis, according to the revised Wilson and Cleary model of HRQoL.
Conclusion
A cross-sectional, correlational study was conducted in 4-phases. Phase-1 explored the conceptual basis of QoL and how that has been assessed in ESKD patients. Phase-2 explored the understanding and acceptability of the concepts of patients using cognitive interviewing and individualized QoL instrument. Phase-3 tested the feasibility of the main study design. Phase-4 assessed the level and predictors of QoL/HRQoL from 13hemodialysis units; and tested psychometric adequacy of key measures using exploratory and confirmatory factor analysis.
Keywords
Cognitive interviewing; Correlational study; Cross-sectional; End-Stage Kidney Disease (ESKD); Health-related QoL; Hemodialysis; Methodology; Psychometric adequacy; Quality of Life (QoL)
INTRODUCTION
End-Stage Kidney Disease (ESKD) is a serious and chronic disease that negatively impacts patients’ Health Related Quality of Life (HRQoL) mainly due to the accompanied impairment or to the imposed limitations in almost all aspects of their lives. Despite the considerable progress which has been made in treating ESKD patients and Hemodialysis (HD) procedures, HRQoL remains a significant problem for HD patients [1]. Numerous studies using different measures have examined both the concepts of HRQoL and overall Quality of Life (QoL) in patients with ESKD and have revealed that the multiple physiological and psychological factors that patients may experience could impair their lifestyle which in turn might change and subsequently lower their QoL level.
Treating ESKD and its related complications consumes large amounts of the health budget. The determination of successful health outcome for patients with ESKD has been limited to clinically focused measures including HD adequacy, acceptable laboratory values and intradialytic management [2,3]. Alternative outcome measures of the efficacy of provided treatment and HD are therefore required [4]. This may be through assessing HRQoL which could be included when health care professionals assess the benefits of different ESKD treatment options. This however might not be enough because dialysis patients may not experience satisfaction with their HRQoL despite physiological measures being met [3,5,6]. Thus, more subjective, patient-focused, disease-focused measures are needed to enhance the health outcome assessment for patients on HD.
Many studies that assessed QoL and HRQoL among ESKD patients were conducted within developed countries culture, with only two studies found within Arab countries [7,8]. The results of these studies showed low scores of HRQoL among the patients with ESKD. Like other Middle East countries, no studies were found that had been conducted in the Gulf Cooperation Countries (GCC) including Oman to assess QoL in this specific population, or on how these patients perceive their QoL. Also, no studies were found that have used the approach of assessing the biological function, symptoms, functional status, general health perceptions, and various characteristics of the individual and their environment. Even with studies that used more than one measure of QoL there searchers did not articulate a holistic conceptual framework to guide their study, providing little justification of the independent variables used to explain QoL [9-17].
This study will try to fill these gaps in knowledge. Accordingly, three different phases will be employed to answer the research questions. Phase one explored the meaning of QoL to our population, using a cognitive interviewing and a measure of individual QoL. In the second phase, the practicality of the study measures was piloted and tested within our population of ESKD population. This phase also assessed the feasibility of the third phase, informed the sampling size, assessed the likely success of the proposed recruitment approaches, and identified possible logistical problems. This third phase involved conducting a large cross-sectional study to determine the factors that affect QoL and HRQoL in patients with ESKD and investigating the associations between symptom burdens and physical, psychological, clinical and socio-demographical factors.
RESEARCH METHODS
Study design
This non-experimental study used a cross-sectional, correlational design to explore and assess QoL and HRQoL and to identify its related predictors regarding Omani patients with ESKD. Since the study is not examining the changes in QoL and HRQoL over time, a cross-sectional design was deemed appropriate and data were collected at one point in time from patients to examine their level of QoL and HRQoL as well as related factors. The benefit of employing a cross-sectional design is to describe the status of phenomena or the relationship among phenomena [18]. The correlation design was used to examine the association among study variables.
Population
Inclusion criteria: The characteristics of patients included in all three phases were: adult patients with ESKD receiving HD; those aged ≥ 18 years; and those who have been on HD for at least three months so that they are adjusted to life on dialysis.
Exclusion criteria: Patients who did not survive on HD longer than three months; patients with acute renal failure; patients aged under 18; patients diagnosed with dementia or any other condition that could impair their ability to answer questions; patients who have recently been diagnosed with cancer; and patients who have recovered their kidney function.
Sample size
Phase one sample size was informed by literature that used the cognitive interviewing and individual QoL instruments method in data collection as well as studies using individual QoL measures [19-21]. An average of eight to 15 participants is considered applicable. Accordingly, it was planned for 16 patients to be approached and interviewed during their waiting time before starting their routine HD sessions.
The target sample in Phase two was 50 participants in total, during their presence for HD, at a rate of 15 patients from Site 1, 15 patients from Site 2 and 20 from Site 3. Based on the response rate obtained from phase one, 60 patients were approached to avoid any inaccuracy in the list as patients sometimes stop HD for transplants or from personal preference. Participants from phase one were excluded from this phase by excluding their names from the randomization list. The statistical analyses in Phase three required the calculation of a sample meeting the assumptions of factor statistical analysis and sequential multiple regression analysis. Thus, in order to perform the planned statistical analyses a sample size of 448 is needed.
Ethical approval and data management
The study was conducted in accordance with the ethical approval granted by the Directorate of Research and Ethical Review and Approve Committee, Ministry of Health, Oman, MH/DGP/R&S/PROPOSAL_APPROVAL/16/2015.
It was expected that no potential risks would occur and that any risk of physical or psychological harm was at a minimum level because this study did not involve any clinical interventions. Also, there was no risk of social or economic harm because the participants who took part in this study did not travel to take part, as they were recruited during their regular attendance at HD. The researcher ensured that patients had complete autonomy to decide whether to participate without any pressure being applied. They were informed that their participation was entirely voluntary, and they could withdraw from the study at any time without negatively affecting the treatment or care they received. The confidentiality of the participants was preserved throughout the study and participants were reassured that interview content was kept confidential and would be used for study purposes only without identities being mentioned in any documents related to this study.
Participants were informed verbally and in writing, using the information sheet approved by both ethical committees. For patients who could not read, the information sheet and consent form were explained verbally. Participants were required to sign the consent form and for those who could not write, an available witness was required to sign instead. The witness had to be a family member (husband, wife, sister, brother, or close relative such as a cousin).
Confidentiality was maintained on all data-collection forms by using codes to identify patients instead of names or any other personal identifiers. The main list of patients’ names was kept separate from the data-collection forms. This list was used during the data-collection period to ensure that patients were not recruited twice.
The data-management process was ensured in compliance with The Research Council Royal Decree (No. 54/2005) and the Directorate of Research and Studies, Ministry of Health, Oman. Participants were notified of this in the information sheets. To empower the ethical part of this study, the researcher has undertaken amaster-level research course for one academic year prior to commencing the study. Also, continuous professional development sessions, seminars and conferences were attended, including Good Clinical Practice (GCP) training to gain the necessary skill to carry out this study GCP is the ethical and practical standard to which all clinical research is conducted.
DATA COLLECTION PROCESS
Measurements
Eight instruments were used to collect the data:
1) Background data sheet
2) Short-Form 36v2 (SF36v2)
3) Quality of life Index-Dialysis (QLI-D)
4) Hospital Anxiety Depression Scale (HADS)
5) Fatigue Severity Scale (FSS)
6) Itch Scale (5-D Itch)
7) Spiritual Wellbeing Scale (SWB)
8) The Schedule for the Evaluation of Quality of Life-Direct weighting (SEIQoL-DW).
Background data sheet: For this study, the variables that determine the characteristics of the individual are: age, gender, educational level, monthly income, region and marital status that might influence health outcomes. A background data sheet was used to collect the patients’ characteristics. This sheet was developed based on the structured reviews conducted among ESKD patients. Data relating to marital status, educational level, current employment status and income status were collected from the patients themselves, as this data is not usually recorded on patient file.
All socio-demographic data were classified according to the Oman norm, using the National Centre for Statistics and Information, Oman (2016). These data are detailed as follows: The category for marital status (single, married, widowed, divorced); level of education (illiterate, low-intermediate, intermediate, high-intermediate, high); employment status (employed or unemployed); and income, measured in Omani Rials (OMR), One Rial = £2.00 (< OMR 250/month; OMR 250-600/month; OMR 601-1000/month; OMR 1001-1500/month; OMR >1500/month).
The treatment characteristics factors related to HD prescription which might affect patients’ HRQoL were collected by the background data sheet. The determinants of treatment characteristics are time since starting HD in months; time to reach HD in minutes; duration of HD session in hours; and adequacy of HD. The adequacy of HD is measured by the urea-reduction ratio which measures the reduction in blood urea in percentage as a result of HD and the effectiveness of HD treatment in removing waste products from the body.
Biological function (hemoglobin, albumin, hematocrit levels). Biological function can often be measured by the lab tests of Hb, HCT and Albumin. The normal range values of these investigations are adopted from The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (2007).
Short-Form 36v2 (SF36v2): It consists of 36 items that make up eight health domains: physical functioning, social functioning, physical role limitations, emotional role limitations, bodily pain, mental health, vitality and general heath perceptions. The scoring range of theSF-36 is 0-100 for each of the eight domains, zero indicating poor health status and 100 indicating very good health status. As there is no Omani study that has used the SF-36v2 to establish a norm-based standard of comparison between patients with ESKD and normal individuals, a cut-off score of a mean of 50 and a standard deviation of 10 was used [22]. That is, any score above or below 50 (standardized score) can be considered above or below the population’s average health status for that domain. Two subscales of the SF36v2 were also used independently to measure: a) Bodily Pain (BP) which refers to the unpleasant sensory and emotional experience associated with ESKD and its related treatment. The BP subscale consists of two items, one of which assesses the rating of the severity of bodily pain during the past four weeks. Its response choices range from “none” to “very severe”. The second item assesses the level of bodily pain’s effect on/interference in daily life activities including in house and out-house activities. The total scores of both items are reverse scored, that is, the higher value indicates less bodily pain. b) General health perception which describes patients’ perceptions of their overall health status [23]. It is a one item subscale asking patients to rate their general health on a five-point scale ranging from 1 = "excellent" to 5 = "poor".
Quality of life Index-Dialysis (QLI-D): Quality of Life Index-Dialysis version (QLI-D) was used as a disease-specific measure of ESKD and consists of 68 items (14 items are disease-specific and four items are related to dialysis treatment). For each pair of items, the first item asks the degree to which patients are satisfied with an aspect of their life and the answer is measured on a six-point Likert-type scale (1 = very dissatisfied, 6 = very satisfied). The second item asks the level of importance of that aspect of their life and this is likewise measured on a six-point Likert-type scale (1 = very unimportant, 6 = very important). The pair of items are finally added together to produce an overall score.
Hospital Anxiety Depression Scale (HADS): HADS was used to test mood symptoms, anxiety and depression. It consists of14 items; seven items related to anxiety and seven related to depression, forming two subscales (HADS-A and HADS-D). Responses are measured on a four-point Likert scale, from 0-3, with 0 representing no symptoms and 3 representing the presence of symptoms related to anxiety or depression. Each subscale is summed up separately providing a sum of 21, and the overall score can be obtained by adding up subscales, providing a sum of 42. The possible scores range are 0-6 normal, 7-10 mild, 11-14 moderate, and 15-21 severe [24].
The Fatigue Severity Scale (FSS): Fatigue is defined as extreme and persistent tiredness and weakness that patients experience due to ESKD [25]. It is measured by the Fatigue Severity Scale (FSS) that measures the severity of fatigue and its effect on patients’ daily life activities and overall QoL. These items are scored on a seven-point scale with 1 = strongly disagree and 7 = strongly agree. The minimum score is nine and the maximum possible score is 63. The higher the score, the greater the fatigue severity.
Itch Scale (5-D Itch): The 5-D Itch Scale was used to assess itching in a brief, easy-to-complete, easy to-score format that is sensitive to the multidimensional nature of pruritus and its effect on HRQoL. The scores of each of these five items are calculated separately and then added together to obtain a total 5-D score. Scores can possibly range between 5 (no pruritus) and 25 (most severe pruritus).
The religious and Spiritual Wellbeing Measure (SWB): SWB has 20 items, with 10 items to Reflect Religious Wellbeing (RWB) and 10items for Existential Wellbeing (EWB). The RWB subscale contains the word “God” - “Allah” in the Arabic version - to assess the patients’ relationship with “God or higher spiritual power” in whatever sense is meaningful to them. The EWB contains no specific religious terms and is instead worded in terms of connection and general satisfaction to assess the patients’ sense of life purpose and life satisfaction. Items are scored on a Likert-scale from 1-6 with a higher number reflecting higher wellbeing. The SWB scale produces three scores: 1) a global SWB score; 2) a score for the religious-wellbeing subscale; and 3) a score for the existential-wellbeing subscale. The SWB overall scores range from 20 to 120, with 20-40 considered low spiritual wellbeing, 41-99 moderate, and 100-120 high spiritual wellbeing. The scores of religious and existential wellbeing subscales range from 10 to 60, with 10-20considered as low, 21-49 moderate, and 50-60 high religious and existential wellbeing [26].
Individualized QoL instrument: The Schedule for the Evaluation of Quality of Life-Direct Weighting (SEIQoL-DW)was used to assess patients’ own perspective on, and perception and understanding of, QoL [27]. It assesses three elements of QoL by asking patients to: a) first nominate five aspects of life they value most; b) rate their functioning/satisfaction level with each aspect of life; and c) rate the importance of each aspect of life in judging overall QoL.
Overview of clinical settings
In phase one, data collection took place in the Nizwa Dialysis Unit. This unit is in central Oman and is one of the largest dialysis units. There are around 73 patients regularly attending out-patient HD. In phase two, three HD units were involved. These units were located in: a)northern Oman (Sumail Dialysis Unit, Site 1), central Oman (Nizwa Dialysis Unit, Site 2) and western Oman (Ibri Dialysis Unit, Site 3) to provide a representative sample across Omani culture and to test the feasibility of the main study. In phase three, 13 HD units across the Sultanate were involved. These units provide routine HD for out-patients affected by ESKD from a variety of regions and are managed by the Omani Ministry of Health (MOH). The MOH provides HD therapy for a total of 1,381 registered patients with ESKD (Annual Health Report,2016, Oman). These patients have varied socio-economic, cultural and educational backgrounds and are likely to provide a representative cross-section of the population, thus allowing generalization of the study results.
Recruitment process
Data collection assistant: To assist with conducting phases two and three of the study, nurses from all study sites were recruited and trained to administer the study measures and to apply research ethics principles. Initially, the researcher approached dialysis-unit managers to explain the study with the ethical-approval letter and the participants’ information sheets, and also to recruit nephrology nurses to assist in the study.
The intention here was that the unit managers would circulate the recruitment request to nurses. The nurses who expressed interest in assisting the study were asked to indicate this to their managers who in turn informed the researcher to approach these nurses. To avoid the possible risk of a low recruitment rate for nurses assisting in the study, an advertisement strategy for the study was considered through posters and presentations including an inclusive description and explanation of the study aims, inclusion criteria and methods of data collection.
Training on measures administration was provided to the recruited nurses by the researcher to ensure efficiency in conducting this procedure, as shown in appendix 1. It was stressed to nurses that participation in the study was voluntary and that patient care should not be affected in any way. In addition, the participation information sheet contained the number of the “complaints call center” so that patients could use it in case any coercion occurred during their participation in the study.
They were also trained in correct data-management procedures to maintain confidentiality. The training session took the form of a practical introduction and consisted of a series of short lectures interspersed with practical activities. Topics covered included: the aims and objectives of study, patient recruitment, obtaininga consent form, and method of questionnaire administration. Subsequently, each research assistant was observed in a simulated data-collection session by the researcher and then both researcher and assistant simultaneously collected data from two patients before the research assistant was able to administer the measures independently. The recruited nurses were not directly responsible for the outcome of the study.
Identification of participants and data collection procedure: Patients were identified from the National Renal Registry of patients treated with ESKD in Oman [28-30]. This registry records registered patients affected by ESKD based on a numerical identifier, contact information, medical history and updated lab results and is organized in a logical and systematic fashion. The authorization to access the registry was based on the letter from the Directorate of Research and Ethical Review and Approve Committee, Oman. Phase-one comprised a semi-structured interview process to test the validity of the concept and to explore the understanding of QoL within Omani patients with ESKD. For this phase, patients were approached by the researcher in the allocated waiting area for their regular HD sessions. An explanation about study objectives and an information sheet was provided, as shown in appendix 2. Patients had48 hours in which to indicate their interest in participating in the study to the researcher, who was physically available in the dialysis unit when they arrived for their next session, and sign consent form, as shown in appendix 3. For patients who agreed to participate, an interview venue and time were decided based on the participant’s preference. All the interviews were audio-recorded with the patients’ permission and the recordings included the administration process of the individualized QoL instrument and the interactions between the patients and the researchers during this process.
Data in this phase were obtained in two ways: Firstly; a cognitive interviewing method was used to explore the cognitive and sociocultural processes associated with answering the HRQoL measures. Data were collected in a semi-structured interview and transcribed based on the “think aloud” technique – patients are asked to think out loud while completing the measure – and the “verbal probing” technique in which the researcher searches for potential problems and explores the basis of the participant’s answers with in the course of the interview [31].
The Question Appraisal System (QAS), a coding form of probed interviews was used to record the process [32-34], as shown in appendix 4. Patients were asked to complete each measure exactly as normal, but also to “read aloud” each item and to “think aloud” their thoughts as they responded to these items. After each interview, field notes and audio recordings were reviewed to identify any potential problems with the measures so that any issues could be addressed.
Secondly; an individualized QoL instrument (SEiQoL-DW) was administered to identify the aspects of life that Omanis value in relation to their QoL and to measure the current satisfaction with these aspects. SEiQoL-DW was administered in a form of a semi-structured interview, in which the researcher first introduced QoL as an individually defined construct, then asked the patients to nominate their own five most important aspects of QoL. Patients were asked to think about which aspects of life determined their own happiness, or QoL, and then nominated aspects were rated numerically.
Phase-two comprised a pilot study to: a) test the acceptability and practicality of use of the SF36v2 and QoLID within Oman; b) test recruitment, participation and feasibility to ensure that any variations in the research design were effectively managed; and c) identify issues of concern for the main study; for instance, whether the allocated time for field work was enough to recruit a large enough sample for the main study.
Applying inclusion and exclusion criteria, 60 (10% of the sample size for the main study) patients were selected randomly using the “RANDOM” function in the Excel program [35-37]. The patient information sheet, as shown in appendix 5, that explained the purpose of the pilot study, the advantages and disadvantages of participation, the expected duration of participation and the researcher’s contact details was provided to eligible patients who were then approached by nephrology nurses during their attendance for regular HD to obtain consent forms, as shown in appendix 6 and to respond to study measures. Patients were asked to complete the study measures before starting their HD session. However, if data were to be collected during the HD session, 60 minutes were allowed before data collection began. This gap in time was designed to prevent any possible errors that might occur, as patients might experience cognitive changes due to fluid and electrolyte shifts.
Using the National Renal Registry, patients in Phase three were identified based on an opt-in strategy from 13 dialysis units across Oman. Those who agreed to participate were identified by nurses who were recruited and trained by the researcher to assist in obtaining consent forms, administering the questionnaire package, following up and collecting the completed study questionnaires. These were then stored in a secure box provided by the researcher. As with phases one and two, patients were given 48 hours to decide on their participation so that, when they attended their next HD session, they could indicate their decision to nephrology nurses and sign the consent form.
Data analysis
Phase one: Data obtained from the cognitive interviewing process were analyzed using amatrix-based method of data analysis proposed by Knafl et al. [38]. The matrix illustrated items in a tabular form in which items appeared in a row and patients appeared in a column. The issues identified with were entered the appropriate intersecting cells, promoting systematic analysis and decision-making regarding item revisions. The matrix-display approach was then used to construct item summaries, linked to all the participating patients, including acoding scheme to reflect problem types specific to each item tested. The findings were then aggregated across all patients, producing a summarized item-by-item analysis of the results associated with the SF-36v2 and QoLI-D. Data obtained from SEiQoL-DW were presented in a tabular form for each patient including the nominated aspects of life, their levels and weights. The levels of each elicited aspect of life were measured by asking the respondents to draw five bars on the “Levels Record Form”. Levels were then scored by measuring the vertical height of each bar in millimeters, using a roller. This produced five scores, which were independent continuous measurements, ranging from 0 to 100, whereas the measuring of the weights of elicited aspects of life was achieved by asking patients to quantify the importance of each aspect, represented by five differently colored areas of a disc weighing system which was produced by the SEiQoL-DW developers specifically for this purpose.
The disc consisted of five interlocking laminated circular discs of different colors on a percentage base which could be rotated independently. Each disc was labelled with one of the cues elicited by the respondent. The weight of each aspect was divided by 100since the weights, when calculating the SEiQoL-DW Index, range from 0.00-1.00. This was so that the overall SEiQoL-DW Index could be calculated by multiplying the level by weight of each aspect and then adding these products across the five aspects [SEiQoL Index = ∑ (levels x weights)].
Phase two and three screening and cleaning data: Collected data were entered into the SPSS software and doubled-checked to avoid any possible errors. Initial analysis outputs were checked for missing, invalid, and extreme values that might have fallen out of the range of normal possible values. The nominal and categorical data were inspected by running frequency tables, while continuous data were inspected by running descriptive statistics. The frequency and descriptive tests outputs were checked to correct any errors before starting data analysis. Missing data and extreme values of categorical variables were checked visually by observing frequencies in output tables. Missing data were examined and were managed by individual mean substitution: if they were found not to be significant or ≤ 10% and if > 10%, then the affected scale/subscale was excluded in related analyses [39].
Computing scales scores: Following data cleaning and missing-data replacement, five measures - Short form 36v2 (SF36v2), Hospital Anxiety Depression Scale (HADS), Fatigue Severity Scale (FSS), Itch-5D and Spiritual Wellbeing Scale (SWB) - were computed and a syntax was created using the SPSSv22 program. For the Quality of Life Index-Dialysis (QoLI-D), the syntax developed by Ferrans et alwas used to score the QoLI-D and its four subscales [40]. This was developed specifically to fit the SPSS program. It should be noted that the scores of the bodily-pain scale are reverse scored: the higher the value, the less the bodily pain.
Checking data normality and outliers: Data normality were checked by running a frequency distribution for each variable and if data-normality assumptions were violated, data were transformed by using square root, logarithm and inverse function, respectively. Outliers were checked by Q-Q Plot. Multivariate outliers were inspected by running standardized residual values, and if greater or less than 3.0, values were categorized as an outlier [39]. Where presented, outliers were handled by being rescored or deleted or by creating separate regression models. Data linearity was checked using scatter-plots to illustrate differences between each of the independent variables compared with the dependent variable.
Statistical analysis procedures
Descriptive analysis: The SPSS (Statistical Package for the Social Sciences) software (Version 22) was used to compute the frequency for nominal and categorical variables, and mean and standard division for continuous variables. The Pearson product correlation coefficient (r) was conducted to assess the relationship between two parametric variables and the Spearman's rank order correlation (rho) was used to assess the relationship between non-parametric variables.
Reliability: To test the reliability of measures within the Omani context, a Cronbach's alpha was computed to examine the internal consistency of the SF36v2 and HADS measures. Cronbach’s alphas, as indicators of internal consistency, were computed for each item and the whole scale. Reseachers have suggested 0.60 and above as an acceptable reliability coefficient since smaller reliability coefficients are inadequate [41-43]. This value was used since the aim of reliability test is to measure a trait with enough accuracy to establish the existence of a relationship with other traits.
Factor analysis: Exploratory Factor Analysis (EFA) and Confirmatory Factor Analysis (CFA) tests were performed to explore the factor structure underpinning the mood measure (HADS) and health outcome measure (SF-36v2). The “Mplus” statistical software version 7 was used for these analyses [44-46]. To perform this test, the ratio of 4-10 cases per item was the rule of thumb employed [35,47]. Among the study measures, QoLI-D has the highest number of items (64). Thus, for QoLI-D, around 7 cases x 64 items = 448 participants and this total was deemed enough to provide a reliable factor structure.
EFA was done on the 14 items of HADS to explore the best fit among the Omani ESKD patients. Likewise, an EFA series was carried out to explore a range of possible factor structures (from 1-8) on the 35 items of SF36v2. The best structures to come out of EFA were verified by conducting CFA. The approach used to determine and retain the number of extracted factors was eigenvalue (>1) and visual investigation of screed plot (against which the eigenvalues were plotted). The Weighted Least Squares (WLSMV) method, chosen for factor extraction, was selected on the basis that this approach is particularly useful in extracting at least one factor indicator of categorical variables [45,46]. The oblique and orthogonal factor rotation procedures were used to determine the best fit between variables and latent factors. The determination of a significant item-factor loading was set at a coefficient level of ≥ 0.307) [39].
The best factor structure identified from HADS and SF36v2 was then verified by CFA. The parameters used to assess the fit of the CFA models were as follows: the chi-square (c2); the Comparative Fit Index (CFI); the Tucker-Lewis Index (TLI); and the Root Mean Square Error of Approximation (RMSEA), plus the Chi-Square Test of Model Fit for the Baseline Model and the Weighted Root Mean Square Residual for the Bi-Factor Model (WRMR). The adequacy of the model fit is considered when: the chi-square is less than 2 or 3 [48]; the RMSEA is below 0.08 [49]; the CFI is greater than 0.95; the TLI is over 0.90 [50,51].
Regression analysis
Using the BM SPSSv22, three main sequential multiple regression analyses were conducted to test the predictive value of the demographic, treatment, clinical, socio-environmental, symptoms, functional status and general health perception on HRQoL in patients with ESKD: SF36v2 physical component summary-PCS(Two-factor standard model); 2) SF36v2 mental component summary-MCS (Two-factor standard model); 3) QoLI-dialysis; and 4.a) SF36v2-PCS (Three factor model), SF36v2-MCS (Three-factor model), and SF36v2-Role-functioningcomponent summary-RCS (Three-factor model).
Six of the nominal variables in this analysis (gender, marital status, education status, job, monthly income, and region) required dummy coding prior to being entered into the model. These variables were coded as dichotomous variables according to the most frequent response obtained from respondents [52]. The order of entry of study variables into the sequential regression models was informed by the order of the study questions, the literature reviews underpinning this study and the result of the pilot study conducted in phase-two.
A ratio of cases-to-independent variables is suggested by Tabachnick and Fidell [39], to test the overall fit of the model (R2): N ≥ 50 + 8m (m is the number of IVs) and to test the contribution of each IVs variable to explain dependent factors: N = 104 + m. There were 22 IVs in this study and the minimum required sample sizes were 226 to test the overall fit of the model and 126 to test the individual independent variable. These sample-size suggestions were based on detecting a medium effect size β = 0.20 with α = or < 0.05 with a power of 80% [39]. Therefore, a sample size of 451 was considered enough for developing four main regression models.
Next, all independent variables were correlated with each other and checked for multi-co-linearity. In the case of two variables correlating at 0.85 or higher, one variable was eliminated from the regression analysis. When all variables were examined together, the tolerance level and variance inflation factor of all IVs were calculated to determine multi-co-linearity. A tolerance value < 0.10 and a variance inflation factor >10 was used to identify multi-co-linearity for possible elimination of variables [53].
A Mahalanobis Distance was computed for each case to detect any extreme multivariate outliers and, once that was done, the Mahalanobis scores were screened in the same manner as the univariate outliers. Hence, frequency distributions were run for each variable and examined for outliers. In addition, multivariate outliers were detected through “standardized residual” values > 3.0 or < -3.0. Outliers would either be rescored or deleted, or separate regression models were created [39].
All variables were checked for data distribution (multivariate normality, linearity and homo-scedasticity) by visually examining standardized residual scatter plots. Violations of any of the assumptions for multiple regression usually reveal a different scatter plot shape. In case any assumptions were violated, data were transformed in an attempt to stabilize the variance and to achieve linearity and normality.
The statistical significance for a variable inclusion into a statistical model was setat α = 0.15 [39]. This determined liberal probability level was to avoid excluding important variables from the model. Independent variables were entered simultaneously into a sequential multiple regression model to determine how well biological factors, symptoms, functional status, general health perception and socio-demographic and treatment factors predicted a patient’s overall HRQoL.
Variables were entered in sequence in six steps: 1) Patient age, Gender, Education, Job, Income; 2) Time since starting HD, Time to reach HD; 3) Social and Economic, Family; 4) Itch, Fatigue; 5) Anxiety, Depression; 6) Perceived general heath.
The improvement in the regression model at each step was evaluated by the R square (R2) and Adjusted R2 values. The process of adding more variables would stop when all the potential variables had been included or when it was not possible to make a statistically significant improvement in R and R2 [54]. To evaluate which variables included in the model contributed to the prediction of the dependent variables, the un standardized regression coefficients (B), the standard errors (SE B), the standardized regression coefficients (b), the t-statistic, the significance of the t-statistic, the R, the F statistic (F), R2 and the change in R2 (DR2) were reported.
Finally, the accuracy of each regression model was evaluated by conducting a Bootstrapping test. Bootstrapping is considered a sound test which can be performed to obtain robust estimates of the intercept and beta weights [39]. It is a process by which regression weights are generated over a very large number of replications (up to 1,000 bootstraps) with samples drawn and replacement from the available data set. Each case may be selected more than once, or not at all, because of replacement [39]. Conclusions were drawn based on the bootstrapped coefficients’ parameter estimates of the overall final model: the un standardized regression coefficients (B), bias, bootstrapped standard errors (SE B), significance of the regression coefficients, the normal approximated 95%confidence intervals (OLS 95% CI) and the bootstrapped confidence interval (BCa 95% CI).
Risk assessment: As part of the project planning process, a risk analysis took place to anticipate potential risks to the project, with the aim being to formalize actions to prevent or manage these risks. A table outlining each identified risk, and actions to prevent or manage the risk, is presented in appendix 7.
DISCUSSION
This is the first study of its type to evaluate QoL and HRQoL in hemodialysis population where more than one measure of QoL were assessed and a holistic conceptual framework was utilized to guide the study, providing justification of the independent variables used to explain QoL.
For the purpose of conducting this study objectively and systematically, the Research Onion Diagram by Saunders et al. was adapted and followed as shown in figure 1 [55,56]. This diagram illustrates the steps of the research process which was followed to produce valid and replicable data. It consists of philosophical paradigm; selected study method; selected study design; determined time horizon according to the study; and planned data collection procedure and analysis.
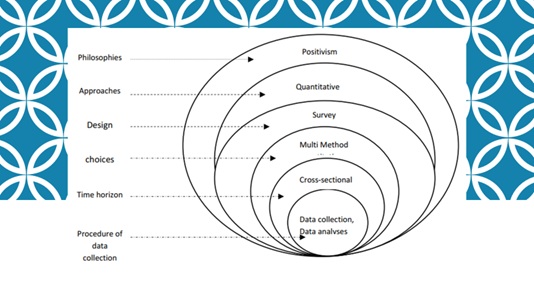 Figure 1: Research Onion Diagram (adapted from Saunders et al. 2009).
Figure 1: Research Onion Diagram (adapted from Saunders et al. 2009).
The term paradigm refers to the roadmap that directs a research journey [57,58]. Different philosophical paradigms are available that represent different ideas about reality and how knowledge can be gained, such as positivism, realism and pragmatism [58]. These paradigms include specific methodological strategies which allow researchers to use the research approach and method, and to recognize any limitations that might disrupt the research [59,60].
For this study, the positivist paradigm was adopted based on the assumption that nature is basically ordered and has antecedent causes as is the case with perceived low HRQoL which can be caused by more than one factor. Quantitative research assumes that phenomena are stable and can be predicted, thus, they can be measured [61]. The outcomes of this study such as physical health, mental health, symptoms, and even spiritual life, can be measured, therefore, the quantitative approach was considered appropriate for this study to find the frequency and association between factors.
In healthcare research, quantitative approach is an essential part and most the common method [62]. It is a good approach to minimize bias and also to maintain an objective view while studying a phenomenon to develop valid results [61]. However, social issues, economical consideration, gender inequality, and political and administrative guidelines would all impact on various aspects of the population health [63-66].
A conceptual framework could act as a heuristic device to provide a better understanding and clarity of QoL and HRQoL. It also can help in specifying research concepts and selecting the appropriate measurements for testing these concepts. The revised version of Wilson and Cleary’s model [23], for health-related quality of life was used to guide this study [40]. This conceptual framework incorporates important health-related factors, as well as individual and environmental characteristics which address the difference between the clinical reported outcomes and the patient reported outcomes.
These health-related factors are biological functioning, symptoms, functional status, and general health perception. A summary of all the study variables are combined and shown in figure 2.
Biological function includes the physiological processes that support life [40]. This describes the patient's biophysical status because of the ESKD condition in terms of the status of anemia and malnutrition. The symptoms experience is the patient's perception of the presence of physical and emotional problems that reflect the severity of their symptoms [23].
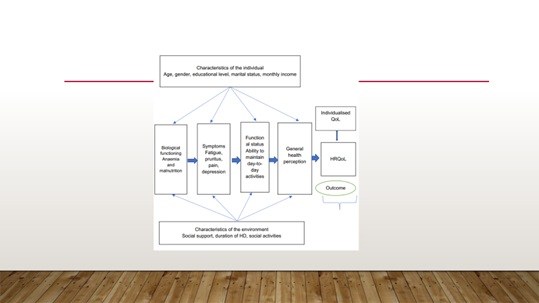 Figure 2: Summary of factors that may contribute to explain HRQoL in patients affected by ESKD.
Figure 2: Summary of factors that may contribute to explain HRQoL in patients affected by ESKD.
Although functional status is usually influenced by biological function and symptoms, it is important that it is measured as a separate variable because it may not be completely correlated with biological function or with symptoms. Two aspects of functioning were measured in this study: (1) physical functioning and (2) psychological functioning using the SF36v2 measure. Socio environmental characteristics were defined as the perceived family, socioeconomic and spiritual life, from a patient's perspective, and their influence on the patient’s health. For this study, the characteristics of the family and socio environmental variables were measured by the subscales of QoLI-dialysis.
As the religious and spiritual domain appeared to be important within our population context, it was essential that it be tested as a separate variable in this study. It refers to the affirmation of an individual’s life in relation to God, self and community [67,68]. It falls very much in line with patients’ personal values and the spiritual beliefs that shape their lives. Thus, the spiritual wellbeing variable will refer to a patient’s sense of wellbeing in relation to God and to a patient’s perception of life’s purpose and satisfaction. General health perception is considered to be central to patients’ health, a representation of all health concepts together, and contains measurable aspects involving an overall assessment of any individual’s life [69,70]. The cognitive appraisal about health, and emotional reactions of patients about their life events are integrated into the overall assessment of HRQoL. Such a combination of personal well-being, life satisfaction and emotional reactions to life events can all be seen as a subjective well-being which might present an ‘umbrella’ term of different valuations that patients make regarding their lives, the events happening to them, their bodies and minds, and their circumstances in which they live [71]. General health perception is most measured with a single global question, indicating an overall health rating on a Likert-type scale from poor to excellent.
The components of this conceptual model acknowledge that health exists on a continuum from simple to complex outcomes with four determinants, each having multiple variables [72]. These determinants, as well as overall quality of life, are ultimately affected by the characteristics of the individual and the environment [40,73]. Further details on the level of the revised version of Wilson and Cleary’s model for HRQoL need to be further studied especially in our region [23,40].
CONCLUSION
A three-phase, cross-sectional, correlational study was conducted to explore the meaning of QoL and to determine factors affecting QoL and HRQoL in patients with ESKD. A targeted sample of around 450 patients undergoing HD at outpatient dialysis units located across the country was used. Eight measures, in total, were administered to patients undergoing regular HD sessions. Data analyses included descriptive statistics and exploratory and confirmatory factor analysis, as well as various sequential multiple-regression models, to determine the influence of study-predictor variables on physical-component summary, mental component summary, role-functioning component summary and QoL index dialysis, according to the revised Wilson and Cleary model of HRQoL.
COMPLIANCE WITH ETHICAL STANDARDS
Disclosure of potential conflicts of interest
The study was approved by the Directorate of Research and Ethical Review and Approve Committee, Ministry of Health, Oman, MH/DGP/R&S/PROPOSAL_APPROVAL/16/2015 and certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments ethical standards. https://mohcsr.gov.om/my-researches/
Informed consent
Each participant was freely given, informed consent to undergo various assessment and laboratory investigations.
Availability of data and material
Data of this paper is not available publicly but can be requested from the corresponding author in a reasonable time.
Funding
No funding available for both authors.
Conflict of interest
Authors declare no conflict of interest.
Acknowledgment
We would like to thank all our patients for their trust on us. Also, authors appreciate all the help and support of the Ministry of Health, Muscat, Oman. In addition, we would like to thank all the staff responsible for the delivery of patients’ care.
REFERENCES
- Cukor D, Ver Halen N, Fruchter Y (2013) Anxiety and quality of life in ESRD. Semin Dial 26: 265-268.
- De Geest S, Moons P (2000) The patient's appraisal of side-effects: The blind spot in quality-of-life assessments in transplant recipients. Nephrol Dial Transplant 15: 457-459.
- Al Salmi I, Larkina M, Wang M, Subramanian L, Morgenstern H, et al. (2018) Missed hemodialysis treatments: international variation, predictors, and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 72: 634-643.
- Brekke FB, Waldum B, Amro A, Østhus TBH, Dammen T, et al. (2014) Self-perceived quality of sleep and mortality in Norwegian dialysis patients. Hemodial Int 18: 87-94.
- Al Rahbi F, Al Salmi I (2017) Commercial kidney transplantation: Attitude, knowledge, perception, and experience of recipients. Kidney Int Rep 2: 626-633.
- Al Salmi I, Al Rukhaimi M, Al Sahow A, Shaheen FA, Al-Ghamdi SM, et al. (2016) Mineral bone disorder and its management among hemodialysis patients in the Gulf Cooperation Council: Initial findings from the dialysis outcomes and practice patterns study (2012-2015). Saudi J Kidney Dis Transpl 27: 62-80.
- A AL-J, Al-Onazi K, Binsalih S, Hejaili F, Al-Sayyari A (2011) A study of quality of life and its determinants among hemodialysis patients using the KDQOL-SF instrument in one center in Saudi Arabia. Arab J Nephrol Transplant 4: 125-130.
- Abd ElHafeez S, Sallam SA, Gad ZM, Zoccali C, Torino C, et al. (2012) Cultural adaptation and validation of the "Kidney Disease and Quality of Life--Short Form (KDQOL-SF™) version 1.3" questionnaire in Egypt. BMC Nephrology 13: 170.
- Saffari M, Lin CY, Chen H, Pakpour AH (2019) The role of religious coping and social support on medication adherence and quality of life among the elderly with type 2 diabetes. Qual Life Res 28: 2183-2193.
- Saffari M, Pakpour AH, Naderi MK, Koenig HG, Baldacchino DR, et al. (2013) Spiritual coping, religiosity and quality of life: A study on Muslim patients undergoing haemodialysis. Nephrology (Carlton) 18: 269-275.
- Green J, Fukuhara S, Shinzato T, Miura Y, Wada S, et al. (2001) Translation, cultural adaptation, and initial reliability and multitrait testing of the Kidney Disease Quality of Life instrument for use in Japan. Qual Life Res 10: 93-100.
- Ramirez SP, Macedo DS, Sales PM, Figueiredo SM, Daher EF, et al. (2012) The relationship between religious coping, psychological distress and quality of life in hemodialysis patients. J Psychosom Res 72: 129-135.
- Griva K, Kang AW, Yu ZL, Mooppil NK, Foo M, et al. (2014) Quality of life and emotional distress between patients on peritoneal dialysis versus community-based hemodialysis. Qual Life Res 23: 57-66.
- Yang F, Griva K, Lau T, Vathsala A, Lee E, et al. (2015) Health-related quality of life of Asian patients with End-Stage Renal Disease (ESRD) in Singapore. Qual Life Res 24: 2163-2171.
- Chiang JK, Chen JS, Kao YH (2019) Comparison of medical outcomes and health care costs at the end of life between dialysis patients with and without cancer: A national population-based study. BMC Nephrol 20: 265.
- Kao TW, Lai MS, Tsai TJ, Jan CF, Chie WC, et al. (2009) Economic, social, and psychological factors associated with health-related quality of life of chronic hemodialysis patients in northern Taiwan: A multicenter study. Artif Organs 33: 61-68.
- Cleary J, Drennan J (2005) Quality of life of patients on haemodialysis for end-stage renal disease. J Adv Nurs 51: 577-586.
- Polit DF, Beck CT (2008) Is there gender bias in nursing research? Res Nurs Health 31: 417-427.
- Tavernier SS, Totten AM, Beck SL (2011) Assessing content validity of the patient generated index using cognitive interviews. Qual Health Res 21: 1729-1738.
- McGee HM, O'Boyle CA, Hickey A, O'Malley K, Joyce CR (1991) Assessing the quality of life of the individual: The SEIQoL with a healthy and a gastroenterology unit population. Psychol Med 21: 749-759.
- Becker G, Merk CS, Meffert C, Momm F (2014) Measuring individual quality of life in patients receiving radiation therapy: The SEIQoL-Questionnaire. Qual Life Res 23: 2025-2030.
- Ware JE (2008) Improvements in short-form measures of health status: introduction to a series. J Clin Epidemiol 61: 1-5.
- Wilson IB, Cleary PD (1995) Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA 273: 59-65.
- Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67: 361-370.
- Pawlikowska T, Chalder T, Hirsch SR, Wallace P, Wright DJ, et al. (1994) Population based study of fatigue and psychological distress. BMJ 308: 763-766.
- Bufford RK, Paloutzian RF, Ellison CW (1991) Norms for the spiritual weil-being scale. Journal of Psychology and Theology 19: 56-70.
- Kolewaski CD, Mullally MC, Parsons TL, Paterson ML, Toffelmire EB, et al. (2005) Quality of life and exercise rehabilitation in end stage renal disease. CANNT J 15: 22-29.
- Al Ismaili F, Al Salmi I, Al Maimani Y, Metry AM, Al Marhoobi H, et al. (2017) Epidemiological transition of end-stage kidney disease in Oman. Kidney Int Rep 2: 27-35.
- Al Alawi I, Al Salmi I, Al Mawali A, Al Maimani Y, Sayer JA (2017) End-stage kidney failure in Oman: an analysis of registry data with an emphasis on congenital and inherited renal diseases. Int J Nephrol 2017: 6403985.
- Al Alawi IH, Al Salmi I, Al Mawali A, Sayer JA (2017) Kidney disease in Oman: A view of the current and future landscapes. Iran J Kidney Dis 11: 263-270.
- Willis J (1995) Fatal attraction: Do high-technology treatments for end-stage renal disease benefit aboriginal patients in central Australia? Aust J Public Health 19: 603-609.
- Paul CB, Willis GB (2007) The practice of cognitive interviewing. The Public Opinion Quarterly 71: 287-311.
- Willis G (2006) Cognitive interviewing as a tool for improving the informed consent process. J Empir Res Hum Res Ethics 1: 9-24.
- Willis G, Zahnd E (2007) Questionnaire design from a cross-cultural perspective: An empirical investigation of Koreans and non-Koreans. J Health Care Poor Underserved 18: 197-217.
- Field AP (2005) Is the meta-analysis of correlation coefficients accurate when population correlations vary? Psychol Methods 10: 444-467.
- Newcombe RG (1988) Two-sided confidence intervals for the single proportion: Comparison of seven methods. Stat Med 17: 857-872.
- Julious SA (2005) Issues with number needed to treat. Stat Med 24: 3233-3235.
- Knafl K, Deatrick J, Gallo A, Holcombe G, Bakitas M, et al. (2007) The analysis and interpretation of cognitive interviews for instrument development. Res Nurs Health 30: 224-234.
- Tabachnick B, Fidell L (2007) Using multivariate statistics. (5th edn.). Allyn & Bacon/Pearson Education, Boston, USA.
- Ferrans CE, Zerwic JJ, Wilbur JE, Larson JL (2005) Conceptual model of health-related quality of life. J Nurs Scholarsh 37: 336-342.
- Cope AB, Ramirez C, DeVellis RF, Agans R, Schoenbach VJ, et al. (2016) Measuring concurrency attitudes: Development and validation of a vignette-based scale. PLoS One 11: 0163947.
- DeVellis RF, DeVellis BM, Blanchard LW, Klotz ML, Luchok K, et al. (1993) Development and validation of the parent health locus of control scales. Health Educ Q 20: 211-225.
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, et al. (1994) Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 151: 1132-1136.
- Hamaker EL, Asparouhov T, Brose A, Schmiedek F, Muthen B (2018) At the frontiers of modeling intensive longitudinal data: Dynamic structural equation models for the affective measurements from the COGITO study. Multivariate Behav Res 53: 820-841.
- Muthen B, Asparouhov T, Rebollo I (2006) Advances in behavioral genetics modeling using Mplus: Applications of factor mixture modeling to twin data. Twin Res Hum Genet 9: 313-324.
- Muthen B, Muthen LK (2000) Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res 24: 882-891.
- Field AP, Gillett R (2010) How to do a meta-analysis. Br J Math Stat Psychol 63: 665-694.
- Stanek EJ, Kline G (1991) Estimating prediction equations in repeated measures designs. Stat Med 10: 119-130.
- MacCallum RC, Hong S (1997) Power analysis in covariance structure modeling using GFI and AGFI. Multivariate Behav Res 32: 193-210.
- Bentler PM, Huang W (2014) On components, latent variables, PLS and simple methods: Reactions to Rigdon's rethinking of PLS. Long Range Plann 47: 138-145.
- Hu LT, Bentler PM, Kano Y (1992) Can test statistics in covariance structure analysis be trusted? Psychol Bull 112: 351-362.
- Munro N, Denney ML, Rughani A, Foulkes J, Wilson A, et al. (2005) Ensuring reliability in UK written tests of general practice: The MRCGP examination 1998-2003. Med Teach 27: 37-45.
- Mertler C, Vannatta R (2016) Advanced and multivariate statistical methods: Practical application and interpretation. Taylor & Francis, Los Angeles, USA.
- Pallant J (2010) SPSS survival manual: A step by step guide to data analysis using SPSS program. Allen & Unwin, Australia.
- Saunders M, Lewis P, Thornhill A (2009) Research methods for business students. Prentice Hall, New Jersey, USA.
- Saunders M, Thornhill A (2012) Research methods for business students. Pearson Education Limited.
- Black C, Sharma P, Scotland G, McCullough K, McGurn D, et al. (2010) Early referral strategies for management of people with markers of renal disease: A systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis. Health Technol Assess 14: 1-184.
- Black T (1999) Outcomes: What's all the fuss about? Rehabil Nurs 24: 188-189.
- Willis C, Kernoghan A, Riley B, Popp J, Best A, et al. (2015) Outcomes of interorganizational networks in canada for chronic disease prevention: Insights from a concept mapping study, 2015. Prev Chronic Dis 12: 199.
- Broom A, Willis E (2007) Competing paradigms and health research. In: Researching health: Qualitative, quantitative and mixed methods. Sage, London, UK.
- Matthews B, Ross L (2010) Research methods: A practical guide for the social sciences. Longman, Harlow, UK.
- Sapsford R (2007) Survey Research. SAGE Publications, California, USA.
- Al Salmi I, Hannawi S (2016) The World Health Report-health systems empowering citizens and improving performance. Research on Humanities and Social Sciences 6: 1-6.
- Al Salmi I, Hannawi S (2018) Health Workforce in the Sultanate of Oman: Improving performance and the Health System. J Int Med Pat Care 1: 6.
- Hannawi S, Al Salmi I (2014) Health workforce in the United Arab Emirates: Analytic point of view. Int J Health Plann Manage 29: 332-341.
- Hannawi S, Al Salmi I (2018) Time to address gender inequalities against female physicians. Int J Health Plann Manage 33: 532-541.
- Johnson ME, Piderman KM, Sloan JA, Huschka M, Atherton PJ, et al. (2007) Measuring spiritual quality of life in patients with cancer. J Support Oncol 5: 437-442.
- Piderman KM, Johnson ME, Frost MH, Atherton PJ, Satele DV, et al. (2014) Spiritual quality of life in advanced cancer patients receiving radiation therapy. Psychooncology 23: 216-221.
- Schmitt M, Juchtern JC (2001) The structure of subjective well-being in middle adulthood. Aging Ment Health 5: 47-55.
- Stanley M, Cheek J (2003) Well-being and older people: A review of the literature. Can J Occup Ther 70: 51-59.
- Diener E, Lucas RE, Scollon CN (2006) Beyond the hedonic treadmill: Revising the adaptation theory of well-being. Am Psychol 61: 305-314.
- Peterson SJ, Bredow TS (2009) Middle range theories: Application to nursing research. Lippincott, Williams & Wilkins, Philadelphia, USA.
- Kring DL, Crane PB (2009) Factors affecting quality of life in persons on hemodialysis. Nephrol Nurs J 36: 15-24.
APPENDIX
For Appendix file please click below link:
https://www.heraldopenaccess.us/fulltext/Nephrology-&-Renal-Therapy/HNRT-20-021-Appendix.docx
Citation: Al-Rajhi W, Al Salmi I (2020) Quality of Life and Health Related Quality of Life Among End-Stage Kidney Disease Patients: Methodology Assessment. J Nephrol Renal Ther 6: 039.
Copyright: © 2020 Waleed Al-Rajhi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

