
Rapid Recovery of Reintubated COVID-19 Patient with Acute Respiratory Distress Syndrome Following Plasmapheresis Treatment Supplanted by Infusion of Convalescent Plasma: Case Report
*Corresponding Author(s):
LT Michael A. TalalaevLarkin Palm Springs Hospital, Hialeah, FL, United States
Tel:+1 6128123622,
Email:michael.a.talalaev@navy.mil
Abstract
Background: Acute Respiratory Distress Syndrome (ARDS) is associated with significant mortality causing death in 46 percent of the patients with severe COVID-19 disease. In these cases, respiratory distress appears to include a crucial vascular insult that mandates a different treatment approach. Attenuating circulating cytokines, and other mediators, by blood purification techniques with the removal of bioactive molecules via therapeutic plasma exchange (TPE) can be a useful adjunct to mechanical ventilation in COVID-19 induced ARDS in conjunction with convalescent plasma.
Methods: Therapeutic plasma exchange treatment was administered to a critically ill patient with confirmed COVID-19 infection complicated by Adult Respiratory Distress Syndrome, after obtaining consent from the Health Proxy.
Results: This report describes rapid improvement of a critically ill patient with confirmed COVID-19 infection following therapeutic plasmapheresis procedure administered in an ICU after re-intubation and supplementedby infusion of convalescent plasma at the end of the plasma exchange procedure.
Conclusions: This abstract demonstrates therapeutic potential of plasma exchange in patients with COVID-19 multi-focal pneumonia and Adult Respiratory Distress Syndrome, directs attention to currently available treatment modalities, and calls for further research of therapeutic plasma exchange in patients with SARS-CoV2 pneumonia.
Keywords
COVID-19; Adult respiratory distress syndrome; Plasmapheresis; Critical care
ABBREVIATIONS
ARDS: Adult Respiratory Distress Syndrome
ASFA: American Society for Apheresis
AST: Aspartate transaminase
BMI: Body Mass Index
CT: Computer Tomography
COVID-19: Corona Virus Disease-19
Hb: Hemoglobin
HNH: Hemophagocyticlymphohistiocytosis
Ht: Hematocrit
ICU: Intensive Care Unit
INR: International Normalized Ratio
IVIG: Intravenous Immunoglobulins
MAS: Macrophage Activation Syndrome
MOD: Multiorgan Dysfunction
MRI: Magnetic Resonance Imaging
PEX: Plasma Exchange
PT: Prothrombin Time
RNA: Ribonucleic Acid
SARS: Severe Acute Respiratory Syndrome
SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2
CSF: Cerebrospinal Fluid
CSR: Cytokine Resolution Score
TPE: Therapeutic Plasma Exchange
USFDA: United States Food and Drug Administration
WBC: White Blood Count
WHO: World Health Organization
BACKGROUND
Acute Respiratory Distress Syndrome (ARDS) is associated with significant mortality causing death in 46 percent of the patients with severe COVID-19 disease. In these cases, respiratory distress appears to include a crucial vascular insult that mandates a different treatment approach. Attenuating circulating cytokines, and other mediators, by blood purification techniques with the removal of bioactive molecules via therapeutic plasma exchange (TPE) can be a useful adjunct to mechanical ventilation in COVID-19-induced ARDS in conjunction with convalescent plasma.
METHODS
Therapeutic plasma exchange treatment was administered to a critically ill patient with confirmed COVID-19 infection complicated by Adult Respiratory Distress Syndrome. Consent was obtained from Health Proxy.
CASE ABSTRACT
A 58-year-old female with confirmed COVID-19 infection, weight 80.7 kg, height 162 cm, BMI 30.7, with history of hypertension, was admitted to the ICU in severe respiratory distress. She was unable to tolerate prone positioning and non-invasive bi-level positive pressure ventilation and was intubated within seven days after ICU admission. Following eight days on mechanical ventilation, she was extubated, but within 72 hours patient developed respiratory distress. Arterial blood gases values were pH 7.52, PaCO2 32mmHg, PaO2 93mmHg, HCO3- 25mEq/L. Worsening O2 saturation to 80-82% range shortly after the patient was re-intubated, and sedated. Ventilation required proning according to our protocol. Patient also required vasopressors. Chest x-ray revealed bilateral low lung volumes and airspace disease of the left upper lobe apex consistent with pneumonia, and no change in the coarsening of interstitial markings, consistent with viral infection or edema. There were no pleural effusions or pneumothoraces and the mediastinum was normal (Figure 1). Post extubation, after plasmapheresis, radiographic improvement followed clinical recovery (Figure 2).
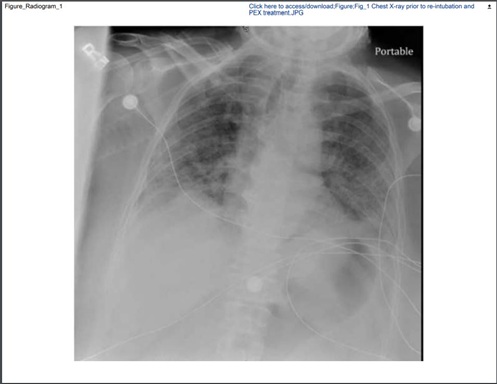 Figure 1: Chest X-ray prior to PEX treatment-note bilateral low lung volumes and airspace disease of the left upper lobe apex consistent with pneumonia, and no change in the coarsening of interstitial markings, consistent with viral infection or edema.
Figure 1: Chest X-ray prior to PEX treatment-note bilateral low lung volumes and airspace disease of the left upper lobe apex consistent with pneumonia, and no change in the coarsening of interstitial markings, consistent with viral infection or edema.
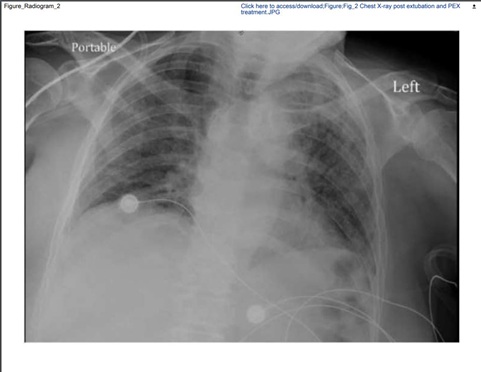 Figure 2: Chest X-ray after PEX procedure and extubation demonstrating significant improvement of the radiologic pulmonary presentation.
Figure 2: Chest X-ray after PEX procedure and extubation demonstrating significant improvement of the radiologic pulmonary presentation.
Laboratory evaluation demonstrated WBC of 9,900 cells/mcL, Hb 11.2 grams/dL, Ht 35.1%, platelets 195,000/mL, normal creatinine; PT/INR was 13 sec/1.1; AST was mildly elevated (46U/L). A consult for Hematology/Oncology and Nephrology services was requested, and within 12 hours, the patient underwent plasmapheresis (TPE) of approximately 1.25 plasma volume with 4.75L of albumin (since there were no signs of active bleeding), based on the rounded up calculation of [1.25 x 0.07 x weight in kg x (1-hematocrit)]/[1.25 x 0.07 x 80.7 x (1-.351)]=4.575) and followed by infusion of 1 unit of convalescent plasma. Plasmapheresis was given over 5 hours at low output and input power levels. Within 24 hours, the patient showed rapid clinical and laboratory improvement, including arterial blood gas measurements and inflammatory indicators; Rapid Shallow Breathing Index (RSBI) was estimated at 53 (RR of 24 per minute, tidal volume of 450ml), and the patient was extubated. She was initially maintained on oxygen via a non-rebreather mask, followed by O2 via nasal cannula. Vasopressors and sedatives were weaned over a 24-hour period. She was able to follow commands and converse in her native language. She was downgraded to step down and subsequently to telemetry observation. The patient started ambulating and was discharged home within 14 days after leaving the ICU with a referral for pulmonary rehabilitation.
DISCUSSION
While there are large clinical trials being conducted by the World Health Organization (WHO) to determine which therapies will be efficacious against SARS-CoV-2, doctors need urgent solutions that will save their gravely ill patients in the ICU [1]. In our study, we decided to treat one patient with both convalescent plasma and plasmapheresis or therapeutic plasma exchange (TPE) based on the following data.
Plasmapheresis or therapeutic plasma exchange
Plasmapheresis or therapeutic plasma exchange is an extracorporeal treatment that selectively removes abnormal larger molecules from the blood. The American Society for Apheresis (ASFA) 2019 guidelines designated sepsis with multiorgan failure (MOD) as category 3 and thus qualified it for TPE therapy in order to improve organ function by eliminating excess cytokines, inflammatory mediators, and viral RNA particles similar to its use with hepatitis C that also has larger particle sizes [2-7].
Therapeutic plasma exchange (TPE) may be useful in a wide variety of illnesses characterized by cytokine storms, microvascular thrombosis, auto-antibodies, and in some infections complicated by ARDS [8]. A comprehensive review of the evidence conducted by Tabibi et al. concluded that the following conditions qualify for TPE [9].
1.Early acute lung injury/early ARDS; or
2.Severe disease, defined as:
a.Dyspnea
b.Respiratory frequency ≥ 30/minute
c.Blood O2 sat ≤ 93%
d.Partial pressure of arterial O2 to fraction of inspired O2<300
e.Lung infiltrates >50% within 24 to 48 hours
3. Life-threatening disease, defined as:
a.Respiratory failure
b.Septic shock, and/or
c.Multiple organ dysfunction or failure
Similar to the overactive, autoimmune, cytokine storm of COVID-19, there have been instances where Secondary Hemophagocytic Lymphohistiocytosis (HLH) occurs from an infectious trigger which results in pathologic hyperactive inflammation due to unchecked immune activation [10].
In these cases, TPE has been shown to be beneficial in reducing the cytokine storm and providing hematologic support in patients with primary and secondary HLH [11-20]. Another small study found a significantly improved survival in seventeen patients with secondary HLH who received plasma exchange, steroids, and IVIG compared to six patients who received plasma exchange, steroids, and/or cyclosporine, and/or etoposide [21].
In a three-case study of pediatric patients with H1N1 Influenza A and acute respiratory distress syndrome combined with hemodynamic compromise, therapeutic plasma exchange via the filtration method appeared to mitigate the associated cytokine storm, and all survived despite statistics suggesting an abysmal outcome [22].
In another case report, nano-membrane-based apheresis was used to cleanse the plasma from the harmful inflammatory bioactive mediators in a 41-year-old man with myasthenia gravis and pneumonia with worsening hypoxemia. After 3 sessions of plasmapheresis, his condition improved, and he was weaned from mechanical ventilation [23].
In a non-peer-reviewed, retrospective propensity-matched controlled study of 90 patients with a median duration of symptoms of 7 days with 93.4% having either severe or critical disease, TPE significantly improved survival (91.1% versus 61.5%), reduced days in the hospital (10 vs.15) and showed reduced cytokine resolution scores (CSR) from 12 to 6 days [24]. Dogan et al. recently demonstrated that plasmapheresis helped extremely ill COVID-19 patients with autoimmune meningoencephalitis. In their series of cases, they showed that a cytokine storm was occurring in the severely ill ICU patients based on the following points:
1) Increased level of inflammatory markers, such as ferritin that can be a useful marker for disease severity;
2) High protein levels and albumin in CSF without pleocytosis;
3) Reversible MRI findings; and
4) Dramatic improvement following plasmapheresis in both clinical and laboratory findings [25,26].
Convalescent plasma or Immunoglobulins (IVIG)
Likewise, convalescent plasma therapy or intravenous immunoglobulins (IVIG) has been used in other cases of acute respiratory distress (ARDS) from viral infections. The United States Food and Drug Administration (US FDA) has issued guidelines for investigating this therapy for use in COVID-19 patients due to the global pandemic emergency [27].
IVIG is plasma rich in bacterial and viral IgG antibodies, and the use of IVIG influences the process of differentiation and maturation of lymphocytes, impairs the normal immune response, and inhibits the production of inflammatory factors [28-30]. In certain patients, IVIG can be used as adjuvant treatment for COVID-19 pneumonia, by reducing the use of mechanical ventilation, hospitalizations, and results in earlier recovery of patients [31].
A meta-analysis of 1703 patients in 8 different studies during the Spanish flu epidemic in the first quarter of the twentieth century showed better results with fewer deaths by using convalescent blood products [32]. Furthermore, a comprehensive review from 2015 of convalescent plasma and immunoglobulin for ARDS showed a 75% reduction in the risk of mortality [33]. However, seven studies using convalescent plasma or IVIG for SARS did show such a benefit [34].
A study by Mohtadi et al. demonstrated that shortly after IVIG administration, there was an improvement in clinical and respiratory status in all patients, translated into higher oxygen levels, faster extubations, and improved pulmonary lesions by lung CT [35] Similar results with IVIG were obtained in China during this pandemic [36,37].
IVIG and TPE
Demirkol et al. concluded their study that children with elevated plasma ferritin and secondary HLH/sepsis/MODS/MAS can be successfully treated with plasma exchange, intravenous immunoglobulin, and methylprednisone [38].
RESULTS
This report describes rapid improvement of a critically ill patient with confirmed COVID-19 infection following therapeutic plasmapheresis procedure administered in an ICU after re-intubation and supplanted by infusion of convalescent plasma at the end of the plasma exchange procedure.
CONCLUSION
This case illustrates that plasmapheresis treatment combined with transfusion of convalescent plasma may be a viable treatment modality for COVID-19 patients with ARDS with endothelial injury. Although this is a single example of a successful treatment, further research is indicated to obtain statistically significant outcomes. Plasma exchange treatment presents minimal risks with potentially a substantial reduction of morbidity and mortality in COVID-19 patients.
DISCLOSURES
Ethics approval: This manuscript constitutes a Case Report, and meets or exceeds 2017 CARE Guidelines. This submission does not report studies involving human participants, human data or human tissue, and as such does not require ethics committee approval.
Consent for publication: Health Care Proxy has provided consent for both treatment and publications of the treatment result at the point of service.
Availability of data and materials: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Competing interests: The authors declare that they have no competing interests.
Funding: No funding was either requestedor received by the authors.
Authors' contributions: Each co-author has made substantial contributions to the conception and design of this case report and have drafted the work or substantively revised it. Each co-author has approved the submitted version for publication.
Acknowledgements: Dr. Talalaev, DO, Dr. Dungo, DO, Dr. Islas, MD are US NAVY Reserve Medical Corps officers, who were deployed to New York City, NY in support of US NAVY COVID-19 relief mission, Operation Gotham 2020.
REFERENCES
- Brown BL, McCullough J (2020) Treatment for emerging viruses: Convalescent plasma and COVID-19. Transfus Apher Sci 59: 102790.
- Tan MS-TE, Lim B (2005) ARDS in SARS: Cytokine mediators and treatment implications. Cytokine 29: 92-94.
- Donnelly SC, Haslett C, Reid PT, Grant IS, Wallace WA, et al. (1997) Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nat Med 3: 320-323.
- Connelly-Smith SC, Haslett C, Reid PT (1997) Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nat Med 3: 320-323.
- Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, et al. (2019) Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: The eighth special issue. J ClinApher 34: 171-354.
- Ishikawa T, Abe S, Kojima Y, Sano T, Iwanaga A, et al. (2015) Prediction of a sustained viral response in chronic hepatitis C patients who undergo induction therapy with double filtration plasmapheresis plus interferon-β/ribavirin. ExpTher Med 9: 1646-1650.
- Liu X, Zhang Y, Xu X, Du W, Su K, et al. (2015) Evaluation of plasma exchange and continuous veno-venous hemofiltration for the treatment of severe avian influenza a (H7N9): A cohort study. Ther Apheresis Dial 19: 178-184.
- Nguyen TC, Kiss JE, Goldman JR, Carcillo JA (2012) The role of plasmapheresis in critical illness. Crit Care Clin 28: 453-468.
- Tabibi S, Tabibi T, Conic RRZ, Banisaeed N, Streiff MB (2020) Therapeutic Plasma Exchange: A potential Management Strategy for Critically Ill COVID-19 Patients. Journal of Intensive Care Medicine 35: 827-835.
- Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, et al. (2007) HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 48: 124-131.
- Coman T, Dalloz MA, Coolen N, Hesmati F, Pene F, et al. (2003) Plasmapheresis for the treatment of acute pancreatitis induced by hemophagocytic syndrome related to hyper triglyceridemia. J Clin Apher 18: 129-131.
- Imashuku S, Hibi S, Ohara T, Iwai A, Sako M, et al. (1999) Effective control of Epstein-Barr virus-related hemophagocytic lymphohistiocytosis with immunochemotherapy. Histiocyte Society. Blood 93: 1869-1874.
- Ladisch S, Ho W, Matheson D, Pilkington R, Hartman G (1982) Immunologic and clinical effects of repeated blood exchange in familial erythrophagocytic lymphohistiocytosis. Blood 60: 814-821.
- Nakakura H, Ashida A, Matsumura H, Murata T, Nagotaya K, et al. (2009) A case report of successful treatment with plasma exchange for hemophagocytic syndrome associated with severe systemic juvenile idiopathic arthritis in an infant girl. Ther Apher Dial 13: 71-76.
- Raschke RA, Garcia-Orr R (2011) Hemophagocytic lymphohistiocytosis: A potentially under recognized association with systemic inflammatory response syndrome, severe sepsis, and septic shock in adults. Chest 140: 933-938.
- Sanada S, Ookawara S, Shindo T, Morino K, Ishikawa H, et al. (2004) A case report of the effect of plasma exchange on reactive hemophagocytic syndrome associated with toxic shock syndrome. Ther Apher Dial 8: 503-506.
- Satomi A, Nagai S, Nagai T, Nilkura A, Ideura T, et al. (1999) Effect of plasma exchange on refractory hemophagocytic syndrome complicated with myelodysplastic syndrome. Ther Apher 3: 317-319.
- Song KS, Sung HJ (2006) Effect of plasma exchange on the circulating IL-6 levels in a patient with fatal hemophagocytic syndrome associated with bile ductopenia. Ther Apher Dial 10: 87-89.
- Zhang XY, Ye XW, Feng DX, Han J, Li D, et al. (2011) Hemophagocytic Lymphohistiocytosis Induced by Severe Pandemic Influenza A (H1N1) 2009 Virus Infection: A Case Report. Case Report Med 2011: 951910.
- Demirkol D, Yildizdas D, Bayrakci B, Karapinar B, Kendirli T, et al. (2012) Hyperferritinemia in the critically ill child with secondary HLH/sepsis/MODS/MAS: What is the treatment? Critical Care 16: R52.
- Ma J, Xia P, Zhou Y, Liu Z, Zhou X, et al. (2020) Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin Immunol 214: 108408.
- Patel P, Nandwani V, Vanchiere J, Conrad SA, Scott LK (2011) Use of therapeutic plasma exchange as a rescue therapy in 2009 pH1N1 influenza A--an associated respiratory failure and hemodynamic shock. Pediatr Crit Care Med 12: e87-e89.
- Yamakova Y, Ilieva VA, Petkov R, Yankov G (2019) Nanomembrane-Based Therapeutic Plasmapheresis after Non-Invasive Ventilation Failure for Treatment of a Patient with Acute Respiratory Distress Syndrome and Myasthenia Gravis: A Case Report. Blood Purif 48: 382-384.
- Kamran SM, Mirza ZH, Naseem A, Liaqat J, Fazal I, et al. (2020) PLEXIT-Therapeutic plasma exchange
- Dogan L, Kaya D, Sarikaya T, Zengin R, Dincer A, et al. (2020) Plasmapheresis treatment in COVID-19-related autoimmune meningoencephalitis: Case series. Brain Behav Immun 87: 155-158.
- Keith P, Day M, Perkins L, Moyer L, Hewitt K, et al. (2020) A novel treatment approach to the novel coronavirus: An argument for the use of therapeutic plasma exchange for fulminant COVID-19. Crit Care 24: 128.
- https://www.fda.gov/vaccines-blood-biologics/investigational-new-drugind-or-device-exemption-ide-process-cber/investigational-covid-19-convalescent-plasmaemergency-inds
- Takashi T, Hiroki M, Kiyohide F (2015) Intravenous immunoglobulin and mortality in pneumonia patients with septic shock: An observational nationwide study. Clin Infect Dis 61: 385-392.
- Hung IFN, To KKW, Lee CK, Lee KL, Yan WW, et al. (2013) Hyperimmune IV immunoglobulin treatment: A multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest 144: 464-473.
- Diebel LN, Liberati DM., Diglio CA, Brown WJ (2005) Immunoglobulin a modulates inflammatory responses in an in vitro model of pneumonia. J Trauma 59: 1099-1106.
- Lanza M, Polistina GE, Imitazione P, Annunziata A, Spirito VD, et al. (2020) Successful intravenous immunoglobulin treatment in severe COVID-19 pneumonia. IDCases 21: e00794.
- Luke TC, Kilbane EM, Jackson JL, Hoffman SL (2006) Meta-analysis: Convalescent blood products for Spanish influenza pneumonia: A future H5N1 treatment? Ann Intern Med 145: 599-609.
- Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, et al. (2015) The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J Infect Dis 211: 80-90.
- Stockman LJ, Bellamy R, Garner P (2006) SARS: Systematic review of treatment effects. PLoS Med 3: e343.
- Mohtadi N, Ghaysouri A, Shirazi S, Ansari A, Shafiee E, et al. (2020) Recovery of severely ill COVID-19 patients by intravenous immunoglobulin (IVIG) treatment: A case series, Virology 548: 1-5.
- Cao W, Liu X, Bai T, Fan H, Ke H, et al. (2020) High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with Corona virus Disease 2019. Open Forum Infect Dis 7: ofaa102.
- Xie Y, Cao S, Dong H, Li Q, Chen E, et al. (2020) Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect 81: 318-356.
- Demirkol D, Yildizdas D, Bayrakci B, Karpinar B, Kendirli T, et al. (2012) Hyperferritinemia in the critically ill child with secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction syndrome/macrophage activation syndrome: What is the treatment? Crit Care 16: R52.
Citation: Talalaev LTM, Popilevsky F, Douen A, Lee W, Frolova E, et al. (2020) Rapid Recovery of Reintubated COVID-19 Patient with Acute Respiratory Distress Syndrome Following Plasmapheresis Treatment Supplanted by Infusion of Convalescent Plasma: Case Report. J Clin Immunol Immunother 6: 043.
Copyright: © 2020 LT Michael A. Talalaev, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

