
Radiation Exposure Appraisal to Public, Caregivers and Families in Contact with Thyroid Diseases Patient Undergoing Radioactive Iodine-131 Therapy: A Systematic Review
*Corresponding Author(s):
Buhari SamailaDepartment Of Physics With Electronics, Federal University, Birnin Kebbi, Nigeria
Email:buhari.samaila@fubk.edu.ng
Abstract
Based on world health organization, cancer is the second leading cause of mortality in the world, accounting for 9.6 million deaths in recent years. In low- and middle-income countries, cancer-related fatalities account for about 70% of all deaths. Thyroid cancer is currently the ninth frequent cancer in the world according to the American Cancer Society. Radioactive chemicals like iodine-131 are the most beneficial for treating individuals with thyroid cancer and thyrotoxicosis as well as researching thyroid physiology. Iodine-131 use must conform to safeguards, stringent safety measures, and specific instructions to avoid unwanted exposure to radiation for populace, family members, the environment, and medical professionals who are in close proximity to patients getting a therapeutic dose. Knowledge of the precautions and recommendations is necessary to comprehend radiation protections. The objective of the current exploratory evaluation was to examine past and current research that used radioiodine-131 to treat a variety of thyroid conditions in connection to the radiation doses received by patients' members of family, caregivers, and general public. The results of the measurements of the radiation are disclosed to the patients, member of family, caregivers, and general public who are in charge of the non-self-supporting patients receiving radioactive iodine therapy at the radionuclide therapy ward. A variety of electronic digital dosimeters were given to the caretakers of numerous patients who had radioiodine therapy for thyroid tumors or hyperthyroidism in accordance with a defined protocol to assess radiation exposure constantly over the course of some days in the hospital. According to historical evidence, it is commonly acknowledged that oxidative stress contributes significantly to the development of DNA damage brought on by iodine's radiation, which is naturally ionizing. The activity of 131I (1850MBq) given to thyroid illness patients was connected to DNA damage and chromosomal abnormalities. To assess thyroid uptake on the day when patients were released, the caregivers, the general public, and the medical staff underwent in vivo bioassays. According to reports, patients who received iodine-131 treatments of 7.4 GBq and 5.55 GBq emitted radiation to their immediate families and nearby members of the general public of roughly 176 and 1920 Sievert, respectively. With the 5.0 mSv overall dosage limit, this result was above the 1.0 mSv/y public dose limit. The radiation dosage that hospital workers got when administering radioiodine therapy to a non-self-supporting patient was significantly below recommended values by the ICRP and IAEA; this may be due to their brief proximity to the patient. Knowing that the caregivers' dose won't go over the 5-mSv limit gives the patients a sense of security. Patients receiving radioiodine-131 therapy for thyroid-related disorders with administered activity ranging from 195 to 800 MBq emitted radiation that potentially threatens the general public and medical personnel. The dose that the general public is allowed to receive as a result of patients’ treatment has been reduced in Europe in accordance with newly updated ICRP standards. The dose limit to the public is 1.0 mSv with the provision that a maximum of 5.0 mSv should not be exceeded throughout a 5.0 year period, even thought adult family members of the patient are permitted to receive higher doses. Unless it can be demonstrated that the doses they received during outpatient radioiodine therapy complied with these new regulations, patients will need to be admitted to the hospital. Family members of patients receiving radioiodine treatment had their radiation exposure measured using thermal luminescence dosimeters attached to wrist bands, radiation survey meters, and analytical methods. After therapy, families were encouraged by their treatment center to limit the patient's close contact. Additionally, it was discovered that administering low doses of radioactive iodine was convenient for the patient, moderately priced, and had few side effects.
Keywords
Exposure to iodine; Iodine radiation measurement; Iodine therapy
Introduction
The safest and most efficient long-term treatment for thyroid diseases is radioiodine (iodine-131). However, there is serious radiation protection concerns related to the therapy. These include radiation emissions and radioiodine loss from sweat, saliva, breast milk, and urine. People in the public who the patient might come into close contact with those who could be at danger of being exposed to radiation, because of their shorter life expectancies than adults, the radio sensitivity of growing tissues, and the difficulty in avoiding intimate contact between young child and their parents, children are thought to be the group most at danger. Avoiding personal contact with patient in the first few days following treatment can usually minimize contamination by radioactive secretions and any dose absorbed should be minimal compared to that which may be obtained by radiation emissions [1]. In many parts of the universe, I131 was frequently used to treat thyroid-related diseases. The benefits of 131I therapy for patients are undeniable, but there should also be careful consideration for the family or caregivers who exposed to radiation due to iodine-131use. The benefits of 131I therapy have to be balanced against the radiation risk to caregivers, whose efforts in ensuring that patients receive proper care following radioiodine administration cannot be overstated [2]. The half-life of 131I, which decays by beta-emission, is 8.03 days. Its beta emission energy [606 KeV] can kill cell up to a few millimeters away by penetrating them.
Furthermore, it produces higher-energy gamma radiation (364 keV, 81.5%), which can be utilized to image the entire body and detect thyroid issues. Iodine-131 is therefore commonly used for both disease diagnosis and treatment in clinical nuclear medicine. Iodine-131 is yet another fission product with a large yield. The International Atomic Energy Agency typically advises quick thyroid screening for Iodine-131 contamination because the significant release of Iodine-131 that could occur in the event of a catastrophic nuclear accident could contaminate a significant portion of the on-site workforce as well as the general public off-site. Assessment of Iodine-131 activity in the human thyroid is consequently restricted for nuclear medicine applications as well as for screening due to the possibility of ingestion following a release of Iodine-131 into the environment [3]. Over the past few decades, a variety of methods and monitoring systems have been developed for in vivo assessments of Iodine-131 in the thyroid. In Whole-Body Counters (WBCs) with Sodium Iodide doped with Thallium (NaI(Tl)), High Purity Germanium (HPGe), or Low Energy Germanium (LEGe) detectors, which are believed to be the most sensitive in detecting Iodine-131 in the thyroid, the Minimum Detectable Activity (MDA) of Iodine-131 could reach 5-8 Bq for a 20-minute measurement. However, due to their expensive cost and restricted mobility, WBCs are impractical for many field measurements. In order to detect Iodine-131 in the thyroid in the field, several types of portable or hand-held equipment, such as survey meters or gamma spectrometers, have recently been developed. Since they can discriminate between the gamma counts of Iodine-131 and other radionuclides, hand-held gamma spectrometers are considered to be the most promising of them all [3]. But it has generally been acknowledged that in addition to counting data, it is crucial to consider the uncertainties brought on by changes in the detecting position, background signal, body dimension, thyroid tissue overlay, and device calibration. In order to eliminate the impact of detector location fluctuation, a two-dimensional detector, the Imaging Plate (IP), which may be attached directly to the neck, was also investigated for qualitative or semi-quantitative measurements of radioactive iodine in the thyroid. However, the specific requirements for light shielding and signal fading correction limit the scope of its applicability. To ensure that the safety of persons who come into contact with radioactive materials, such as I-131, for a variety of reasons, including treatment, the International Atomic Energy Agency (IAEA) has set a few standards. Individuals receiving radioactive treatment at levels lower than 1100 MBq are excused from hospitalization under their standard [4]. These precautions are necessary because radioiodine therapies using higher dosages may be harmful to both patients' families and medical personnel [5-9]. Family members or other caregivers who are present for the patient's treatment will almost certainly be exposed to radiation. Consequently, there is ongoing worry that these individuals will be revealed [2].
The discovery of radiologic techniques for disease diagnosis in humans had a big impact on the medical field. Nearly 2.4 million medical diagnostic radiation workers employ ionizing radiation for nuclear imaging operations in more than 11,000 institutions worldwide. These ionizing radiations can lead to environmental dangers, genetic mutations, hematological system dysfunction, oxidative stress, and immunological dysfunction. Radioisotopes like (I-131) are generated as artificial sources in the medical field to treat some life-threatening illnesses. The first radioisotope used in medical therapy was radioactive iodine-131, and as a result, nuclear medicine as an area of study was created [10]. The energy, type, and range of radiation are of highest relevance when integrating several fundamental scientific domains, such as inorganic chemistry, with clinical applications, physics, physiology, and biochemistry, which provides a difficult field for nuclear medicine in tissues. Iodine-131 and other radioactive substances are applied both inside and externally. Radioactive chemicals are the most beneficial for treating individuals with thyroid cancer and thyrotoxicosis, as well as for researching thyroid physiology [10]. Iodine-131 use must conform to precautions, stringent safety regulations, and specific instructions in order to avoid unwanted radiation exposure for patients, family members, the environment, and medical workers who are in close proximity to patients getting a therapeutic dose.
Understanding radiation protection involves familiarity with the warnings and recommendations [10]. Administration of low dose radioactive iodine is patient-friendly, moderately priced, and has few side effects. One of the risks of using Iodine-131 to treat thyroid cancer is that patients themselves could act as mobile radiation sources. If protections are not put in place, radiation exposure to family members, carers and the general public is inevitable. Standard regulations are therefore established for the discharge of those patients from the hospital. The International Atomic Energy Agency (IAEA) Safety Series No. 63 states that patients shouldn't be released from the hospital unless the Iodine-131 activity is less than or similar to 1100 MBq (30 mCi). Additionally, the 400 MBq (11 mCi) safety level serves as a guideline for best practices in many countries [11]. The current analysis set out to examine previous studies that used radioiodine I-131 to treat a variety of thyroid conditions in connection to the radiation doses that patients' family members and caregivers who work with them were exposed to.
Thyroid Related Diseases that require iodine-131 therapy
The second leading cause of death worldwide, according to the World Health Organization (WHO), was cancer, which claimed 9.6 million lives in 2018. In low- and middle-income countries, almost 70% of cancer-related mortality occur. The American Cancer Society (ACS) reports that thyroid malignancy is currently the tenth most frequent cancer worldwide. Graves disease, toxic multinodular goiter, and toxic adenoma are the three most common causes of excessive thyroid hormone production. In 2018, thyroid cancer is the sixth most common malignancy among Sri Lankans. According to data from the WHO's International Agency for Research on Cancer, it is also the second most common cancer in women in Sri Lanka [11].
Radioactive iodine (iodine-131) therapy is the thyroid cancer treatment method that is most frequently used globally. Patients with thyroid carcinoma who have undergone a (full or nearly complete) thyroidectomy are given Iodine-131 to destroy any cancer cells that may still exist as well as any functional thyroid tissues. The majority of the radioactive iodine that is administered is absorbed by the thyroid tissues, and the renal system then excretes it. There may be individual variations in the radiation dosage absorbed and the radiation emission (dose rate) by the thyroid tissues [11]. Thyroid cancer, hyperthyroidism, medullary, follicular, and papillary malignant tumors of the thyroid glands are the four different forms of thyroid disorders (Thyrotoxicosis). Tumors of the papillary and follicular types are very common [10].
Thyrotoxicosis, Grave diseases, and thyroid cancer were the three main and most-researched thyroid conditions (Hyperthyroidism). Thyrotoxicosis (hyperthyroidism), an endocrine disorder, is defined by the thyroid gland producing an excessive amount of thyroid hormone [12]. Thyrotoxicosis is a common illness that is more prevalent in women. It is brought on by an overproduction of thyroid hormone. The most common cause of thyrotoxicosis is Graves' disease. Additional causes include toxic nodules and thyroiditis. The typical symptoms of a disease include fatigue, weight loss, shaking, anxiety, palpitations, disturbed sleep, sweating, heat intolerance, and polydipsia. It is possible to treat these conditions and slow or stop the development of the disease using Anti-Thyroid Drugs (ATD), thyroid surgery, radioactive iodine-131 (radioactive iodine), and other treatment approaches for treating thyrotoxicosis [13].
Women are 10 times more likely than men to develop hyperthyroidism, with a prevalence of up to 2%, according to a follow-up community survey on thyroid disease carried out in the United Kingdom. Radioactive Iodine (RAI) has been used to treat solitary dangerous nodules, multi-nodular goitres, and cases of hyperthyroidism brought on by Graves' disease for more than 70 years. Radioactive iodine-131 can be used as a first- or second-line therapy after an antithyroid drug has failed. This procedure makes use of the beta-emitting iodine radioisotope iodine-131. When radioactive iodine is concentrated specifically on the thyroid gland, the thyroid gland finally dies. With RAI therapy, cure rates vary from 80% to 100%, with some patients requiring two or more doses [12].
Radioiodine-131 therapy side effects on patient
The side effects that have been taken into consideration, such as nausea, headaches, lacrimal and salivary dysfunction, and changes in taste and smell, appear to be acute effects that are more common in therapies with 100 mCi and less frequent for dosages between 30 and 50 mCi. According to studies by, the prevalence of sialadenitis ranges from 2 to 67% and that of nasolacrimal duct obstruction is 3.4%. In terms of Sexual Sphere Side Effects, male and female infertility have sub-acute latency, which is different from the previously mentioned sialadenitis but also from nasolacrimal duct obstruction, and the onset of secondary malignancy. These late effects appear to only be visible for iodine-131 activity, which is primarily 100 mCi. Notably, a temporary drop in Follicle-Stimulating Hormone (FSH) levels and reduced sperm motility were seen in men, while a lower birth rate in the 35-39 year age range was seen in women. Exocrine and endocrine testicular function may be affected by 3.7 GBq iodine doses, it has been noted. Three months and thirteen months following treatment, they noticed Sertoli cell activity and produced sperm chromosomal aberrations.
Observations from around the world
Dose limitations are often not employed for medical radiation because the ALARA principle is used to provide a therapeutic effect. The International Commission on Radiological Protection publication 94 (ICRP-94) [7] and the International Atomic Energy Agency Safety Reports Series No. 40 (IAEA-SRS-40) both recommend a maximum effective dose for caretakers of 5 mSv per treatment session. Additionally, these reports offer safety advice, such as the ban of caregivers who are expecting or under the age of 20, as well as the demand that the ALARA criterion be satisfied. Additionally recommended in National Council on Radiation Protection & Measurements Report No.155 (NCRP-155) was the dosage cap of 5 mSv for a single treatment session for caretakers. The manner in which patients are released from hospitals after receiving radioactive therapy varies significantly, according to the IAEA Safety Reports Series No. 63 (IAEA-SRS-63) [8]. IAEA report K9010241 also provides some helpful guidance, including the suggestion that physicians exercise their professional judgment when determining whether to admit or release patients from hospitals based on each patient's condition. To ensure the safety of radiation from patients receiving nuclear medicine, radiation dose control can therefore be implemented based on important safety and health recommendations, dose measurement and assessment, and expert judgment.
Criteria used in this review
Search techniques: PRISMA, or Preferred Reporting Items for Systematic Reviews and Meta-Analyses, was followed for this study's systematic review. The MDPI, Research Gate, PubMed, and Google Scholar databases were used to search the English literature for recent studies on iodine therapy. The terms Differentiated Thyroid Cancer (DTC), Thyroid Cancer (thyroid carcinoma), Second Primary Malignancy (SPM), Radioiodine Therapy (RIT), Radioactive Iodine Therapy (RAI), and RAI therapy were used in this search. Other search terms included: side effects of radioiodine-131 therapy on patients, iodine-131 therapy for thyroid-related diseases, Radiometric Analysis of Patients with Diseases Associated with the Thyroid, administered Iodine-131 activity, Iodine uptake in the remaining thyroid in the postoperative bed, Maximum Tolerable Activity administered to the patient, Iodine's effective half-life after administration to the patient, measurement of the dose rate to the general public and caregivers, estimation of the total radiation dose, and dos rate release criteria of patients treated with iodine-131.
Study selection: A complete systematic review and meta-analysis of published papers were conducted to assess the clinical results of radioiodine therapy for thyroid-related illnesses with respect to the radiation absorbed doses to the thyroid. Only studies that included information on radiation exposure to patients, their relatives, caregivers, and the general public as well as treatment outcomes for patients were taken into consideration. Only full-text articles that appeared in peer-reviewed journals were assessed. Two to three reviewers looked at the study titles and abstracts to make sure they were appropriate before accessing the complete article. In a subsequent step, the same reviewers independently selected all of the accepted papers in accordance with the prerequisites. Additionally, prospective additional citations were carefully inspected in the bibliographies of the papers that were acquired.
Eligibility for the study and data extraction: Each study was initially grouped according to the authors, publication year, and journal. If a study met all of the following criteria, it was considered qualified to harmonize the predictors of interest: These findings were cited in studies (1) Patients with TC and TH who have received RAI and an estimate of the radiation dose received (2) the patient's iodine-131 treatment's criterion release activity. The information that was recovered covered the study's design in great detail, the number of patients who received RAI treatment, and the exclusion of those who did not. The figure below indicates the details of inclusion and exclusion criteria used (Figure 1).
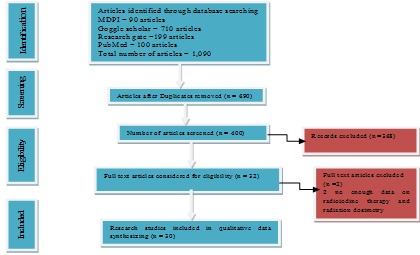 Figure 1: Methodological flow chart.
Figure 1: Methodological flow chart.
Data analysis
The number of patients, type of patient diseases, radioactive iodine-131 activity provided to patients, range of radiation absorbed dose to patients and the public were all extracted for each study. Additionally, dosimetry techniques were extracted
Radiometric analysis of patients with thyroid related diseases [Dosimetry in iodine-131 therapy]: Benua & Leeper and the blood absorption method were used to determine the Maximum Activity of Iodine-131 that could be given to the patient without going over the Bone Marrow Tolerance Limit of 2 Gy. The Medical and Internal Radiation Dosimetry (MIRD) scheme is used in the blood dosimetry method to determine the radiation dose to the blood per Giga-Becquerel of radioactivity utilizing full body scans and blood samples. The following equations calculate the blood dose that was absorbed. 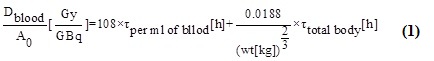 Dblood stands for average blood dose, Ao for amount of activity administered, τ for time-integrated activity coefficients derived from areas under the decay curve, and wt for patient weight.
Dblood stands for average blood dose, Ao for amount of activity administered, τ for time-integrated activity coefficients derived from areas under the decay curve, and wt for patient weight.
The dose that is absorbed for red bone marrow: Using the provided equation, the absorbed dose for red bone marrow was determined.
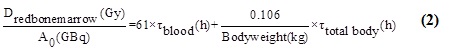
Total body activity's residence time was calculated by integrating whole-body retention from zero to the maximum time point.
Blood dosimetry and 48-hour retention methods were both used in various studies to calculate the maximum allowable activity. The concepts of Medical and Internal Radiation Dosimetry (MIRD) are the foundation of blood dosimetry, which calculates the radiation dose received in blood per GBq of iodine-131.
Equation 2 below is used to compute the retained activity:
Retained Activity = Count C.F (3)
C.F is the calibration factor having units of mCi/counts.
Administered activity of iodine-131
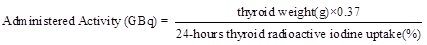 Radioactive Iodide Uptake (RAIU) was measured 24- hours after oral ingestion of iodine.
Radioactive Iodide Uptake (RAIU) was measured 24- hours after oral ingestion of iodine.
Geometric mean: On the anterior image, a Region of Interest (ROI) rectangle was formed around the entire body. The number of total pixels and counts/pixel in the anterior and posterior photographs were obtained. Equation 3 was used to get the geometric mean of the counts per pixel for the complete body scan (cpp).
 The G.M. assists in removing the ambiguity related to the patient's geometry and shape. Without the patient on the sofa and at the same normal scan speed, a whole-body image was acquired to adjust for background radiation. To get an accurate representation of the background, all adjacent radiation sources were eliminated. To ascertain the count/pixel, the anterior and posterior background images were examined. The following equation is used to compute the retained activity.
The G.M. assists in removing the ambiguity related to the patient's geometry and shape. Without the patient on the sofa and at the same normal scan speed, a whole-body image was acquired to adjust for background radiation. To get an accurate representation of the background, all adjacent radiation sources were eliminated. To ascertain the count/pixel, the anterior and posterior background images were examined. The following equation is used to compute the retained activity.
Retained Activity = C.F {(GM of Cpp – background Cpp) No.of Pixels} (5)
Where Cpp stands for count per pixel and C.F for conversion factor, the temporal activity curve is integrated to determine the time-integrated activity coefficient. Plotting the % activity versus the interval between injections of activity and scanning time yields the curve, which exhibits the exponential decay that is typical of radioactive decay.
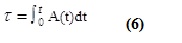 Administered decay correction for iodine-131: Blood samples from the patients were collected at the time of the scans and analyzed in a gamma counter to determine the background corrected counts. Decay correction was used to account for the lags between administration, sampling, and analysis times. In this methodology, blood samples were examined on days two or three, and each measurement's decay correction was determined as follows.
Administered decay correction for iodine-131: Blood samples from the patients were collected at the time of the scans and analyzed in a gamma counter to determine the background corrected counts. Decay correction was used to account for the lags between administration, sampling, and analysis times. In this methodology, blood samples were examined on days two or three, and each measurement's decay correction was determined as follows.
Countdecaycorrected = Counttotal (7)
Maximum tolerable activity administered to patient: By calculating the maximum activity that could provide the radiation absorbed dosage of 2 Gy to blood, the maximum activity to be administered to the patient can be computed as shown in equation (8).
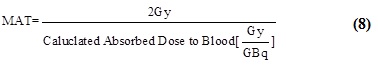 Thyroid weight and volume calculation: Calculation of thyroid volume and weight
Thyroid weight and volume calculation: Calculation of thyroid volume and weight
It is unlikely that the oral route of administration of the diagnostic activity may produce differing readings for 24-hour RAIU compared to the intravenous approach because gastrointestinal absorption of radioiodine is rapid and thorough. Using the Doering and Kramer formula, the thyroid weight was calculated from the planimetric surface of a rectilinear thyroid scintigram:
Thyroid weight (g) = 0.326 (Surface in cm2)3/2 (9)
Before starting treatment, ultrasonography was used to measure the thyroid volume. By assuming that both lobes are ellipsoidal in shape and measuring three orthogonal diameters, the thyroid volume was roughly estimated. One lobe's volume was determined using the formula:
Volumelobe = 1/2 [length * thickness * width] (10)
The target volume was assumed to be equal to the total thyroidal volume for Graves' disease, non-toxic goiter, and toxic multinodular goiter; for toxic uninodular goiter, the target volume was assumed to be equal to the adenomal volume. The tissue was thought to have a density of 1 g/cm3.
Iodine uptake in the residual thyroid in the recovery bed
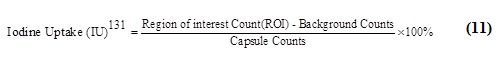 IU131 is the amount of iodine absorbed by the leftover thyroid in the postoperative bed after n hours (n = 6, 24, 48, or 72 h), and ROI Counts is the total number of counts in the area of interest (ROI) throughout the measurement (i.e. 5 min). The ROI in the patient's postoperative bed was an iodine-rich residual thyroid. A 131I-capsule (3700 MBq) inserted in a neck phantom was counted over a five-minute measurement period. Backdrop counts are the number of counts that are marked in the postoperative bed as the background for evaluation. Murielle's formula can be used in addition to the previously mentioned technique to determine the therapeutic activity of 131I. (3700 MBq).
IU131 is the amount of iodine absorbed by the leftover thyroid in the postoperative bed after n hours (n = 6, 24, 48, or 72 h), and ROI Counts is the total number of counts in the area of interest (ROI) throughout the measurement (i.e. 5 min). The ROI in the patient's postoperative bed was an iodine-rich residual thyroid. A 131I-capsule (3700 MBq) inserted in a neck phantom was counted over a five-minute measurement period. Backdrop counts are the number of counts that are marked in the postoperative bed as the background for evaluation. Murielle's formula can be used in addition to the previously mentioned technique to determine the therapeutic activity of 131I. (3700 MBq).
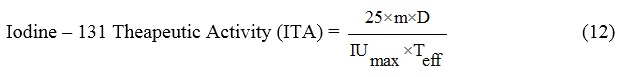 The absorbed dose from patients treated with radioiodine can be calculated from the above formula.
The absorbed dose from patients treated with radioiodine can be calculated from the above formula.
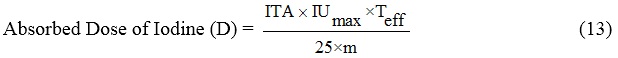
where Teff is the effective 131I half-life in remnant thyroid and A is the therapeutic activity of iodine-131 of 3700 MBq, 25 is the unit conversion coefficient, and m is the mass of the remnant thyroid measured using an ultrasound scanner (days).
Effective half-life of iodine administered to patient: The effective half- life of iodine delivered to the patients can be calculated using equation below:
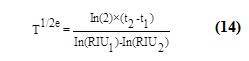 Where T1/2e is the effective half-life during the radioiodine test [days] and RIU is the radioiodine uptake in percent at time point t=t1 or t2, respectively.
Where T1/2e is the effective half-life during the radioiodine test [days] and RIU is the radioiodine uptake in percent at time point t=t1 or t2, respectively.
Maximum reduction of radiation: For each type of sample, the maximum radiation reduction percentage was computed using the equation (15) below:
 Measurement of the dose rate from iodine-131treated patients: The thyroid cancer dosage rates were tracked daily for a preset period of time after the administration of the iodine-131 at varied distances from the patient. Surface contamination levels from the room surfaces, clothing, door handle, fan switches, and restroom were assessed after the release of Iodine-131 patients [14]. Several survey meters, including the GRAETZ X5C plus, which was calibrated to measure the ambient dose equivalent, H, were used to measure dosage rates. It is presumable that the value of H (10) provides a reliable estimation of the radiation dosage needed to offer sufficient radiation protection. The CoMo 170 mobile contamination meter was calibrated to test radioactive contamination for alpha, beta, and gamma radiation. The area survey meter was calibrated at the Secondary Standard Dosimetry Laboratory using a well-known technique that is described in the IAEA report, however the contamination monitor was calibrated by the manufacturer [14]. Patients receiving Iodine-131 therapy frequently need to stay in the hospital for a few days to avoid an accumulation of Iodine-131 in their body that could otherwise expose others to excessive radiation. There are currently few quantifiable metrics that can be used to evaluate scientifically whether the length of hospitalization genuinely guarantees patient safety following discharge. As long as radiation safety rules are followed, medical facilities strive to minimize hospital stays. A respectable outpatient management system will be set up, according to the responsible party. The 1-m dose rate measurement among patients following hospital discharge has been widely utilized as a metric to ascertain a patient's eligibility for release. Here, survey meters were used in medical facilities to determine the dosage rate [14].
Measurement of the dose rate from iodine-131treated patients: The thyroid cancer dosage rates were tracked daily for a preset period of time after the administration of the iodine-131 at varied distances from the patient. Surface contamination levels from the room surfaces, clothing, door handle, fan switches, and restroom were assessed after the release of Iodine-131 patients [14]. Several survey meters, including the GRAETZ X5C plus, which was calibrated to measure the ambient dose equivalent, H, were used to measure dosage rates. It is presumable that the value of H (10) provides a reliable estimation of the radiation dosage needed to offer sufficient radiation protection. The CoMo 170 mobile contamination meter was calibrated to test radioactive contamination for alpha, beta, and gamma radiation. The area survey meter was calibrated at the Secondary Standard Dosimetry Laboratory using a well-known technique that is described in the IAEA report, however the contamination monitor was calibrated by the manufacturer [14]. Patients receiving Iodine-131 therapy frequently need to stay in the hospital for a few days to avoid an accumulation of Iodine-131 in their body that could otherwise expose others to excessive radiation. There are currently few quantifiable metrics that can be used to evaluate scientifically whether the length of hospitalization genuinely guarantees patient safety following discharge. As long as radiation safety rules are followed, medical facilities strive to minimize hospital stays. A respectable outpatient management system will be set up, according to the responsible party. The 1-m dose rate measurement among patients following hospital discharge has been widely utilized as a metric to ascertain a patient's eligibility for release. Here, survey meters were used in medical facilities to determine the dosage rate [14].
Measurement of the dose rate to the public and caregivers: The personal dosimetry badges were used by persons to calculate the radiation dosage received by caregivers and the general public. The personal dosimetry badges include the following, to name a few: Model type 7776, Thermo luminescence dosimetry card, and TLD-100 (LiF:Mg,Ti) chips are placed in the Thermal Fisher Scientifics model type 8814 dosimetry holder between polytetrafluoroethylene plates. The holder contains the TLD chips and offers four attenuation filters for each chip to make it easier to calculate the individual dose equivalents at tissue depths of 0.07, 3, and 10 mm (0.004 inch in Cu, 0.162 inch in PTFE, 0.0015 inch in Mylar, and one filter without attenuation). Tracking photons with energy more than 1 keV, with a detection threshold of 0.07 mSv and 10 Pico Sievert to 10 Sv-level dosages. Therefore, the custom dosimetry badges can be used in the study to measure the 365-keV gamma ray from iodine-131. The performance of the dosimetry measurement system has also received ISO/IEC-17025 certification for a section of its operation. A respectable outpatient management system will be set up, according to the responsible party. The 1-m dose rate measurement among patients following hospital discharge has been widely utilized as a metric to ascertain a patient's eligibility for release. Here, survey meters were used in medical facilities to determine the dosage rate [14].
Evaluation of cumulative dose: The radiation dose was supplied to the caretakers when patients were released from the hospital, and the effective dose was monitored using personalized dosimetry badges. The duration of wearing the personalized dosimetry badges was decided based on the patients' conditions and was approximately 1-2 weeks after release. Caretakers were continuously exposed to radiation because Iodine-131 that had only partially attenuated or been digested remained in the patient's tissue. The radiation that the badges might absorb if caregivers wore them continuously was therefore referred to as the cumulative dose. The cumulative doses among caregivers based on Hp (10) were calculated using the formula below, which took the wearing time period (7-11 days) and effective half-life (Teff) into consideration:
D2 = D1 (1-e-nλ) (16)
D1 is Hp(10) measured with the customized thermo luminescence dosimetry badge, n is the exposure duration (usually, n = 7-11 days), λ is the effective attenuation constant = 0.693/Teff, and Teff = 7.3 days. D2 is the cumulative dose to caregivers per therapy session upon hospital release.
Calculation of the radiation dose using NCRP-155: NCRP-155 previously described a dose assessment procedure for family members in accordance with the dose rate of patients following Iodine-131 treatment. In agreement with an earlier study published in INER, the formula was modified to take into account the relationship between the radiation dose received by caregivers and the radiation dose at 1 m from patients after hospital discharge. A dose assessment process for family members was previously detailed by NCRP-155 in accordance with the dose rate of patients after receiving Iodine-131 treatment. The formula was changed to consider the link between the radiation dose received by carers and the radiation dose at 1 m from patients after hospital release in accordance with an earlier study published in INER:
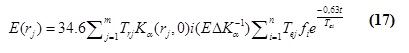 Where (E(r(j)))is the effective dose from patient after taking radioiodine at distane rj m, Tej is the occupancy factor from patient at the distance rj m, Kα (rj, 0)i is the air kerma rate from the patient at the distance rj m in the i-th term, EΔK(-1) is the conversion factor from the air kerma to effective dose Tej effective half- life of the i-th exponential term in a multiple exponential function, Fi is the absorption fraction of the metabolic compartment, t is the length of time, and n, m is the radioactivity with time dependent.
Where (E(r(j)))is the effective dose from patient after taking radioiodine at distane rj m, Tej is the occupancy factor from patient at the distance rj m, Kα (rj, 0)i is the air kerma rate from the patient at the distance rj m in the i-th term, EΔK(-1) is the conversion factor from the air kerma to effective dose Tej effective half- life of the i-th exponential term in a multiple exponential function, Fi is the absorption fraction of the metabolic compartment, t is the length of time, and n, m is the radioactivity with time dependent.
Result and Discussion
Six hundred and ninety (690) research studies out of a total of 1,090 were removed from the systematic review, because they presented duplicate data. 368 more studies were disqualified for not meeting the eligibility requirements based on title and abstract. Following independent analysis, 30 full-text publications from the remaining 32 research were determined to be appropriate for the systematic review (Figure 1). Table 1, contained the study's summary of the range of radiation dose measured by a dosimeter device at a certain distance in meter from patients and the activity of iodine-131, along with the patient disease and methods of anthropometric findings, whereas table 2, which comprised 3.3% of patients with Graves' illness, 76.7% of patients with thyroid cancer, and 23.3% of patients with hyperthyroidism, was eligible and used in this analysis. IAEA dose restrictions for iodine-131 for various groups of people were stated in table 3 in 2009. The studies reporting thyroid cancer outcomes used a variety of dosimetry approaches (Table 1), with the majority (76.7%) utilizing a variation of the approach advocated by many scientists. The progress of the iodine-131 therapy at the time of the patient's release was also documented in table 4. For patients who had iodine-131 treatment, table 5 listed the dos rate release requirements.
|
Patient no |
Patient diseases |
Radioactive iodine-131 activity administered |
Radiation dose |
Method used in measurement |
References |
|
Measured (mSv) |
|||||
|
76 |
TH |
7.02 to 16.2 (mCi) |
0.07-3.04 |
TLD |
Cappelen et al., [15] |
|
23 |
GD |
10 to 20 (mCi) |
0.08-0.09 |
TLD |
Salman et al., [16] |
|
20 |
TC |
100 to 150 (mCi) |
0.20-2.80 |
TLD |
Carvalho et al., [17] |
|
10 |
TC |
100 to 150 (mCi) |
0.021-0.67 |
TLD |
Nantajit et al., [18] |
|
20 |
TC |
150 to 200 (mCi) |
176×10-7-0.33 |
TLD |
Tonnonchiang et al., [19] |
|
51 |
TC |
100 to 200 (mCi) |
0.1-3.75 |
TLD and Analytical method |
Ebrahimi et al., [20] |
|
- |
TH & |
5 to 13.5 (mci) |
0-2.4 |
TLD |
Pant et al., [21] |
|
TC |
25 to 200 (mCi) |
0-5 |
|||
|
- |
TC |
100 to 200 (mCi) |
0.02-0.5 |
TLD & K-Factor |
Jeong et al., [22] |
|
31 |
TC |
30 (mCi) |
0.01-2.17 |
TLD & K-factor |
Lee et al., [23] |
|
33 |
TC |
100 to 200 (mCi) |
0.1-1.88 |
TLD & K-factor |
Lee et al., [23] |
|
57 |
TC |
100 to 200 (mCi) |
0.1-3.64 |
TLD & Neural Network |
Ebrahimi et al., [24] |
|
122 |
TH |
195 to 800 (MBq) |
Adult: 0.2-5.8 |
TLD |
Sally et al., [1] |
|
Child: 0.2-7.2 |
|||||
|
74 |
TC |
100 - 150 (mCi) |
Oh: 74-220 |
Aloka |
Altay et al., [25] |
|
12h: 21-116 |
TGS-172 Survey meter |
||||
|
24h: 9.6-75 |
|||||
|
36h: 0-48 |
|||||
|
48h: 29.2-36 |
|||||
|
60h: 29-33 |
|||||
|
1065 |
TC |
18 50 to 9250MBq |
Adult: 0.1-117.2 |
ALMO 3, MED Nuklear-Medizintechnik GmbH, Dresden (Rad meter) |
Hatziioannou et al., [26] |
|
Babies: 1.2-196.1 |
|||||
|
Children:0.8-100.7 |
|||||
|
Traveller: 0.2-114.9 |
|||||
|
65 |
TC |
60 to 200 (mCi) |
9.26 to 27.0 μSv/hr |
RDS 200 Multi-Purpose Radiation Survy |
LAHFI et al., [27] |
|
63 |
TC |
100-200 (mCi) |
4.76 mSv |
TLD |
Li-Yen et al., [28] |
|
35 |
TH |
100-200 (mCi) |
1h: 130 |
Dose Rate Measuring System Graetz X5C plus |
Nafal et al., [29] |
|
24h: 40.56 |
|||||
|
48h: 20.90 |
|||||
|
Female thyroid |
|||||
|
32 |
TC |
810±246 MBq |
7.1±2 mSv |
TLD-100 |
Hassan [30] |
|
796.8±58.2 MBq |
4.6±0.31 mSv |
||||
|
6 cases |
TC |
1.1 to 7.0 GBq |
123-6.75 mSv |
Monte Carlo N-Particle software |
Foreman et al., [31] |
|
25 |
TC |
30 to 100 mCi |
24h: 45-78 μSv/hr |
RDS-120 meter |
El-Sayed [32] |
|
48h: 48-65 |
|||||
|
72h: 17-53 |
|||||
|
96h: 11-37 |
|||||
|
141 |
TC |
400 MBq |
30 µSv/h |
RM- 905a |
Pingyan et al., [33] |
|
Radiation monitor |
|||||
|
60 |
TH |
370 to 1110 MBq |
20 to 66 μGy h-1 |
GRAETZ X5C plus |
Essam et al., [14] |
|
TC |
3700 to 7400 MBq |
3 to 55 μSv h-1 |
RAD meter |
||
|
176 |
TC |
600-2200 mCi |
- |
- |
Kaewputa et al., [34] |
|
24 |
TC |
2959 to 4045 MBq |
128-674 mGy |
OLINDA |
Fatin et al., [35] |
|
130-680 mGy |
IDAC |
||||
|
141-725 mGy |
EANM |
||||
|
200 |
TH |
15 to 250 mCi |
0.5 m: 7.02 mR/h |
Pocket dosimeters, |
Humara et al., [36] |
|
TC |
150 mC to above |
1m: 5.11 |
Film badges & TLDs indirect |
||
|
1.5m: 3.81 |
|||||
|
2m: 2.83 |
|||||
|
62 |
TH |
50 mCi |
9-24 µSv/h |
M-FISH Micronucleus & Comet Assa |
Alberto et al., [10] |
|
TC |
RAD51 G315C |
||||
|
40 |
TC |
30 mCi |
50.01 µSv/hmean |
RadEye™ PRD |
Mubarak et al., [11] |
|
37.3-80.0 µSv h-1 |
|||||
|
65 |
TC |
28.8 to 5.6 GBq |
M: 0.01-1.09 |
Dosimeters |
Perry et al., [37] |
|
P:0.02-1.11 mSv |
|||||
|
R:0.17 -0.58 mSv |
Table 1: An Overview of thyroid diseases, activity administered, dose rate and methods.
M=members, P=pets, R=rooms
|
Thyroid Diseases |
Percentage (%) |
|
Thyroid Cancer/Carcinoma (TC) |
76.70% |
|
Thyrotoxicosis (Hyperthyroidism) [TH] |
23.30% |
|
Grave Diseases (GD) |
3.30% |
Table 2: Descriptive statistics of the articles based on the thyroid diseases.
|
Member of the Public |
Dose Constraints |
Ref |
|
Driver of Patient treated with Iodine -131 |
0.3 mSv |
IAEA [8] |
|
Adult comforters care giver up to 60 years |
3 mSv |
|
|
Adult comforters care giver more than 60 years old |
15 mSv |
|
|
Pregnant family Members |
1 mSv |
|
|
Children family Members up to 2 years old |
1 mSv |
|
|
Children Family members 3-10 years |
1 mSv |
Table 3: Dose Constraints on iodine-131 for different Group of people as proposed by IAEA [8].
|
S/N |
Country/Organizations |
Release time Activity [mCi(MBq)] |
References |
|
1 |
Germany |
6.8(250) |
Mubarak et al., [11] |
|
2 |
Spain & New Zealand |
21.6 (800) |
|
|
3 |
China |
10.8 (400) |
|
|
4 |
Malaysia & Bangladesh |
29.5 (1100) |
|
|
5 |
France, Uk, and Poland |
21.6 (800) |
|
|
6 |
IAEA |
1100 MBq |
IAEA [8] |
|
7 |
EC |
95-800 MBq |
EC [5] |
Table 4: Activity at the time of releases.
|
Organization/Country |
Release Criteria |
References |
|
Japan |
30 µSv/h at 1m |
IAEA [8] |
|
Germany |
3.5 µSv/h at 1m |
IAEA & NRC [8,38] |
|
USA |
70 µSv/h at 1m |
NRC & NRC [9,38] |
Table 5: Dos rate Release Criteria for Patient treated with Iodine-131.
Discussion
Based on the literatures retrieved and used in this review paper was observed to have thyroid Cancer patients treated with iodine-131 accounted for 76.7% and hyperthyroidism account for 23.3% as indicated in table 2. The release criteria of patient treated with iondine-131 based on administered activity of iodine-131 in Germany, Spain & New Zealand, China, Malaysia & Bangladesh, France, UK, and Poland were 6.8, 21.6, 10.8, 29.5 and 21.6 mCi respectively as indicated in table 4. Similarly the release criteria based on dose rate measurement for patient underwent radioactive iodine-131 therapy vary according to the regions and country. A patient treated with iodine-131 in Japan, Germany, and USA will be released on 30 µSv/h, 3.5 µSv/h, and 70 µSv/h dose rate measured at one metre away as shown in table 5. The basis of radiation safety protocols is frequently the measurement of Iodine-131 retention or air absorbed dose-rate data. An individual member of the public should not receive more than 1 mSv of total effective dose from a scheduled activity per year, and family members and caregivers should not receive more than 5 mSv per year (Table 2). To ensure that the aforementioned dose constraints are not exceeded, the maximum dose for a patient receiving Iodine-131 treatment is 1110 MBq [14] and/or the dose rate at 1 m from the patient should be 50 or 70 Sv/h. The current recommendations for the patient release criteria after Iodine-131 treatment are summarized in table 4 and table 5. The patient is trying to avoid unnecessary exposure. Along with the release conditions, the individual and their family often receive extensive instructions. Instructions must be given at a dose of 20 Sv/h and must be given over an activity of 240 MBq [14]. For individuals with Graves' disease, toxic nodular disease, and well-differentiated thyroid cancer, radioactive iodine (iodine-131) therapy is a frequently used therapeutic approach, either for postoperative ablation or for the treatment of metastatic illness. It has been demonstrated to be a secure and reasonably priced kind of treatment. Typically, therapeutic dosages of iodine-131 fall between 100 and 7400 MBq. Higher doses are used to treat metastatic illness in people with well-differentiated thyroid carcinoma whereas lower activity is used to treat toxic goiter [40]. The most low iodine-131 dose reported to be permitted for outpatient therapy in many countries is 30 mCi (1110 MBq), and the use of larger doses typically necessitates hospitalization for a short period of time in a designated isolation room. It is regarded as the highest permitted radioactivity for ambulatory therapy. After receiving iodine-131 therapy, the patient may pose a radiation risk to family members and others in the home [39].
The limit on radiation exposure from patients receiving iodine-131 treatment to children and adolescents is generally agreed upon to be 1 mSv/year (Table 3) by all radiation protection-related associations but adult comforters care giver up to 60 years is 3 mSv and adult comforters’ caregiver more than 60 years old was 15 mSv (IAEA, 2009) [8] as shown in table 3. Because the risk of developing cancer increases with age after radiation exposure, the restriction on children and young adults is less severe. Children have an increased risk of acquiring cancer due to frequent exposure to ionizing radiation, which is a well-known carcinogen, and more cell division in growing and developing tissues with a predicted longer lifespan. Some nations utilize the dose rate recorded at a set distance as a standard for patient release [40,41]. The set distance can vary by up to two meters from the patient; for instance, Poland measures the dose rate at a distance of one meter from the patient, whereas Germany does so at a distance of two meters [42]. However, Iodine-131 treated patients are closely instructed at the time of release on the appropriate safety steps to be taken for the protection of anyone they may come into contact with, particularly pregnant women and children [11]. Because the general public may not be aware of the patient's condition, the radiation exposure from a patient who has received Iodine-131 mostly depends on the patient's actions and comprehension of radiation consequences. Releasing Iodine-131 treated patients is an issue here because of the socioeconomic circumstances, extended family system, nature of the profession, means of transportation, and cultural ties. Many nations currently have their own standards for discharging patients after radioactive iodine therapy based on their unique needs [42]. The yearly maximum dose limit from Iodine-131 treated patients may be exceeded by the family members and caregivers, especially toddlers and young children, while they are staying at home. For instance, utilizing the same bathroom or laundry facilities might cross-contaminate family members with radiation. Before receiving iodine, patients are typically counseled on how to behave during their period of isolation at home [43]. A practical precaution that might be implemented while adhering to these guidelines to lower the exposure risk is covering the patient’s neck area with an appropriate shielding material [11]. Due to lead's high gamma radiation blocking capacity, the use of lead collars by radioactive patients has become common in wealthy nations in recent years for the protection of public and family members from hazards of radioactive iodine-131. But due to drawbacks like high cost, burden, difficult disposal, lead toxicity, etc., different compounds are now used in place of lead for radiation protection [44,45]. Shielding materials are primarily required to be more flexible, pleasant, economical, and non-toxic so that they may be disposed of conveniently [11].
The study conducted by [36] measured dose rate from patient treated with iodine-131. In order to safely release patients who have received radioactive iodine treatment, radiation dose rates must be measured. After receiving radiopharmaceuticals, radioactive patients provide a radiation risk to other people; hence it's critical to assess the radiation dose rate nearby the patients. Although family members are permitted to receive higher doses, they should not exceed 5 mSv over a 5-year period in accordance with IAEA guidelines. The annual public dose limit is 1 mSv. Without taking into account scattered radiation or correction variables, radiation dose rates from radioactive patients were measured in this study. A total of 200 patients receiving radioactive iodine-131 treatment for thyroid cancer and thyrotoxicosis at the PIUNM cancer hospital were assessed. The measurements were made at 0.5, 1.0, 1.5, and 2.0 meters away from the thyroid tissue. The outcomes allowed for more precise evaluation of exposure rates, absorbed doses around radioactive patients, and radiological protection procedures for patient release criteria. Due to the patient's gland size, age, and renal function levels, which affect the biological half-life of I-131, the radiation dose varied from patient to patient even when the same radioactivity was supplied. The outcomes of all patients who received 5 mCi, 10 mCi, 15 mCi, 20 mCi, 25 mCi, 28 mCi, 29 mCi, 30 mCi, 100 mCi, 150 mCi, 200 mCi, and 250 mCi treatments were recorded and statistically analyzed (Table 1). According to the findings of this study, individuals who had 15mCi to 30mCi treatment shouldn't be hospitalized and should instead be sent home with instructions. Patients who receive radioactive Iodine-131 treatments ranging from 30 to 150 microcurie (mCi) should stay in the hospital for 24 to 40 hours following radioactive I-131 administration. After receiving radioactive I-131 treatments of 150 mCi to 250 mCi, patients should stay in the hospital for 60 to 72 hours as shown in (Table 1).
It is widely recognized that oxidative stress plays a significant role in the development of DNA damage caused by ionizing radiation [10]. The research conducted to investigate 62 patients who had either Thyroid Hormone Withdrawal (THW) or rhTSH preparation. The researcher examined stable and unstable genomic changes in both groups of patients prior to Iodine-131 therapy as well as one week and three months following Iodine-131 administration by Comet assay, micronuclei, and multicolor fluorescence in situ hybridization (M-FISH) to assess chromosome abnormalities and determine if thyroid residual ablation with modest activity of Iodine-131 (1850 MBq) is related to DNA damage [10]. The correlation between the genetic damage and a number of factors, such as the level of radiation-induced oxidative stress, genetic polymorphisms of DNA-repair enzymes, and anti-oxidative stress were found. Additionally, comparable numbers of DNA breaks were detected using the Comet assay and the micronuclei test in both patient groups at various time points, but patients who had undergone THW preparation had a significantly higher number of stable chromosome aberrations (breaks and translocations) detected using M-FISH [10]. Overall, increased retained body radioactivity and adverse gene polymorphism were linked to high chromosome damage. At any given time, all patients had high levels of free oxygen radicals and low levels of antioxidants. At 3 months, free oxygen radical levels were especially greater in patients treated with THW than in those prepared with rhTSH. After administering low doses of Iodine-131 to patients prepared with THW but not rhTSH, an increase in stable chromosome abnormalities relative to baseline was found. It is still unknown what these chromosomal changes mean clinically [10].
Conclusion
From the foregoing of this review, reported that increases in the incidence of thyroid cancer can be traced to iodine consumption and environmental factor such as radiation & nitrates as well as chronic lymphocytic thyroiditis. Radio Active Iodine (RAI) is a safe, definitive, and cost-effective modality of treatment that is used as the first line of treatment for thyroid related diseases by most endocrinologists. If patients receiving radioiodine for thyroid diseases adhere to sound advice regarding limiting close contact with others and stay below the updated lower ICRP dose limits, they can be treated safely as outpatients with activities up to 800 MBq. Patients receiving 200, 400, 600, or 800 MBq given activities should be encouraged to sleep alone for 1, 5, or 12 days so that their spouses can adhere to a 3-mSv dose restriction. Parents who get 200 MBq, 400 MBq, 600 MBq, or 800 MBq should be encouraged to intentionally avoid contact with children under the age of 5 for 5, 11, 14 or 16 days, or with children over the age of 5 for 5, 11, 16 or 20 days, in order to adhere to a 1-mSv restriction. The release criteria of patient treated with iondine-131 based on administered activity of iodine-131 in Germany, Spain & New Zealand, China, Malaysia & Bangladesh, France, UK, and Poland were 6.8, 21.6, 10.8, 29.5 and 21.6 mCi respectively. Similarly the release criteria based on dose rate measurement for patient underwent radioactive iodine-131 therapy vary according to the regions and country. A patient treated with iodine-131 in Japan, Germany, and USA will be released on 30 µSv/h, 3.5 µSv/h, and 70 µSv/h dose rate measured at one metre away. Only when patients have children who are 3 years old or less and arrangements cannot be made for a substitute caretaker for the kid, may hospital admission be necessary. After administering low dosages of 131I, patients treated with THW show an increase in stable chromosome abnormalities compared to baseline. These chromosomal changes' clinical significance has yet to be established. Due to lead's high gamma radiation blocking capacity, the use of lead collars by radioactive patients has become common in wealthy nations in recent years for the protection of public and family members from hazards of radioactive iodine-131.
Acknowledgement
The author gave due credit to all the researchers whose scientific work was extremely helpful in completing this review.
Competing of Interest
No competing of interest
References
- Barrington SF, Doherty MJO, Kettle AG, Thomson WH, Mountford PJ, et al. (1999) Radiation exposure of the families of outpatients treated with radioiodine (iodine-131) for hyperthyroidism. Eur J Nucl Med 26: 678-692.
- Kadhim AA, Sheikhzadeh P, Farzanefar S, Yavari S, Jber MM, et al. (2020) Measurement of radiation exposure to caregivers of patients with thyroid diseases treated with Iodine-131: A review. Frontiers in Biomedical Technologies 7: 192-200.
- Liu H, Chen B, Zhuo W (2021) A progress review on methods for in vivo measurement of Iodine-131 in thyroids by using portable gamma spectrometers. Radiation Medicine and Protection 2: 155-159.
- International Atomic Energy Agency (1996) International basic safety standards for protection against ionizing radiation and for the safety of radiation sources. Safety series No.115. International Atomic Energy Agency, Vienna, Austria.
- European Commission (1998) Radiation protection following iodine-131 therapy (exposures due to outpatients or discharged inpatients). Directorate-General for Environment (European Commission), European Commission, Luxembourg, Europe.
- International Commission on Radiological Protection (2008) ICRP Publication 103: Recommendations of the ICRP. Radiation Protection Dosimetry 129: 500-507.
- International Commission on Radiological Protection (2004) Release of patients after therapy with unsealed radionuclides. Ann ICRP 34: 1-79.
- International Atomic Energy Association (2009) Release of patients after radionuclide therapy. International Atomic Energy Associat, Vienna, Austria.
- S. Nuclear Regulatory Commission (1997) Regulatory Guide 8.39: Release of Patients Administered Radioactive Materials. U.S. Nuclear Regulatory Commission, Washington, DC, USA.
- Signore A, Campagna G, Marinaccio J, Vitis M, Lauri C, et al. (2022) Analysis of short-term and stable DNA damage in patients with differentiated thyroid cancer treated with 131I in hypothyroidism or with recombinant human thyroid-stimulating hormone for remnant ablation. J Nucl Med 63: 1515-1522.
- Mubarak S, Nanayakkara D, Jayalath C, Sivakumar V (2021) Low dose radioiodine therapy: A review of dosimetry and evaluation of potential shielding materials for neck collars. J Nat Sc Foundation Sri Lanka 49: 583-591.
- Madu NM, Skinner C, Oyibo SO (2022) Cure rates after a single dose of radioactive iodine to treat hyperthyroidism: The fixed-dose regimen. Cureus 14: 28316.
- Rashidi H, Ghaderian B, Sedaghat A, Latifi M, Naimi Z (2022) Effect of iodine-therapy on hyperthyroidism patients without pre-administration of anti-thyroid therapeutic options. Revis Bionatura 7: 7.
- Mattar E, Salih MA, Alsafi K, Suliman II (2019) Radiation protection in the release of patients receiving 131I treatment. Radiat Prot Dosimetry 187: 499-508.
- Cappelen T, Unhjem JF, Amundsen AL, Kravdal G, Følling I (2006) Radiation exposure to family members of patients with thyrotoxicosis treated with iodine-131. Eur J Nucl Med Mol Imaging 33: 81-86.
- Salman KH, Wagih S, Munshi T, Almalki M, Zatar S, et al. (2018) Measurement of radiation exposure to household contacts of patients with Graves’ disease treated with low dose radioactive iodine (131I) on outpatient basis. The Egyptian Journal of Radiology and Nuclear Medicine 49: 1125-1130.
- de Carvalho JW, Sapienza M, Ono C, Watanabe T, Guimarães MI, et al. (2009) Could the treatment of differentiated thyroid carcinoma with 3.7 and 5.55 GBq of (131I)NaI, on an outpatient basis, be safe? Nucl Med Commun 30: 533-541.
- Nantajit D, Saengsuda S, NaNakorn P, Saengsuda Y (2015) High-dose radioiodine outpatient treatment: An initial experience in Thailand. Asia Ocean J Nucl Med Biol 3: 66-71.
- Tonnonchiang S, Sritongkul N, Chaudakshetrin P, Tuntawiroon M (2016) Radiation Exposure to Relatives of Patients Treated with Iodine-131 for Thyroid Cancer at Siriraj Hospital. J Med Assoc Thai 99: 220-224.
- Ebrahimi M, Changizi V, Kardan MR, Pooya SMH, Geramifar P (2016) Experimental and analytical dose assessment of patient’s family members treated with I-131. Physica Medica 32: 292.
- Pant GS, Sharma SK, Bal CS, Kumar R, Rath GK (2006) Radiation dose to family members of hyperthyroidism and thyroid cancer patients treated with 131I. Radiat Prot Dosimetry 118: 22-27.
- Jeong KH, Jung JW, Kim CB, Ahn BC, Lee HK, et al. (2014) Estimation of external radiation dose to caregivers of patients treated with radioiodine after thyroidectomy. Health Phys 106: 466-474.
- Lee HK, Hong SJ, Jeong KH, Jung JW, Kim SM, et al. (2015) An engagement factor for caregiver radiation dose assessment with radioiodine treatment. Radiat Prot Dosimetry 163: 499-508.
- Ebrahimi M, Kardan MR, Changizi V, Pooya SMH, Geramifar P (2018) Prediction of dose to the relatives of patients treated with radioiodine-131 using neural networks. J Radiol Prot 38: 422-433.
- Myssayev A, Myssayev A, Ideguchi R, Nishi K, Fukuda N, et al. (2020) Estimation of clearance rate of Iodine-131 from thyroid cancer patients based on individual patient’s characteristic. Research square.
- Hatziioannou K, Papanastasiou E, Badiavas K, Zapros A, Iakovou I (2020) Absorbed dose estimation to cohabitants and co-travelers of patients treated with radioiodine for differentiated thyroid carcinoma. Hell J Nucl Med 23: 173-179.
- Lahfi Y, Anjaq O (2018) Radiation exposure assessment from patient treated or diagnostic by radioiodine. IAEA, Syrian Arab Republic, Syria.
- Chen L-Y, Huang Y-Y, Lee P-I, Chan S-C, Lin K-H, et al. (2020) Radiation safety assessment of caregivers of thyroid cancer patients treated with 131I in Taiwan. Radiation Physics and Chemistry 172: 108781.
- Bahjat NN, Abbas ZN (2020) Estimation of radiation exposure to some iraqi patients with hyperthyroidism treated with radioactive Iodine-131 as an outpatient basis. Indian Journal of Forensic Medicine & Toxicology 14: 1328-1333.
- Ibrahim HS (2021) evaluation of radiation exposure for the employees and patients at nuclear medicine departments. PhD thesis in Medical Physics Submitted to Sudan University of Science and Technology College of Graduate Studies, Khartoum, Sudan.
- Foreman C, Dewji S (2020) estimation of external dose rates to hotel workers from bed linens contaminated by 131I patients. Health Phys 118: 615-622.
- El-Sayed Mansour MS (2021) Release timing of thyroid’s carcinoma patients from the hospital after Radiodine (I-131) therapy based on dose rate measurements. J Rad Nucl Appl 6: 151-154.
- Jin P, Feng H, Ouyang W, Wu J, Chen P, et al. (2018) Radiation dose rates of differentiated thyroid cancer patients after 131I Radiat Environ Biophys 57: 169-177.
- Kaewput C, Pusuwan P (2021) Outcomes following I-131 treatment with cumulative dose exceeding or equal to 600 mCi in differentiated thyroid carcinoma patients. World J Nucl Med 20: 54-60.
- Halim F, Yahya H, Hamzah F, Hashim H, Mansor S (2021) Evaluation of absorbed dose estimation accuracy in post radioiodine therapy for differentiated thyroid disease: A comparative study. Malaysian Journal of Medicine and Health Sciences 17: 1-6.
- Humara, N, Saira Z, Muhammad AN (2018) Evaluation of reduction in radiation dose rates from patients receiving Iodine-131 therapy for thyrotoxicosis and thyroid cancer. Journal of Advanced Research Design 44: 20-29.
- Grigsby PW, Siegel BA, Baker S, Eichling JO (2000) Radiation exposure from outpatient radioactive iodine (131I) therapy for thyroid carcinoma. JAMA 283: 2272-2274.
- Nuclear Regulatory Commission (2008) Consolidated Guidance about Materials Licensees: Program Specific Guidance about Medical Use Licenses. Nuclear Regulatory Commission, Maryland, USA.
- Yin L, Li W, Lv X, Lin Y Q, Jia Q, et al. (2022) Effects of high-activity radioactive iodine treatment on renal function in patients with differentiated thyroid carcinoma-retrospective study. Endokrynol Pol 73: 619-626.
- Zanzonico PB, Siegel JA, Germain J St (2000) A generalized algorithm for determining the time of release and the duration of post-release radiation precautions following radionuclide therapy. Health Phys 78: 648-659.
- Hewamanna R, Loganathan N, Perera DKA (2014) Releasing thyroid cancer patients from the hospital based on dose rate measurement after Iodine-131 activity administration. Journal of the National Science Foundation of Sri Lanka 42: 137-142.
- Holian BE (2014) International patient release practices following iodine-131 therapy. European Commision, Strasbourg, France.
- Radiation Protection 97 (1998) Radiation Protection Following Iodine 131 Therapy. European Commision, Strasbourg, France.
- Nambiar S, Yeow JTW (2012) Polymer-composite materials for radiation protection. ACS Applied Materials & Interfaces 4: 5717-5726.
- Nambiar S, Osei EK, Yeow JTW (2012) Polymer nanocomposite-based shielding against diagnostic X-rays. Journal of Applied Polymer Science 127: 4939-4946.
Citation: Samaila B (2022) Radiation Exposure Appraisal to Public, Caregivers and Families in Contact with Thyroid Diseases Patient Undergoing Radioactive Iodine-131 Therapy: A Systematic Review. J Otolaryng Head Neck Surg 8: 076.
Copyright: © 2022 Buhari Samaila, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

