
Journal of Nuclear Medicine Radiology & Radiation Therapy Category: Medical
Type: Research Article
Radiochemical Technology for Production of Preparations of Technetium - 99m on Extraction Centrifugal Semi-Countercurrent Generator
*Corresponding Author(s):
Filyanin ATAn Frumkin Institute Of Physical Chemistry And Electrochemistry Of Ras, Moscow, Russian Federation
Tel:+7 926 8854820,
Email:a.filyanin@yandex.ru
Received Date: Mar 31, 2017
Accepted Date: May 22, 2017
Published Date: Jun 12, 2017
Abstract
The results of the development and long-term operation of the technetium-99m extraction generator and use of Radiopharmaceuticals (RP) prepared on its base are presented. The main advantage of such generator is the possibility of separation of the molybdenum and technetium isotopes obtained with the use of any starting materials (both enriched and natural stable isotopes) as well as any irradiators (reactor and accelerators) to obtain the product - sodium [99mTc] pertechnetate solution with the necessary radioactive concentration and radiochemical purity ≥ 95%.
Keywords
Extraction; Radiopharmaceutical; Semi-countercurrent centrifugal extractor; Technetium-99m
INTRODUCTION
The problems of separation of elements with different chemical properties are solved during production of most short-lived radionuclides, used in medicine as well as in the other fields of science and technology. Mostly, these are elements with different valence state for which selective, well studied and available extraction systems can be found, so that the separation coefficients in those systems are higher than 100. In our opinion, the extraction semi-countercurrent separation method is most suitable for the practical solution of such problems. The essence of this method is that one of the phases (for example, aqueous) is kept in one or several stages not communicating in this phase, while the organic phase (extractant), passes successively through all stages, extracting the extractable components of the mixture of elements in decreasing order of values of the distribution coefficients. The process can also, if necessary, be carried out in inversed form, when the organic phase is stationary and the selective extraction process is carried out by an aqueous solution.
The classical semi-countercurrent process and its calculations are well known [1,2]. The semi-counter current extraction process of separation of elements in a dynamic mode was first described in 1951 [3].
The main feature of semi-countercurrent separation method is the fact that, if the initial mixture of the components is in the aqueous phase, than the less extractable component can be obtained with any purity level, but the yield continuously decreases. At the same time, the more extracted component has a maximum of the concentration rate equal to the value of the separation coefficient at the beginning of the extraction process.
In semi-counter current extractors of the mixer-settler types with gravitational phase separation were used [1-3]. The main disadvantages of such devices are high retention volume of the mobile phase, low efficiency and productivity, as well as the possibility of entrainment of the emulsion.
Semi-countercurrent processes are used in the separation of similar components in Craig apparatuses, but they are of little use for radiochemical purposes for many reasons: low stratification rate, complex and cumbersome construction, long time needed to achieve equilibrium when the process of mixing is simple wiggle.
One of the first papers describing the use of centrifugal semi-countercurrent extractors for the separation of multi-component mixtures requiring a large number of stages was published in 1974 by GV Korpusov et al., [4]. The centrifugal extractor considered is not intended to be used for obtaining individual radionuclide’s, since in this case the requirements for the purity of the product are much more stringent at a high recovery rate [4].
Considering the need to implement the process of isolation of short-lived isotopes in a short period of time, semi-counter flow extractors with forced phase separation in a centrifugal field were developed. The use of such apparatuses makes it possible to shorten the time of the process and increase the purity of the products, since in this case intensive mixing is acceptable (equilibrium in the extraction system is achieved in 2-3 seconds) and phase separation in the centrifugal field excludes the entrainment of the emulsion.
The process of continuous organic solvent feeding into the initial aqueous solution, containing the components to be recovered, is realized in the extractor.
Passing through the mixing chamber and the separator, the solvent sequentially extracts the components of the mixture in accordance with the decrease in their distribution coefficients (D2> D1) (Figures 1 and 2).
The classical semi-countercurrent process and its calculations are well known [1,2]. The semi-counter current extraction process of separation of elements in a dynamic mode was first described in 1951 [3].
The main feature of semi-countercurrent separation method is the fact that, if the initial mixture of the components is in the aqueous phase, than the less extractable component can be obtained with any purity level, but the yield continuously decreases. At the same time, the more extracted component has a maximum of the concentration rate equal to the value of the separation coefficient at the beginning of the extraction process.
In semi-counter current extractors of the mixer-settler types with gravitational phase separation were used [1-3]. The main disadvantages of such devices are high retention volume of the mobile phase, low efficiency and productivity, as well as the possibility of entrainment of the emulsion.
Semi-countercurrent processes are used in the separation of similar components in Craig apparatuses, but they are of little use for radiochemical purposes for many reasons: low stratification rate, complex and cumbersome construction, long time needed to achieve equilibrium when the process of mixing is simple wiggle.
One of the first papers describing the use of centrifugal semi-countercurrent extractors for the separation of multi-component mixtures requiring a large number of stages was published in 1974 by GV Korpusov et al., [4]. The centrifugal extractor considered is not intended to be used for obtaining individual radionuclide’s, since in this case the requirements for the purity of the product are much more stringent at a high recovery rate [4].
Considering the need to implement the process of isolation of short-lived isotopes in a short period of time, semi-counter flow extractors with forced phase separation in a centrifugal field were developed. The use of such apparatuses makes it possible to shorten the time of the process and increase the purity of the products, since in this case intensive mixing is acceptable (equilibrium in the extraction system is achieved in 2-3 seconds) and phase separation in the centrifugal field excludes the entrainment of the emulsion.
The process of continuous organic solvent feeding into the initial aqueous solution, containing the components to be recovered, is realized in the extractor.
Passing through the mixing chamber and the separator, the solvent sequentially extracts the components of the mixture in accordance with the decrease in their distribution coefficients (D2> D1) (Figures 1 and 2).
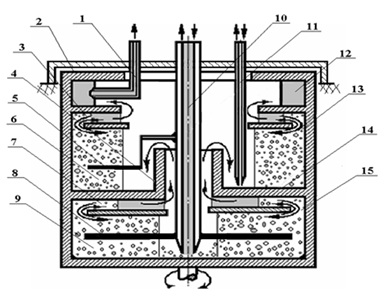
Figure 1: Basic circuit of a two-stage centrifugal extractor.
drain tube for extract withdrawal
rotating housing
stratifying chamber of a washing stage
cylinder for light phase flow from the first to the second stage
angular stirrer of a washing stage
mixing hamber of a washing stage
stratifying chamber of extraction stage
stirrer of extraction stage
mixing chamber of extraction stage
central tube
tube for filling and emptying of washing solution
annular groove
annular gap of a washing stage
annular partition
annular gap of extraction stage
rotating housing
stratifying chamber of a washing stage
cylinder for light phase flow from the first to the second stage
angular stirrer of a washing stage
mixing hamber of a washing stage
stratifying chamber of extraction stage
stirrer of extraction stage
mixing chamber of extraction stage
central tube
tube for filling and emptying of washing solution
annular groove
annular gap of a washing stage
annular partition
annular gap of extraction stage
The organic phase can be washed in the same manner, but in this case the less extractable component of the mixture is washed out first (1/D1; 1/D2).
Both processes can be described by the following equations:
Extraction
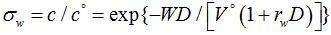
Washing
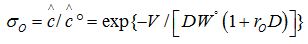
Where: σ&w, σo - relative concentrations of the extracted or washed out components in the aqueous (in the case of extraction) and organic (in the case of washing) phases;
C0, C - initial and final concentrations of the extracted component
?0, ? - initial and final concentrations of the washed out component
D - Distribution coefficient
W - Feed rate of extractant
W0- Volume of initial extract
V0- Volume of initial aqueous phase
V –feed rate of washing solution
rw, ro - the ratio of phases in the emulsion during extraction and washing processes, respectively
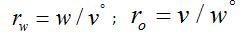
Depending on the task, there are two types of these devices: with stationary aqueous or organic phase. The calculation methods are the same, and there is only one thing that changes: the inverse values of the distribution coefficients (1/D) are used for calculations in the case of a stationary organic phase.
The semi-countercurrent extraction process is carried out as follows: a stationary phase (aqueous or organic) containing a mixture of elements to be separated is introduced into the apparatus in a certain volume. After that a stream of extractant or aqueous solution, respectively is passed through it. The effective operation of the extraction generators of radioactive isotopes is equally depends on the selected extraction system, the properly organized process of isolation and the design features of the extractor.
A number of specific requirements are presented to extraction generators used for the production of radionuclides:
• Complete elimination of phase entrainment
• Absence of stagnation zones
• Possibility of phase inversion
The design of the developed extraction centrifugal apparatus must also satisfy certain functional requirements:
• Possibility of combining the basic typical operations in one device (extraction, washing, re-extraction and fraction collection)
• Functional adjustment of isolated chambers with the possibility of independent filling, emptying and rinsing
• Creation of conditions for additional clarification of outflow solutions
MODERN APPROACHES TO OVERCOMING THE “MOLYBDENUM CRISIS”
Technetium-99m is one of the most widely used radionuclides in diagnostic nuclear medicine during the entire period of formation and development of nuclear medicine. Technetium-99m decays emitting only gamma rays with photon energy of 0.14 MeV. This low energy, as well as the absence of β - radiation and a short half-life (6.02 h) minimizes radiation hazard of technetium-99m for the patient [6].
The parent isotope for the production of technetium-99m is molybdenum-99, which can be obtained by irradiation of natural or enriched molybdenum-98 with neutrons and also can be isolated from the mixture of fission products of uranium-235. Obtaining 99Mo from irradiated uranium is an independent, expensive, complex problem requiring deep purification of the final product from radionuclide impurities, such as 131I, 103Ru, 95Zr, etc. Practically all survey papers on 99mTc and 99Mo contain information on the advantages and disadvantages of both nuclear reactions. In both cases, the target must be irradiated in a nuclear reactor with a neutron flux of more than 5×1013 n/ (cm2.s) [7].
Since 2007, in the context of the world-wide “Molybdenum crisis” (which was caused by the almost simultaneous shutdown of reactors in Canada and Holland, that providing more than 60% of the world production of 99Mo), alternative ways of molybdenum-99 production using accelerators took another development [8-10].
99Mo can be produced by the reaction 100Mo (p, pn) 99Mo, as well as 99mTc directly, with high yields, using the charged particle accelerators (for example see [9]). Several studies have been published on the determination of the yields of various radionuclide’s during irradiation of molybdenum targets by protons [11], deuterons [12] and photonuclear reactions were also studied [13,14]. In the review the problems, advantages and disadvantages of all currently available sources and possibilities for obtaining 99mTc are discussed in detail, since the exceptional value and necessity of this radionuclide in modern nuclear medicine does not lose its significance [15].
Now several research projects are being implemented in this field and leading manufacturers of medium-energy cyclotrons (GE Healthcare, IBA and Advanced Cyclotron Systems Inc.) actively offer to their customers to consider the possibility of technetium-99m production in operating PET-centers.
The most complete studies were carried out by a group of Canadian specialists who has now completed preclinical studies of specific radiopharmaceuticals with cyclotron produced 99mTc. The.The results of first clinical trials were published as well. The effect of radionuclide impurities, formed during nuclear reactions on accelerators, on radiation dose received by patients and the quality of SPECT imaging were studied [16-18]. When 99mTc is obtained from 99Mo produced on the reactor or accelerator, or when 99mTc is directly obtained as a result of the nuclear reaction, there is a problem of the subsequent separation of technetium and molybdenum isotopes. The use of a semi-countercurrent centrifugal extractor for solving this problem is discussed below.
The parent isotope for the production of technetium-99m is molybdenum-99, which can be obtained by irradiation of natural or enriched molybdenum-98 with neutrons and also can be isolated from the mixture of fission products of uranium-235. Obtaining 99Mo from irradiated uranium is an independent, expensive, complex problem requiring deep purification of the final product from radionuclide impurities, such as 131I, 103Ru, 95Zr, etc. Practically all survey papers on 99mTc and 99Mo contain information on the advantages and disadvantages of both nuclear reactions. In both cases, the target must be irradiated in a nuclear reactor with a neutron flux of more than 5×1013 n/ (cm2.s) [7].
Since 2007, in the context of the world-wide “Molybdenum crisis” (which was caused by the almost simultaneous shutdown of reactors in Canada and Holland, that providing more than 60% of the world production of 99Mo), alternative ways of molybdenum-99 production using accelerators took another development [8-10].
99Mo can be produced by the reaction 100Mo (p, pn) 99Mo, as well as 99mTc directly, with high yields, using the charged particle accelerators (for example see [9]). Several studies have been published on the determination of the yields of various radionuclide’s during irradiation of molybdenum targets by protons [11], deuterons [12] and photonuclear reactions were also studied [13,14]. In the review the problems, advantages and disadvantages of all currently available sources and possibilities for obtaining 99mTc are discussed in detail, since the exceptional value and necessity of this radionuclide in modern nuclear medicine does not lose its significance [15].
Now several research projects are being implemented in this field and leading manufacturers of medium-energy cyclotrons (GE Healthcare, IBA and Advanced Cyclotron Systems Inc.) actively offer to their customers to consider the possibility of technetium-99m production in operating PET-centers.
The most complete studies were carried out by a group of Canadian specialists who has now completed preclinical studies of specific radiopharmaceuticals with cyclotron produced 99mTc. The.The results of first clinical trials were published as well. The effect of radionuclide impurities, formed during nuclear reactions on accelerators, on radiation dose received by patients and the quality of SPECT imaging were studied [16-18]. When 99mTc is obtained from 99Mo produced on the reactor or accelerator, or when 99mTc is directly obtained as a result of the nuclear reaction, there is a problem of the subsequent separation of technetium and molybdenum isotopes. The use of a semi-countercurrent centrifugal extractor for solving this problem is discussed below.
SELECTION OF THE EXTRACTION SYSTEM FOR THE SEPARATION OF 99MO AND 99MTC
In addition to sorption methods, which are extremely useful in the case of preparing a generator for clinical use, the extraction can also be used to separate molybdenum and technetium. The regularities of the extraction separation of 99Mo and 99mTc in various systems have been studied in detail as early as the 1960s [19]. For the extraction separation of this pair of elements, it is possible to use various extractants, such as MEK, methyl isobutyl ketone, as well as organ phosphorus compounds [20,21]. In this case, the distribution coefficients of these elements differ by 2-3 orders of magnitude, which make it possible to achieve high values of Separation Coefficients (Ks). For example, when extracting 99mTc from 5 M NaOH solution in MEK (100%), the value of Ks = 2×105 and from 5 M K2CO3 ?s= 4×105. Therefore, a semi-countercurrent process can be performed on a limited number of steps (from 1 to 3). It is rational to use a mixture of alkali and carbonate, which plays the role of a salting out agent. One of the first technological schemes on the basis of this system for obtaining 99mTc of pharmaceutical quality in a countercurrent glass column was published by R Boyd in 1982 [22]. Later this scheme was applied in different countries. During the extraction separation of 99mTc and 99Mo using 100% MEK, the extract is evaporated to dryness, and the dry residue containing 99mTc is dissolved in 0.9% NaCl solution. According to the requirements of US Pharmacopeia a relatively high MEK content (≤ 0.1%) in the product (sodium [99mTc] pertechnetate) is allowed [23].
It should be noted that in the case of use of enriched molybdenum targets for irradiation with necessity of following their regeneration for repeated irradiation, only potassium should be used as the alkali metal. This is necessary to exclude the possibility of sodium traces presence in the target and correspondingly, radionuclide impurities presence in the final product.
It should be noted that in the case of use of enriched molybdenum targets for irradiation with necessity of following their regeneration for repeated irradiation, only potassium should be used as the alkali metal. This is necessary to exclude the possibility of sodium traces presence in the target and correspondingly, radionuclide impurities presence in the final product.
TWO-STAGE EXTRACTION CENTRIFUGAL SEMI-COUNTERCURRENT GENERATOR FOR 99MTC PRODUCTION
To implement the process of 99mTc production for radiopharmaceuticals, a centrifugal two-stage extractor integrating the extraction and washing stages was created (Figure 1). The generator works as follows; A certain volume of the initial solution in the form of sodium (or potassium) molybdate is introduced into the mixing chamber (9) of the lower stage through the central tube (10). The second stage is filled with a washing solution (5 M K2CO3) through the tube (11).
After filling the apparatus, an electric motor is started using a starting device. Electric motor rotates the generator housing (2). During these operations the tube (11), as well as the central tube (10), does not come into contact with the solutions in the chambers (6) and (9), because they are thrown to the periphery by centrifugal forces. Then, the dosing device is turned on and the extractant (MEK) is fed to the mixing chamber (9) via the central tube (10) with a predetermined flow rate. In the mixing chambers (9) and (6) uniform mixing of the phases occurs with the stirrers (8) and (5) respectively. The resulting emulsion exits from the mixing chamber (9) through the annular gap (15) and enters the stratifying chamber (7) where it is stratified to form light and heavy phases under the action of centrifugal forces.
As the light phase exits the mixing chamber (9) to enter into the stratifying chamber (7), it reaches the level of the water seal (14) and then it rises along the inner surface of the cylinder (4) and enters the second mixing chamber (6). The height and inner diameter of the cylinder (4) are preliminarily determined by calculation. Depleted on the light phase emulsion in the stratifying chamber (7) through the annular gap (15) returns again to the mixing chamber (9), creating recirculation.
In the second (washing) stage, the hydrodynamic processes are repeated and the light phase (99mTc extract) comes from the stratifying chamber (3) into the annular groove (12) from which it is withdrawn from the extractor with the tube (1).
The main structural material of the extractor was stainless steel. Stirring devices which contacting with solutions was made of inert metals: niobium, tantalum and zirconium.
The extractor, with stages located on one rotation axis in the vertical position, is attached to the electric motor shaft. The operating speed is 2000-2500 rpm.
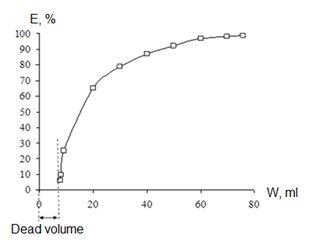
It should be noted that the extractor has high reliability in operation due to the fact that there are no rubbing points or contact points between the rotating drum and fixed parts (tubes, mixers). The operating time of the apparatus is determined by the engine life. The radiation dose from 99Mo and 99mTc virtually do not affect the life of the engine. Figure 2 shows the dependence of the 99mTc extraction degree on the fed extractant volume. As can be seen from the data given, in the chosen mode, 50-60 ml of extractant is sufficient to extract 95% of 99mTc. At an organic phase feed rate of 600-1000 ml/h, the time of the extraction course will be 5-10 minutes, respectively.
After filling the apparatus, an electric motor is started using a starting device. Electric motor rotates the generator housing (2). During these operations the tube (11), as well as the central tube (10), does not come into contact with the solutions in the chambers (6) and (9), because they are thrown to the periphery by centrifugal forces. Then, the dosing device is turned on and the extractant (MEK) is fed to the mixing chamber (9) via the central tube (10) with a predetermined flow rate. In the mixing chambers (9) and (6) uniform mixing of the phases occurs with the stirrers (8) and (5) respectively. The resulting emulsion exits from the mixing chamber (9) through the annular gap (15) and enters the stratifying chamber (7) where it is stratified to form light and heavy phases under the action of centrifugal forces.
As the light phase exits the mixing chamber (9) to enter into the stratifying chamber (7), it reaches the level of the water seal (14) and then it rises along the inner surface of the cylinder (4) and enters the second mixing chamber (6). The height and inner diameter of the cylinder (4) are preliminarily determined by calculation. Depleted on the light phase emulsion in the stratifying chamber (7) through the annular gap (15) returns again to the mixing chamber (9), creating recirculation.
In the second (washing) stage, the hydrodynamic processes are repeated and the light phase (99mTc extract) comes from the stratifying chamber (3) into the annular groove (12) from which it is withdrawn from the extractor with the tube (1).
The main structural material of the extractor was stainless steel. Stirring devices which contacting with solutions was made of inert metals: niobium, tantalum and zirconium.
The extractor, with stages located on one rotation axis in the vertical position, is attached to the electric motor shaft. The operating speed is 2000-2500 rpm.
It should be noted that the extractor has high reliability in operation due to the fact that there are no rubbing points or contact points between the rotating drum and fixed parts (tubes, mixers). The operating time of the apparatus is determined by the engine life. The radiation dose from 99Mo and 99mTc virtually do not affect the life of the engine. Figure 2 shows the dependence of the 99mTc extraction degree on the fed extractant volume. As can be seen from the data given, in the chosen mode, 50-60 ml of extractant is sufficient to extract 95% of 99mTc. At an organic phase feed rate of 600-1000 ml/h, the time of the extraction course will be 5-10 minutes, respectively.
EXTRACTION APPARATUS FOR THE SEPARATION OF TECHNETIUM-99M FROM THE IRRADIATED MOLYBDENUM MATRIX
To obtain the final sodium [99mTc] pertechnetate, it is required to use an installation that includes, apart from the extractor, additional processing equipment. The diagram of such an installation is shown in figure 3.

Figure 3: The scheme equipment layout for obtaining sodium [99mTc] pertechnetate.
1- Reactor-glass vessel for dissolving of irradiated ???3 in 5 ?NaOH
2- Waste collector
3- two-stage semi-countercurrent centrifugal extractor
4- Intermediate vessel for initial sodium molybdate solution
5- Measuring vessel for extractant feeding
6- Condenser for MEK vapor
7- Collector –glass flask with a ground joint for receiving the extract (MEK)
8- Peristaltic pump
9- Ultratermostat
10- Evaporator
11- Column with Al2O3
12- Aerosol filter (air)
13- Dispensing vessel
14- Collector for washing solution
15- 16, 17 External vessels for feeding K2CO3; MEK; 0,9 % NaCl into the system
16- Dispensing devices
2- Waste collector
3- two-stage semi-countercurrent centrifugal extractor
4- Intermediate vessel for initial sodium molybdate solution
5- Measuring vessel for extractant feeding
6- Condenser for MEK vapor
7- Collector –glass flask with a ground joint for receiving the extract (MEK)
8- Peristaltic pump
9- Ultratermostat
10- Evaporator
11- Column with Al2O3
12- Aerosol filter (air)
13- Dispensing vessel
14- Collector for washing solution
15- 16, 17 External vessels for feeding K2CO3; MEK; 0,9 % NaCl into the system
16- Dispensing devices
The connection of all vessels and pumps is carried out by chemically stable and flexible polymer pipes with an internal diameter of 2-5 mm.
The initial solution of sodium molybdate is introduced into the lower extraction stage of the extractor, and then the process of extraction (and washing of the extract) of technetium-99m is realized as described above. At the end of the process, the extract enters the evaporator and gets evaporated to dryness. The dry residue is dissolved in 0.9% NaCl solution and, after analysis, is dispensed for transfer to consumers. The subsequent cycles of technetium extraction are carried out according to the scheme above, taking into account the half-life of the parent molybdenum-99.
The first facility for the production of 99mTc radiopharmaceuticals was placed in the Khlop in Radium Institute in a line of two hot cells and one laminar box, arranged in series.
In the first hot cell, the irradiated target was disclosed and the molybdenum trioxide was dissolved. An extractor and an evaporator were set in the second hot cell. The dissolution of the irradiated target is carried out once a week, and the extraction of technetium-99m is carried out daily. In the laminar box, the final product is dispensed in vials, sterilized and after receiving the results of quality control is sent to the clinics.
Evaluation of the cost of radiopharmaceuticals obtained with the extraction generator showed that they are much cheaper (2-5 times) than sodium pertechnetate, obtained from sorption generators. This is primarily because of the huge material costs associated with the irradiation of uranium-235 and the processing of waste generated. In the case of the use of enriched molybdenum-98, it was regenerated and re-used after processing.
To date, more than 20 years of experience have been accumulated in the operation of such a facility for the needs of nuclear medicine laboratories in St. Petersburg. The increase in the demand for technetium-99m in clinics led to the necessity of the increase in production volume and later the parallel work of the two extractors was organized.
Usually, a fresh molybdenum-99 solution is loaded into the extractor on Friday evening. At the same time, the “blank” extraction by MEK is carried out to remove all recoverable chemical and radionuclide impurities from the molybdenum-99 solution. The separation of technetium-99m from the solution of molybdenum-99 for the run-up of a series of preparations is carried out on Monday morning (after 2.5 days after “blank” extraction) and then every other day for 5 days. At the moment of molybdenum dissolution and loading into the extractor, its activity is usually 200-300 GBq (~ 6.0 - 8.0 Ci). The yield of technetium-99m into the final prepackaged product is 85 to 90%, taking into account the decay at the beginning of the extraction of technetium-99m from the molybdenum-99 solution. There was no decrease in the yield of technetium-99m during the whole working week.
A study of the possible consequences of MEK radiolysis in the extractor was carried out in [24,25]. Low boiling radiolysis products are removed together with MEK during its evaporation and do not affect the quality of the finished product. The main products of radiolysis with a boiling point higher than those of MEK are its dimmers, the amount of which in the extract (with the above-mentioned 99Mo loads) are comparable to their content in the initial reagent and practically do not affect the quality of the preparation obtained.
The product is delivered to medical institutions with in no more than 2 hours. The number of such institutions is from 5 to 15 depending on their applications. The amount of the product delivered varies from 40 to 150 GBq (1-4 Ci). According to statistics, unscheduled stops that led to the termination of deliveries throughout the workweek occurred on average 1 time in three years, which was due to various reasons: the problem with the supply of irradiated targets, the failure of auxiliary equipment, the human factor, etc. During the whole operation of the system, no series of products was rejected because of a lack of quality. The quality indices of the five series of technetium-99m preparations at the time of manufacture and at the end of its shelf life (18 hours) are presented in comparison with the chromatography generator eluate quality indicators (Table 1).
| Requirements to the quality of pertechnetate solution | Batch No | Elute from chromatographic generator | ||||||
| 1 | 2 | 3 | 4 | 5 | ||||
| Appearance | Clear colorless liquid | Clear colorless liquid | Clear colorlessliquid | |||||
| Identificationtest | 99mTc | corresponds | corresponds | corresponds | ||||
| Na | corresponds | |||||||
| ?? | 5.0 - 7.5 | 6.6 | 6.7 | 6.5 | 6.8 | 6.1 | 4.0 - 7.5 | |
| Volumetric activity on the day and time of production, MBq/ml | 740 - 2960 | 2220 | 2070 | 2950 | 2950 | 2950 | 74 - 1480 | |
| Radionuclide impuritiesat the dateand time of production,%*) | 99?? | £2×10-3 | <2×10-3 | £2×10-2 | ||||
| Otherg-nuclides | £1×10-4 | <1×10-4 | <1×10-3 | |||||
| Radiochemicalpurity, %**) | ³99,0 | 99.5 | 99.4 | 99.4 | 99.1 | 99.4 | ³99.0 | |
| 99.4 | 99.3 | 99.4 | 99.2 | 99.2 | ||||
| NaCl concentration, mg/ml | 8.0 - 10.0 | 8.9 | 9.2 | 8.9 | 8.9 | 9.0 | 8.0 - 10.0 | |
| MEK concentration, mg/ml | £0.5 | <0.5 | - | |||||
| Nonradioactive impurities concentration,µg/ml***) | ?? | £ 0,2 | < 0.2 | Mo ≤ 0.1; Mn ≤ 0.05; Fe ≤ 0.1; Cu ≤ 0.05; Si ≤ 1.0; B ≤ 0.3 | ||||
| Cu | £ 0,1 | < 0.1 | ||||||
| Si | £ 5.0 | < 5.0 | ||||||
| Bacterialendotoxins, EU/ml | ≤ 8.75 | < 8.75 | ≤ 8.75 | |||||
| Sterility | Sterile | Sterile | Sterile | |||||
Note:*) The actual content of radionuclide impurities in the preparations obtained in the extraction generator does not exceed < 1×10-5 %;
**)at the beginning and end of shelf life (18 hours)
***)B, As, Ba, Be, Bi, Fe, Cr, Cd, Mn, Hg, Ni, Pb, Sn, Sb, Te, Zn, Al are not detected in quantities exceeding the limits of their detection in accordance with the State Pharmacopoeia of the Russian Federation.
**)at the beginning and end of shelf life (18 hours)
***)B, As, Ba, Be, Bi, Fe, Cr, Cd, Mn, Hg, Ni, Pb, Sn, Sb, Te, Zn, Al are not detected in quantities exceeding the limits of their detection in accordance with the State Pharmacopoeia of the Russian Federation.
All control tests were carried out in accordance with the methods described in the State Pharmacopoeia of the Russian Federation [26], the admissible values of the quality indicators are included in the manufacturer's Pharmacopoeia monograph of Khlopin Radium Institute.
Comparison of the characteristics of the preparations obtained on different days after molybdenum-99 solution loading shows the absence of the influence of the molybdenum-99 solution lifetime on the quality of the preparations obtained during this period. At the same time, the preparation obtained is significantly (by an order of magnitude) purer in terms of 99Mo and other radionuclide’s content. The established limit of MEK content is 20 times lower than that adopted in the United States Pharmacopeia. This technology allows varying the volumetric activity of the product changing the volume of sodium chloride solution used to dissolve technetium-99m. Usually, at the request of consumers, the volumetric activity of the final product is closer to the upper limit of the permissible values. Thus, the long experience of the extraction generator has shown its high efficiency and the promise of the technical solutions contained in it.
The developed apparatus allows obtaining steadily the product of proper quality.
AUTOMATION THE OF TECHNETIUM-99M EXTRACTION PROCESS
After successful tests of centrifugal extractors in the facilities for the production of radionuclide’s used in nuclear medicine (99mTc [24], 188Re [25,27,28], 90Y [5]), extraction technology was commercially develop at Medradiopreparat Plant (FSUE “Federal?enter of Designing and Development of nuclear medicine objects” of FMBA of Russia) for the serial production of sodium [99mTc] pertechnetate and supplies to clinics in Moscow. To master the technology, a semi-automatic installation was developed. The installation included a centrifugal extractor, a preparation module and a remote control panel (Figure 4).

Figure 4: 1H NMR spectra for (a) EM53, (b) AEA, and (c) AEA/EM53 micelle in D2O containing 0.1 M NaCl at 20°C. Assignments are indicated for the resonance peaks.
The operations were controlled by electromagnetic valves, peristaltic pumps and a vacuum line. The extractor and the preparation module were placed in a two-section radiochemical box and the control unit was located in the operator zone. Glass elements, fittings and pipes were provided only by industrial manufacturers and allowed for use in pharmaceuticals. A thermo - and cryostat for the evaporation process were built into the system. All operations are carried out in a semi-automatic mode, including washing process and the drug preparation process. Control of the working valves is carried out from the touch screen using a program designed specifically for sodium [99mTc] pertechnetate preparation. Ready solutions of initial reagents are fed into the chamber just before production starts. The duration of the process is 40 minutes.
There are two modes of equipment operations:
Manual mode:
The operations in the auxiliary stage of work and the main technological process are carried out by pressing the on-screen display buttons.
Auto mode:
The operations in the auxiliary stage of work and the main technological process are carried out according to the algorithm in the control program. The operator monitors the performed process confirming the execution of the main operations.
The module is intended for operation in radiochemical laboratories of medical institutions or in the RP producing facilities. A demonstration version of the installation (the extractor is located behind the frame on which the vessels for the initial reagents, the evaporator, and the receiver of the product are mounted) and the screen of the display panel figure 5.
The module is intended for operation in radiochemical laboratories of medical institutions or in the RP producing facilities. A demonstration version of the installation (the extractor is located behind the frame on which the vessels for the initial reagents, the evaporator, and the receiver of the product are mounted) and the screen of the display panel figure 5.
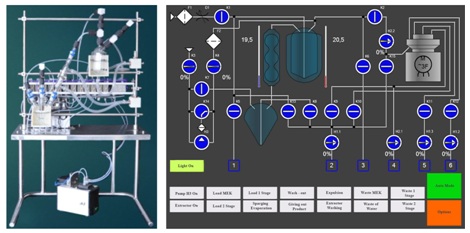
Figure 5: (a) Light scattering intensities and (b) Rh for PIC micelles of AEA/EM106 (?), AEA/EM53 (?), and AEA/M27 (?) as a function of fAMPS (= [AMPS]/([AMPS] + [MAPTAC])) in 0.1 M NaCl aqueous solutions. [AMPS] and [MAPTAC] represent the concentrations of the AMPS and MAPTAC units, respectively. The total polymer concentration was kept constant at 1 g/L.
In working condition, the initial reagents and extractor are placed in a separate section of the hot cell.
The software allows to:
• set the operating modes of the module and promptly make changes to the mode from the touch screen display
• control the execution of the process occurring in the module according to the mnemonic scheme and the information displayed on the screen in real time
• control the operation time of the replaceable module elements, exposed to radiation and aggressive environments
• There are the functions of the service mode, which allow to search and localize the malfunction of the module elements
Since 2010, the semi-automatic synthesis module has been put into serial operation. 99Mo, obtained by thermal neutrons irradiation of molybdenum oxide enriched in 98Mo in a nuclear reactor was used as the raw material. The initial radioactive raw material was 99MoO3, sealed in quartz ampoules. As a rule, the activity on the date of certification was 300-400 GBq, the specific activity was 75-100 GBq/g. Ampoules were opened with a special device, then the contents were dissolved in a mixture of 5 M KOH and K2CO3 solutions with heating (70-90° C, dissolution time is 15 min). In the resulting 99Mo solution sample (0.2 ml), volume activity was monitored (should be at least 1 GBq/ml on the day of the generator charging) and 99Mo authenticity. The work of the extractor and evaporator is realized in an automatic mode. The process is finished by dispensing and packing of the radiopharmaceutical in the vials with subsequent sterilization in an autoclave according to the rules for the production of parenteral drugs. The technological yield of the product by activity is not less than 95% at the date and time of production. The product quality indexes fully corresponded to those presented earlier (Table 1). The requirements to the radiopharmaceutical quality of the sodium pertechnetate solution are presented in the line 1 of table 1. The methods for quality determination are fully described in European, United States or other National pharmacopoeia. For determination of radiochemical purity we proposed to use thin-layer chromatography in acetone as mobile phase except descending paper chromatography in water - methanol (20:80 v/v) with development time for about 2 h. Time of chromatography is about 15 min. In this system pertechnetate ions migrate with solvent front.
In working condition, the initial reagents and extractor are placed in a separate section of the hot cell.
The software allows to:
• set the operating modes of the module and promptly make changes to the mode from the touch screen display
• control the execution of the process occurring in the module according to the mnemonic scheme and the information displayed on the screen in real time
• control the operation time of the replaceable module elements, exposed to radiation and aggressive environments
• There are the functions of the service mode, which allow to search and localize the malfunction of the module elements
Since 2010, the semi-automatic synthesis module has been put into serial operation. 99Mo, obtained by thermal neutrons irradiation of molybdenum oxide enriched in 98Mo in a nuclear reactor was used as the raw material. The initial radioactive raw material was 99MoO3, sealed in quartz ampoules. As a rule, the activity on the date of certification was 300-400 GBq, the specific activity was 75-100 GBq/g. Ampoules were opened with a special device, then the contents were dissolved in a mixture of 5 M KOH and K2CO3 solutions with heating (70-90° C, dissolution time is 15 min). In the resulting 99Mo solution sample (0.2 ml), volume activity was monitored (should be at least 1 GBq/ml on the day of the generator charging) and 99Mo authenticity. The work of the extractor and evaporator is realized in an automatic mode. The process is finished by dispensing and packing of the radiopharmaceutical in the vials with subsequent sterilization in an autoclave according to the rules for the production of parenteral drugs. The technological yield of the product by activity is not less than 95% at the date and time of production. The product quality indexes fully corresponded to those presented earlier (Table 1). The requirements to the radiopharmaceutical quality of the sodium pertechnetate solution are presented in the line 1 of table 1. The methods for quality determination are fully described in European, United States or other National pharmacopoeia. For determination of radiochemical purity we proposed to use thin-layer chromatography in acetone as mobile phase except descending paper chromatography in water - methanol (20:80 v/v) with development time for about 2 h. Time of chromatography is about 15 min. In this system pertechnetate ions migrate with solvent front.
It was established that when using raw materials with the activity of 200 GBq or higher under conditions of irregular operation of the extractor, it is possible to observe the radiolysis of the extractant, which is accompanied by the production of colored solutions of the product and extractant. However, with daily production of the radio pharmaceutical and regular maintenance of the equipment (complete release of traces of the used reagents and washing once a week), it is possible to load up to 350-400 GBq of raw material. Thus, the main criterion influencing the radiochemical purity of sodium [99mTc] pertechnetate is not the total activity of the feed, but the regularity in production and maintenance of the setup.
EXTRACTION RELEASE OF 99MTC FROM 99MO OBTAINED BY THE PHOTONUCLEAR REACTION
Alikhanyan National Science Laboratory (Yerevan Physics Institute) was founded in 1943 for investigation of the high energy physics and cosmic rays. Firstly it was based on two high altitude cosmic ray stations (~2000 and 3000 m s.l.a.). In 1967 the ring synchrotron with 4.5 GeV energy of electrons has been installed. Till 2000 a lot of fundamental investigation has been done using this accelerator. Besides ring accelerator a few small linear accelerators are working for applied research, technology development and other areas. In particular the injector of ring accelerator is powerful enough with the energy up to Ee=75 MeV (Figure 6).
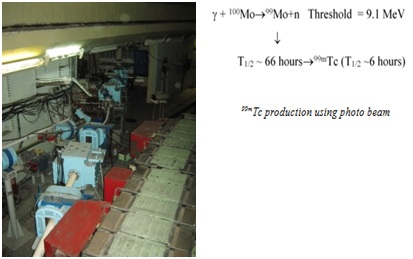
Figure 6: (a) Distributions of Rh for the PIC micelles of AEA/EM106 (?), AEA/EM53 (?), and AEA/EM27 (?) in 0.1 M NaCl aqueous solutions. (b) Relationship between relaxation rate (G) and square of the magnitude of the scattering vector (q2). (c) Plots of Rh as a function of Cp.
It was used as a source of intensive electron beam for 99Mo/99mTc and 123I production technology development.
One of alternative methods of 99mTc production is a photonuclear reaction. 99mTc could be obtained in the photonuclear reaction by irradiation of 100Mo under intensive photon beam (Figure 6). For this aim the electron beam should be converted to a photon beam via bremsstrahlung. This method cannot provide high specific activity and therefore does not permit the production of 99Mo/99mTc generators with standard activity but could cover the demand for regional and city clinics.
To use its electron beam for photon-induced reactions the electron gun had to be upgraded. A new high intensity metallic cathode was installed with slightly modified gun electrodes so that the maximum intensity was increased from 3 µA to ~10 µA. From 3 accelerator sections 2 were used providing Ee= 40 MeV electron energy. The electron beam was transported to the target. Magnetic optics provided the beam spot diameter on the target equal to 12 mm. The beam profile was measured by luminofoor frame or vibrating wire scanner. The beam pulse length was ~1.1 µsec, repetition frequency f=50 Hz.
A special experimental setup has been designed and mounted for material irradiation that provides remote controlled motion of the target module across the beam direction adjusting the center of the target to the beam axis (Figure 7).

Figure 7: A typical example of Zimm plots for AEA/EM106 micelle in 0.1 M NaCl aqueous solution.
The target housing module was fabricated of copper. A thick tantalum plate has been installed on the entrance window to convert the electron beam to photons. A Monte-Carlo simulation of an optimal thickness of the converter has been performed. The optimum thickness of the tantalum radiator is about 2 mm (Figure 8).
Figure 8: TEM images for (a) AEA/EM27, (b) AEA/EM53, and (c) AEA/EM106 micelles.
The oxide of natural molybdenum ???3 was used for the irradiation. The abundance of the stable isotope 100??/natMo is 9.63%. The irradiated material was packed in a special aluminum capsule. Two styles of target materials were used - a stack of pressed pellets and full length pressed powder covered by thin copper foil with 0.045 g/cm2 areal density.
For the low specific activity option only direct extraction of 99mTc from the irradiated material is a reasonable option. For that, a centrifuge extractor with MEK solvent technology was chosen. The complete semi-automated system commissioned from “Medradiopreparat” was installed in a hot cell. The natural MoO3 target of 20 g mass and areal density 0.8 g/cm2 has been irradiated under electron beam with energy Ee = 40 MeV and average intensity i.e., ~ 9.5 µA for a duration of T=100 hours. The irradiated material was then processed by the centrifuge extractor and the first trial amount of 99mTc has been produced. The decay correction to the EOB (End of Bombardment) yielded ~ 80 mCi (~ 3 GBq).The normalized specific activity was 3000 Bq/mg•µA•h, which is close to the maximum value of the previously published results [14].
99M?? RADIOPHARMACEUTICALS
In accordance with the national requirements for the conditions and procedures for the use of new developments in clinical practice, sodium [99mTc] pertechnetate preparations have been tested for suitability for the manufacturing of other 99mTc RPs based on domestic kits to the 99??/99m?? generator. This procedure was carried out every time when a new production site was organized and included the following mandatory studies:
• Determination of the main quality indices of the RPs produced using eluates from extraction and chromatographic generators - volumetric activity, Radiochemical Purity (RCP) or Radiochemical Impurities (RCI) and pH
• A comparative study of the biological distribution of prepared RPs at the most critical time after intravenous administration, which were previously identified in the report on preclinical studies of a radiopharmaceutical approved for clinical use
Tested RPs were prepared by dissolving of lyophilized kits in sodium [99mTc] - pertechnetate solutions obtained from extraction generator (Facility “Medradiopreparat”, Moscow, Russia) or generator of chromatographic type (GT-4K, FSUE “Karpov Institute of Physical Chemistry”, Obninsk, Russia). Quality control of tested RPs (RCP or PCI) was carried out by thin-layer chromatography or descending paper chromatography in different solvents or electrophoreses in agarose gel according to the national monographs.
Since the results obtained in the tests were almost the same, the summary data of such studies are selectively (for the most commonly used RPs) presented in table 2. No difference in labeling efficiency, quality and biological behavior of the radiopharmaceuticals (within the limits of acceptable errors) was observed.
• Determination of the main quality indices of the RPs produced using eluates from extraction and chromatographic generators - volumetric activity, Radiochemical Purity (RCP) or Radiochemical Impurities (RCI) and pH
• A comparative study of the biological distribution of prepared RPs at the most critical time after intravenous administration, which were previously identified in the report on preclinical studies of a radiopharmaceutical approved for clinical use
Tested RPs were prepared by dissolving of lyophilized kits in sodium [99mTc] - pertechnetate solutions obtained from extraction generator (Facility “Medradiopreparat”, Moscow, Russia) or generator of chromatographic type (GT-4K, FSUE “Karpov Institute of Physical Chemistry”, Obninsk, Russia). Quality control of tested RPs (RCP or PCI) was carried out by thin-layer chromatography or descending paper chromatography in different solvents or electrophoreses in agarose gel according to the national monographs.
Since the results obtained in the tests were almost the same, the summary data of such studies are selectively (for the most commonly used RPs) presented in table 2. No difference in labeling efficiency, quality and biological behavior of the radiopharmaceuticals (within the limits of acceptable errors) was observed.
| Trade name | Sodium [99m??] pertec-netate | Phosphotech, 99m?? | Pentatech, 99mTc | Technetril, 99mTc | Technemag, 99mTc | Macrotech, 99mTc | Bromezida, 99mTc | ||
| INN or Abbreviated name | NaTcO4[99m??] | 99m??-HEDP | 99m??-DTPA | 99mTc-MIBI | 99m??-MAG3 | 99m??-??? | 99m??-Br-HIDA | ||
| Vol.activity, MBq/ml*) | 74-1480 | 185-740 | 75-1500 | 37-1850 | 37-185 | 37-148 | 37-185 | ||
| 370 | 196.5 | 150 | 173.3 | 62.9 | 37 | 85.1 | |||
| RCP,%*) | ≥ 99 | > 95 | ≥ 80 | ≥ 90 | ≥ 90 | ≥ 90 | ≥ 94 | ||
| 99.7 | 99 | 85.1 | 93.9 | 94.3 | 95 | 98.8 | |||
| pH*) | 4 - 7.5 | 4.0 - 6.0 | 5.0 - 6.0 | 5.3 - 5.9 | 5.0 - 6.0 | 4.0 - 7.0 | 4.5 - 5.3 | ||
| 6.9 | 4.7 | 5.3 | 5.2 | 5 | 5.8 | 4.7 | |||
| Time, h**) | 2 | 1 | 0.5 | 0.1 | 0.25 | 0.5 | 0.5 | ||
| Biodistribution, % ID*) | Blood | 6.8 ± 0.3 | 0.80 ± 0.06 | 1.3 ± 0.1 | 1.8 ± 0.3 | 1.6 ± 0.4 | 4.0 ± 0.5 | 0.5 ± 0.03 | |
| 5.4 ± 1.3 | 0.54 ± 0.16 | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 4.9 ± 0.4 | 0.3 ± 0.08 | |||
| Liver | 2.5 ± 0.2 | 0.50 ± 0.03 | 0.6 ± 0.07 | 7.3 ± 1.0 | 5.7 ± 0.1 | - | 1.3 ± 0,1 | ||
| 1.9 ± 0.6 | 0.62 ± 0.07 | 0.5 ± 0.01 | 6.4 ± 0.5 | 3.9 ± 0.2 | - | 1.1 ± 0.2 | |||
| Kidneys | 1.6 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.1 | 5.8 ± 0.3 | 6.2 ± 0.6 | 3.8 ± 0.6 | 0.4 ± 0.04 | ||
| 1.8 ± 0.3 | 1.7 ± 0.23 | 1.2 ± 0.05 | 6.6 ± 0.02 | 13.7 ± 1.1 | 4.2 ± 0.5 | 0.2 ± 0.03 | |||
| Bladder | 6.6 ± 0.5 | 50.0 ± 1.9 | 80.0 ± 4.2 | - | - | - | 1.4 ± 0.05 | ||
| 7.8 ± 0.8 | 54.9 ± 5.0 | 83.4 ± 0.5 | - | - | - | 1.1 ± 0.07 | |||
| Skeleton | - | 38.0 ± 3.4 | - | - | - | - | 0.16 ± 0.0 | ||
| - | 41.4 ± 7.2 | - | - | - | - | 0.15 ± 0.0 | |||
| Intestine | 8.5 ± 0.4 | 0.50 ±0.02 | - | - | 4.1 ± 0.,2 | - | 92.0 ± 0.9 | ||
| 7.9 ± 2.1 | 1.43 ± 0.35 | - | - | 5.2 ± 0.3 | - | 91.0 ± 1.4 | |||
| Stomach | 25.3 ± 1.3 | 0.08 ± 0.01 | - | 1.5 ± 0.3 | 0.4 ± 0.03 | - | 0.02 ± 0.0 | ||
| 26.8 ± 1.7 | 0.35 ± 0.13 | - | 0.9 ± 0.04 | 0.3 ± 0.04 | - | 0.04 ± 0.0 | |||
| Heart | 0.23 ± 0.0 | - | - | 1.9 ± 0.2 | Lungs → 85.4 ± 5.7 | - | |||
| 0.2 ± 0.1 | - | - | 2.4 ± 0.2 | Lungs → 89.2 ± 2.1 | - | ||||
| Muscle | 7.7 ± 0.4 | - | - | - | - | - | 0.6 ± 0.02 | ||
| 8.0 ± 0.1 | - | - | - | - | - | 0.4 ± 0.09 | |||
| Thyroid gland | 0.8 ± 0.1 | - | - | - | - | - | - | ||
| 0.9 ± 0.2 | - | - | - | - | - | - | |||
*) first line for every RP - the results obtained using sodium pertechnetate solution from chromatographic generator (GT-4K, FSUE “Karpov Institute of Physical Chemistry”, Obninsk, Russia) and served as a reference source;
The second line of every RP - the results obtained using sodium pertechnetate solution from extraction generator (FSUE “Federal center of nuclear medicine projects design and development”, Moscow, Russia);
**) time after intravenous injection specified in the instruction for medical use of each radiopharmaceutical
The second line of every RP - the results obtained using sodium pertechnetate solution from extraction generator (FSUE “Federal center of nuclear medicine projects design and development”, Moscow, Russia);
**) time after intravenous injection specified in the instruction for medical use of each radiopharmaceutical
CONCLUSION
The centrifugal semi-countercurrent extractor has proven itself as effective, reliable, easy-to-use equipment capable of stable production of a product - sodium [99mTc] pertechnetate solution of proper quality.
Currently, the development of a new synthesis module is in progress. The main differences between the future synthesis modules are its mass-dimensional parameters (Figure 9). Due to the use of new design solutions it was possible to abandon the use of cryo - thermostats included in the current set of equipment. By using compact valves and tubes, the dead zones volume is reduced.
Currently, the development of a new synthesis module is in progress. The main differences between the future synthesis modules are its mass-dimensional parameters (Figure 9). Due to the use of new design solutions it was possible to abandon the use of cryo - thermostats included in the current set of equipment. By using compact valves and tubes, the dead zones volume is reduced.

Figure 9: TEM images for (a) AEA/EM27, (b) AEA/EM53, and (c) AEA/EM106 micelles.
The software of the system is implemented on the base of a computer. To ensure greater operational safety, the application interface is organized in such a way that only necessary and meaningful system settings are available to the operator at each process operation. Along with a completely new interface, the software of the complex includes the following functions:
• Logging all operator actions
• Privileges for different types of users are established (separately for personnel engaged in daily production or maintenance of the installation)
• There is a reminder function for scheduled maintenance
• You can remotely access from different types of devices (PC, tablet) through DRP (Remote Desktop)
Although the set of equipment is self-contained and is no significantly expand of its capabilities is expected, the platform is built in such a way that adding support for new features at the customer's request will not lead to drastic changes (and will not take much time). Among such features can be, for example, duplication of the log file in the factory database (SQL, Oracle), switching to another hardware complex (transfer of software to another hardware platform), and integration into SCADA - the customer's system. It is possible to transfer the software to other platforms running Windows/Linux. Also, there is no problem with the integration of the modern element base (the control PC can be extended with both analog signals and digital ports).
The developed technology and technical devices should be considered promising for the organization of local technetium-99m production facilities, including specialized radiochemical laboratories for medium-capacity medical cyclotrons. In addition, it is possible to organize the simultaneous production of small batches of ready-to-use radiopharmaceuticals with subsequent delivery to radionuclide diagnostic units. This provides an opportunity to guarantee the receipt of high-quality RPs and reduce of the radiation doses obtained by medical personnel.
REFERENCES
- Fischer W, Braune G, Dietz W, Jubermann O, Krause G, et al. (1954) Über die Trennung der Seltenen Erden durch Verteilen zwischen zwei Lösungsmitteln. Angew Chem 66: 317-325.
- Levin VI (1962) Extraction, Theory, Application, Instrument. In: Zefirov AP, Senjavin MM (eds.). Quasi-equilibrium extraction processes. Gosatomizdat, Moscow,Russia. Pg No: 1: 143-162.
- Kies MW, Davis PL (1951) A New Procedure for Fractionation of Mixtures by Solvent Distribution. J Biol Chem Vol 189: 637-650.
- Korpusov GV, Kuznetsov GI, Ye NP, Popkov GP, Yakovlev GN (1974) The study semi-countercurrent extraction methods of separation of actinide elements in the trivalent state. Radiochemistry 16: 695-701.
- Kodina GE, Korpusov GV, Filyanin AT (2002) Production of High-Purity 90Y on Specially Developed Centrifugal Semicounterflow Extractors. Radiochemistry 44: 62-66.
- Zolle I (2007) Technetium-99m Pharmaceuticals. Springer Berlin Heidelberg, New York, USA. Pg No: 345.
- Zykov MP, Kodina GE (1999) Methods of obtaining 99?? (review). Radiochemistry 41: 193-204.
- Einstein AJ (2009) Breaking America's dependence on imported molybdenum. JACC Cardiovasc Imaging 2: 369-371.
- Guérin B, Tremblay S, Rodrigue S, Rousseau JA, Dumulon-Perreault V, et al. (2010) Cyclotron production of 99mTc: an approach to the medical isotope crisis. J Nucl Med 51: 13-16.
- Osso JA, Catanoso MF, Barrio G, Brambilla TP, Teodoro R, et al. (2012) Technetium-99m -- new production and processing strategies to provide adequate levels for SPECT imaging. Curr Radiopharm 5: 178-186.
- Lebedan O, Pruszy?ski M (2010) New measurement of excitation functions for (p,x) reactions on natMo with special regard to the formation of 95mTc, 96m+gTc, 99mTc and 99Mo. Applied Radiation and Isotopes 68: 2355–2365.
- Lebedan O, Fikrle M (2010) New measurement of excitation functions for (d,x) reactions on natMo with special regard to the formation of 95mTc, 96m+gTc, 99mTc and 99Mo. Applied Radiation and Isotopes 68: 2425–2432.
- Sabelnikov AV, Maslov OD, Molokanova LG, Gustova MV, Dmitriev SN (2006) Preparation of 99Mo and 99mTc by 100Mo(γ,n) photonuclear reaction on an electron accelerator, MT-25 Microtron. Radiochemistry 48: 191-194.
- Avagyan R, Avetisyan A, Kerobyan I, Dallakyan R (2014) Photo-production of 99Mo/99mTc with electron linear accelerator beam. Nucl Med Biol 41: 705-709.
- Pillai MR, Dash A, Knapp FF (2013) Sustained Availability of 99mTc: Possible Paths Forward. J Nucl Med 54: 313-323.
- Selivanova SV, Lavallée E, Senta H, Caouette L, Sader JA, et al. (2015) Radioisotopic Purity of Sodium Pertechnetate 99mTc produced with a Medium-Energy Cyclotron: Implications for Internal Radiation Dose, Image Quality, and Release Specifications. J Nucl Med 56: 1600-1608.
- Hou X, Tanguay J, Buckley K, Schaffer P, Bénard F, et al. (2016) Molybdenum target specifications for cyclotron production of 99mTc based on patient dose estimates. Phys Med Biol 61:542-553.
- Selivanova S, Lavallée É, Senta H, Caouette L, McEwan AJ, et al. (2016) Clinical Trial with Sodium 99mTc-Pertechnetate Produced by a Medium-Energy Cyclotron: Biodistribution and Safety Assessment in Patients with Abnormal Thyroid Function. J Nucl Med 58: 791-198.
- Fadeyeva MS, Pavlov ON, Bakunina VV (1958) Extraction method for isolation of technetium from irradiated molybdenum. Russian Journal of Inorganic Chemistry 3: 165-166.
- Lavrukhina AK, Pozdnyakov AA (1996) Analytical chemistry of technetium, promethium, astatine and France. The series "Analytical chemistry of elements". Nauka, Moscow, Russia. Pg No: 360.
- El-Asrag HA, El-Kolay M, Hallaba E (1978) On the Production of 99mTc by Solvent Extraction technique. Microchemical Journal 23: 42-50.
- Boyd RE (1982) Molybdenum-99/Technetium-99m Generator. Radiochim Acta 30: 123-145.
- US Pharmacopeie (2016) Sodium Pertechnetate Tc 99m Injection. USP 29, USP Monographs, US Pharmacopeie, USA.
- Zykov MP, Romanovskii N, Wester DW, Bartenev SA, Korpusov GV,et al. (2001) Use of Extraction Generator for Preparing a 99mTc Radiopharmaceutical. Radiochemistry 43: 297-300.
- Zykov MP, Romanovskiy VN, Filyanin AT, Tsivadze A Yu, Tkachuk DA, et al. (2007) The 188Re extraction generator. Proceedings of the Khlopina radium Institute. 12: 86-95 (in Russian).
- State Pharmacopoeia of the Russian Federation (2008) Current state and strategy for the future. XII edn., State Pharmacopoeia of the Russian Federation, Medistina, Moscow, Russia.
- Tsivadze A Yu, Filyanin AT, Zykov MP, Kodina GE, Filyanin OA (2014) Extraction centrifugal W-188/RE- 188 Generator for radiotherapeutic application. Journal of International Scientific Publications: Materials, Methods and Technologies 8: 639-646.
- Tsivadze A Yu, Filyanin AT, Romanovskiy VN, Zykov MP, Kodina GE. (2016) Extraction centrifugal generator and 188Re and radiopharmaceuticals based on it for radionuclide therapies. Radiochemistry 58: 513-520.
Citation: Tsivadze A Yu, Filyanin AT, Filyanin OA, Avetisyan AE, Zykov MP, et al. (2017) Radiochemical Technology for Production of Preparations of Technetium-99m on Extraction Centrifugal Semi-Countercurrent Generator. J Nucl Med Radiol Radiat Ther 2: 007.
Copyright: © 2017 Filyanin AT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Journal Highlights
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!
