
Repellent Activity of Bark Extracts of Zanthoxylum heitzii Against the Malaria Vector Anopheles gambiae
*Corresponding Author(s):
Bertin MikoloEcole Nationale Superieure Polytechnique, Université Marien Ngouabi, BP 69 Brazzaville, Congo
Tel:+242 068641803,
Email:mikolobertin@yahoo.fr
Abstract
The objective of this study was to evaluate crude extracts of leaves, bark and seeds of the plant Zanthoxylum heitzii using hexane, ethyl acetate, ethanol, water/ethanol and water solvents, and to evaluate the extracts for repellence to the malaria mosquito, Anopheles gambiae.
The results obtained showed that extracts from the bark of the native plant Zanthoxylum heitzii, unlike that of seeds and leaves, contain repellent compounds against Anopheles gambiae. The most active extract was obtained with hexane from the bark, while other solvents gave less active extracts. The Kdr strain of Anopheles gambiae mosquitoes was the most sensitive, followed by Kisumu and with Acer as the least sensitive strain. Effects of leaf and seed extracts with either hexane or other solvents were not significant.
Keywords
Mosquitoes; Repellence; Solvents; Zanthoxylum
INTRODUCTION
Control of insect vectors of diseases remains one of the great concerns in public health, particularly in tropical countries. Until now, insecticidal compound application is one of the main measures recommended by WHO for this purpose. However, due to the side effects of insecticide overuse causing resistance in mosquitoes, intoxication to humans, and environmental damage, efforts are made to produce safer and cheaper chemicals including repellents in addition to insecticides. Many insecticidal and repellent plant materials and some pathogens have been tested on insect vectors of diseases [1]. The purpose is to avoid insect bites either by killing or by repelling the vectors. Bites are currently avoided with insect repellents; most of them made with DEET (N,N-diethyl-m-toluamide) or botanical oils [2]. Different methods used for mosquito repellent applications are reported by Patel et al. [3].
Among the plants studied for their repellent or insecticidal properties are those belonging to the Zanthoxylum genus (also named Fagara) of the Rutaceae family [4,5]. Zanthoxylum is mainly found in tropical regions around the world and is a rich genus with up to 250 species identified. Many of these species are sources of materials used for medicine or pest management purposes. Bioactive constituents of Zanthoxylum genus have been reviewed [6].
In Central Africa, a well known species is Z. heitzii (Aubrev. & Pellegr.) P.G. Waterman; syn. Fagara heitzii Aubrev. & Pellegr. [7] (Figure 1) and so is Zanthoxylum gilletii (De Wild) P.G. Waterman (syn. Fagara macrophylla (Oliv). Engl.), also found elsewhere. The bark of Z. heitzii (Figure 2) is toxic to the American cockroach (Periplaneta americana),stored product pests [8] and mosquitoes [9]. A review of Zanthoxylum species used for medicinal purposes in East Africa can be found in Lye et al. [10]. Other species of this genus such as Z. amratum and Z. alatum can be found in Asia and the Americas.

Figure 1: Zanthoxylum heitzii (By: Ehoarn Bidault, CC BY-NC-ND 4.0).

Figure 2: Bark of Zanthoxylum heitzii (By K.E. Malterud, reproduced by permission).
These plants are traditionally used for controlling a wide range of pests and diseases including mosquitoes, beetles, malaria, sickle-cell anemia and toothache. The plant parts used most often are the bark of stems and roots [11-13].
Because of their traditional use, the plants of this genus have been subject to laboratory investigations. Dried plant materials (leaves, bark and fruit powder), crude extracts and their fractions, isolated compounds, and their synthetic analogues have been tested on insects. These plant materials have been investigated either alone or in mixtures to study potential synergism or antagonism. Extracts of different Zanthoxylum species are used in mixture with other plant materials in pesticide preparations. Most of them have been performed in China. Those prepared with extracts from Z. piperitum [14], Z. bungeanum [15-18] and Z. usambarense [19] have expressed biological activities on some insect pests and microorganisms.
The objective of this study was to test Z. heitzii crude leaf, bark and seed extracts for repellence against A. gambiae and to determine which of hexane, ethyl acetate, ethanol and water were the best solvent for extraction of repellent compounds.
MATERIALS AND METHODS
Botanical material preparation
Leaves, seeds, and bark of Z. heitzii were collected around Douakani village in the southwestern part of the Republic of Congo. The plant material was treated and extracts were made as previously described [9]. Briefly, after drying, grinding and extraction with hexane in a Soxhlet apparatus or in an Accelerated Solvent Extractor (ASE) with hexane (Soxhlet, ASE) followed by ethyl acetate, 96% ethanol, 50% water/ethanol, and water (ASE), extracts were filtered, taken to dryness in vacuo or freeze-dried.
Mosquito strains
Three strains of Anopheles gambiae s.s. Giles were used in this study:
- Kis: Kisumu strain originating from Kenya is free of any detectable insecticide resistance mechanisms.
- kdrKis: A pyrethroid and DDT resistant strain, obtained by introgression of L1014F (kdr mutation) into the genome of the susceptible Kisumu strain through successive backcrosses followed by selection with permethrin (1 mg/L). kdrKis has the same genetic background as the Kisumu strain, but differs by the presence of 1014F allele at homozygous state.
- AcerKis: An organophosphate and carbamate resistant strain, obtained by introgression of insensitive acetylcholinesterase (Ace1R) into the genome of the Kisumu strain through successive backcrosses followed by selection with propoxur (10 mg/L). AcerKis has the same genetic background as the Kisumu strain, but differs by thepresence of Ace1R allele (9S) at homozygous state.
Mosquitoes were kept in the Institut de Recherche de Developpement (IRD), Montpellier, France as previously described [9].
Concentration preparation and paper impregnation
Concentrations of 0% (control), 0.5%, 1%, and 2% were prepared according to the WHO procedures [20]. Concentrations are expressed as percentage of the compound mass per silicone oil mass. An appropriate amount of each extract (13.2 mg extract for 2% concentration, 6.6 mg for 1%, and 3.3 mg for 0.5%) was first diluted in 1.34 ml of acetone, and then mixed with 0.66 ml (648 mg) of silicone oil. The solutions were applied to Whatman filter paper. The impregnated paper was then air-dried for 24 hours and inserted into a cylinder for repellency tests.
Contact repellence tests
Tests were done in LIN (Laboratoire de Lutte contre les Insectes Nuisible), IRD, Montpellier, following a procedure described in Kawada et al. 2014 [20]. Briefly, tests were carried out in standard WHO susceptibility test kits with exposure tubes lined with Whatman papers impregnated with extract preparations or with solvent alone. Ten mosquitoes were introduced into an observation tube lined with unimpregnated filter paper [21]. The observation tube was attached to an exposure tube, but with a door preventing mosquito movement between tubes. Tubes were placed horizontally on a bench in a quiet room at 22°C and left for 2 min for mosquito stabilization. In these conditions, mosquitoes normally stayed on the inner surface of the observation tube if they were not disturbed. Then doors between observation and exposure tubes were opened carefully to allow mosquitoes free movement between tubes. After 10 min of mosquito contact with the impregnated paper, the door between tubes were closed carefully and numbers of mosquitoes in each tube counted. Each assay was replicated three times.
Data analysis
Concentrations were converted into logarithms. Probit was chosen as a mathematical model. The goodness-of-fit was tested by Chi-square tests. The relative repellence rates expressed by effective concentrations of extracts at 50% response level (EC50) were compared by examining their 95% confidence intervals. If the intervals overlap, the lethal doses do not differ significantly. Data were analysed using the PoloPlus program [22]. Graphs of main effects were drawn using Minitab 17.3.
RESULTS
Analysis of the results showed that repellence rates were concentration-dependent (Table 1(A-C); Figure 3).
|
|
Soxhlet Hexane |
ASE Hexane |
ASE Ethyl acetate |
ASE Ethanol |
ASE Aq.ethanol 50% |
ASE Water |
|||||||
|
Strain |
Dose (%) |
Tested |
Responded |
Tested |
Responded |
Tested |
Responded |
Tested |
Responded |
Tested |
Responded |
Tested |
Responded |
|
Kis |
0.00 |
57 |
5 |
62 |
1 |
59 |
3 |
63 |
0 |
60 |
1 |
60 |
1 |
|
0.05 |
52 |
15 |
61 |
1 |
71 |
11 |
63 |
9 |
59 |
1 |
63 |
7 |
|
|
1.00 |
60 |
32 |
62 |
4 |
60 |
21 |
66 |
8 |
59 |
1 |
62 |
5 |
|
|
2.00 |
51 |
35 |
65 |
32 |
63 |
15 |
64 |
9 |
60 |
1 |
61 |
7 |
|
|
Acer |
0.00 |
60 |
3 |
30 |
1 |
30 |
0 |
31 |
0 |
30 |
0 |
30 |
0 |
|
0.05 |
57 |
3 |
78 |
16 |
60 |
1 |
62 |
1 |
57 |
0 |
58 |
0 |
|
|
1.00 |
69 |
36 |
60 |
2 |
60 |
1 |
61 |
1 |
59 |
1 |
62 |
2 |
|
|
2.00 |
63 |
45 |
59 |
7 |
66 |
16 |
58 |
5 |
70 |
4 |
61 |
2 |
|
|
Kdr |
0.00 |
103 |
1 |
64 |
3 |
18 |
0 |
30 |
2 |
30 |
2 |
31 |
2 |
|
0.05 |
68 |
14 |
60 |
3 |
24 |
1 |
31 |
12 |
30 |
3 |
19 |
4 |
|
|
1.00 |
67 |
45 |
62 |
21 |
28 |
7 |
31 |
10 |
19 |
3 |
17 |
6 |
|
|
2.00 |
64 |
64 |
62 |
45 |
21 |
8 |
22 |
13 |
21 |
3 |
21 |
7 |
|
Table 1A: Cumulative numbers of Anopheles gambiae repelled by Zanthoxylum heitzii Bark extracts.
|
|
Soxhlet |
ASE Hexane |
ASE Ethyl acetate |
ASE Ethanol |
ASE Aq.ethanol 50% |
ASE Water |
|||||||
|
Strain |
Dose (%) |
Tested |
Responded |
Tested |
Responded |
Tested |
Responded |
Tested |
Responded |
Tested |
Responded |
Tested |
Responded |
|
Kis |
0.00 |
28 |
3 |
69 |
4 |
59 |
3 |
63 |
0 |
60 |
1 |
60 |
1 |
|
0.05 |
63 |
13 |
58 |
7 |
66 |
5 |
58 |
10 |
59 |
1 |
60 |
1 |
|
|
1.00 |
58 |
16 |
67 |
5 |
63 |
9 |
66 |
7 |
59 |
1 |
59 |
3 |
|
|
2.00 |
62 |
17 |
50 |
10 |
60 |
8 |
65 |
9 |
60 |
1 |
61 |
4 |
|
|
Acer |
0.00 |
30 |
0 |
30 |
0 |
30 |
0 |
31 |
0 |
30 |
1 |
30 |
0 |
|
0.05 |
60 |
2 |
61 |
1 |
66 |
1 |
58 |
0 |
62 |
2 |
62 |
0 |
|
|
1.00 |
60 |
7 |
60 |
2 |
60 |
0 |
60 |
1 |
62 |
2 |
60 |
1 |
|
|
2.00 |
78 |
12 |
64 |
2 |
62 |
0 |
60 |
0 |
62 |
4 |
60 |
0 |
|
|
Kdr |
0.00 |
39 |
1 |
32 |
0 |
31 |
2 |
30 |
2 |
28 |
0 |
30 |
2 |
|
0.05 |
32 |
2 |
32 |
2 |
30 |
4 |
37 |
7 |
29 |
0 |
22 |
2 |
|
|
1.00 |
30 |
4 |
30 |
2 |
32 |
8 |
32 |
13 |
31 |
3 |
14 |
3 |
|
|
2.00 |
35 |
5 |
32 |
2 |
30 |
8 |
27 |
13 |
33 |
3 |
19 |
2 |
|
Table 1B: Cumulative numbers of Anopheles gambiae repelled by Zanthoxylum heitzii Seed extracts.
|
|
Soxhlet Hexane |
ASE Hexane |
ASE Ethyl acetate |
ASE Ethanol |
ASE Aq. ethanol 50% |
ASE Water |
|||||||
|
Strain |
Dose (%) |
Tested |
Responded |
Tested |
Responded |
Tested |
Responded |
Tested |
Responded |
Tested |
Responded |
Tested |
Responded |
|
Kis |
0.00 |
65 |
2 |
63 |
2 |
63 |
3 |
53 |
2 |
60 |
2 |
60 |
2 |
|
0.05 |
60 |
1 |
67 |
3 |
63 |
3 |
44 |
2 |
60 |
2 |
63 |
2 |
|
|
1.00 |
58 |
2 |
69 |
9 |
64 |
4 |
57 |
5 |
62 |
3 |
64 |
3 |
|
|
2.00 |
63 |
3 |
63 |
3 |
64 |
5 |
64 |
2 |
59 |
3 |
59 |
3 |
|
|
Acer |
0.00 |
60 |
1 |
33 |
1 |
33 |
1 |
31 |
1 |
33 |
1 |
33 |
1 |
|
0.05 |
57 |
3 |
62 |
2 |
33 |
1 |
34 |
1 |
30 |
0 |
33 |
1 |
|
|
1.00 |
62 |
5 |
59 |
1 |
29 |
0 |
30 |
0 |
32 |
0 |
33 |
2 |
|
|
2.00 |
61 |
5 |
60 |
0 |
31 |
1 |
34 |
1 |
35 |
1 |
31 |
1 |
|
|
Kdr |
0.00 |
58 |
2 |
18 |
0 |
31 |
2 |
28 |
0 |
28 |
0 |
30 |
1 |
|
0.05 |
60 |
2 |
26 |
2 |
14 |
5 |
30 |
1 |
31 |
1 |
20 |
2 |
|
|
1.00 |
60 |
3 |
28 |
7 |
18 |
5 |
32 |
2 |
29 |
1 |
24 |
2 |
|
|
2.00 |
50 |
0 |
21 |
8 |
24 |
9 |
28 |
1 |
38 |
5 |
18 |
3 |
|
Table 1C: Cumulative numbers of Anopheles gambiae repelled by Zanthoxylum heitzii Leaves extracts.
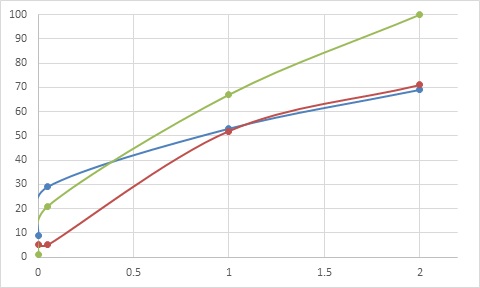 Figure 3: Percent repellence of hexane extract (Soxhlet) of Z. heitzii bark as a function of % concentration. Green: kdr-Kis strain ( pyrethroid resistant), orange: acer-Kis (organophosphate/carbamate resistant), blue: Kis strain.
Figure 3: Percent repellence of hexane extract (Soxhlet) of Z. heitzii bark as a function of % concentration. Green: kdr-Kis strain ( pyrethroid resistant), orange: acer-Kis (organophosphate/carbamate resistant), blue: Kis strain.
The hexane (Soxhlet) bark extract showed the highest activity, with more than 50% repellence towards all strains at 1% concentration. All three mosquito strains were sensitive to this extract, according to the chi-square values obtained. The pyrethroid-resistant strain KdrKis was the most sensitive one, followed by Kisumu and AcerKis. The EC50 values obtained with this extract tested on these strains, KdrKis, Kisumu and AcerKis, were 0.28%, 0.84% and 1.03% respectively (Table 2).
|
Extraction methods |
Parameters |
Kisumu |
Kdr-Kis |
Acer-Kis |
|
Soxhlet |
LC50%m/m silicone |
0.841 |
0.279 |
1.027 |
|
95%CI |
0.533-1.432 |
0.078-to 0.626 |
0.557 -1.365 |
|
|
Χ2 |
10.358 |
13.857 |
0.035 |
|
|
Degree of freedom |
9 |
5 |
1 |
|
|
Heterogeneity |
1.1509 |
2.7715 |
0.035 |
|
|
ASE-Hexane |
EC50%V/V |
2.035 |
1.390 |
50.552 |
|
95%CI |
1.805 -2.461 |
1.152 -1.652 |
8.653- 695165.502 |
|
|
Χ2 |
0.000 |
0.013 |
0.428 |
|
|
Degree of freedom |
1 |
1 |
1 |
|
|
Heterogenity |
0.000 |
0.013 |
0.428 |
|
|
CI = Confidence interval, EC = Effective concentration |
||||
Table 2: Analysis of Z. heiitzii bark extracts repellency to three of A. gambiae strains.
All mosquitoes were repelled at a 2% concentration of the hexane (ASE) bark extract, except for the AcerKis strain (12% repellence). The hexane bark extracts obtained by ASE method were less effective towards the KdrKis and Kisumu strains, with EC50 values of 1.4% and 2.0%, respectively.
No significant correlation was present between the extract concentrations and repellence rates for bark extracts obtained with other solvents (ethyl acetate, ethanol and water) and leaf and seed extracts obtained with all solvents.
DISCUSSION
The bark of Zanthoxylum heitzii contains compounds which are repellent to three strains of A. gambiae. The repellence rates obtained with bark hexane extract were concentration-dependent. The susceptible strain (Kisumu) and the resistant ones (Acer and Kdr) are all susceptible to these extracts. Repellent activity has previously been observed in extracts of related plants such as Z. zanthoxyloides on M. domestica [23] and in Z. limonella (in admixture with clove oil) on Aedes aegypti, Culex quinquefasciatus and Anopheles dirus mosquitoes [24].
Hexane is the best solvent for extracting Zanthoxylum heitzii repellent compounds, thus the active principles are lipophilic, as previously found for the mosquito toxicity of Z. heitzii extracts [9]. Egunyomi et al. [25], working on Nigerian plants, also reported that hexane plant extracts were more repellent than methanol ones.
Compounds from seeds, leaves, barks and roots of several Zanthoxylum species have been studied for toxicity or repellency towards mosquitoes and other insects. Products often studied are powdered, dried material, extracts obtained with diverse solvents (water, ethanol, methanol, acetone or hexane) or essential oils. Thus, the essential oil from Z. setulosum and the lipophilic extracts from Z. tetraspermum and Z. caudatum are toxic to aphids [26,30], and Z. limonella oil is repellent to mosquitoes [24,27]. Lipophilic extracts of Z. usambarense [19] and Z. zanthoxyloides [23] are toxic to the housefly, Musca domestica. The essential oils from Z. limonella [28], Z. armatum [29] and Z gillettii [34] are toxic to mosquito larvae (Anopheles, Aedes and Culex).Larvicidal effects towards mosquitoes is exhibited by the ethanol extract of an unidentified Zanthoxylum species [12], as well.
The chemistry of Z. heitzii has been investigated, and alkaloids [35-39], amides [37,39,40], phenylethanoids [37], lignans [37-40], triterpenoids [38,39] and sesquiterpenoids [39,40] have been reported. Other Zanthoxylum (Fagara) species have been investigated, as well(review, e.g. [6]).Many of the compounds reported exhibit medicinal and pesticidal activities such as insecticidal, larvicidal, molluscicidal, fungicidal and antiplasmodial.
The results reported in this communication represent a continuation of our work on antimalarial properties of Z. heitzii extracts and constituents [9,39-41]. We conclude that this plant shows promising activity, having antimosquito, antiplasmodial and mosquito repellent properties, and it acts on both chloroquine-sensitive and chloroquine resistant strains of A. gambiae. Further work to elucidate its possibility as a drug in practice is needed.
CONCLUSION
Results of this study showed that the lipophilic extracts from Z. heitzii bark obtained with hexane was the most effective repellent material compared to the other extracts obtained using ethyle acetate, ethanol and water as extraction solvents. There was no significant contact repellence observed with the seed and leave extracts. Among the three strains tested, the resistant strains called Kdr Kis was the most sensible unlike the two other strains Kisumu and AcerKis.
ACKNOWLEDGMENTS
Research Council of Norway (RCN) for funding the project. Jasmin MBA| NI from Douakani village for botanical material collecting.
REFERENCES
- Weiser J, Žizka Z (2004) Brachiola gambiae n. the Microsporidian Parasite of Anopheles gambiae and A. melas in Liberia. Acta Protozoologica 43: 73-80.
- Peterson C, Coats J (2001) Insect repellents - past, present and future. Pesticide Outlook 12: 154-158.
- Patel EK, Gupta A, Oswal RJ (2012) A review on: mosquito repellent methods. International Journal of Pharmaceutical, Chemical and Biological Sciences 2: 310-317.
- Kamsuk K, Choochote W, Chaithong U, Jitpakdi A, Tippawangkosol P, et al. (2007) Effectiveness of Zanthoxylum piperitum-derived essential oil as an alternative repellent under laboratory and field applications. Parasitology Research 100: 339-345.
- Datta S, Ghosh A, Sarkar S, Deka P, Choudhuri T. et al. (2010) Herbal mosquito repellents: a review. International Journal of Pharmaceutical Science and Biotechnology1: 195-202.
- Patiño LOJ, Prieto RJA, Cuca SLE (2008) Zanthoxylum genus as potential source of bioactive compounds. In Rasooli, I. (ed.): Bioactive Compounds in Phytomedicine. Intech, Rijeka, pp 185-218.
- Tailfer Y (1989) La forêt dense d'Afrique centrale: identification pratique des principaux arbres. Agence de Cooperation Culturelle et Technique (ACCT), Paris.
- Mikolo B, Matos L, Massamba D, Mamonekene V, Miller T (2009) Extracts from the bark of Fagara heitzii (Aubr. et Pel.)(Rutaceae) tree are toxic to two weevils and the American cockroach. Entomological Research 39: 401-405.
- Overgaard H, Sirisopa P, Mikolo B, Malterud K, Wangensteen H, et al. (2014) Insecticidal activities of bark, leaf and seed extracts of Zanthoxylum heitzii against the African malaria vector Anopheles gambiae. Molecules19: 21276-21290.
- Lye KA, Bukenya-Ziraba R, Tabuti JRS, Waako PJ. In : Plant-Medicinal Dictionary for East Africa. Makerere Herbarium Handbook No. 2. Makerere University, Kampala, 2008.
- Calderon AI, Romero LI, Ortega-Barria E, Brun R, Correa AMD, et al. (2006) Evaluation of Larvicidal and in Vitro Antiparasitic Activities of Plants in a Biodiversity Plot in the Altos de Campana National Park, Panama. Pharmaceutical Biology44: 487-498.
- Garcez WS, Garcez FR, da Silva LM, Hamerski L (2009) Larvicidal activity against Aedes aegypti of some plants native to the West-Central region of Brazil. Bioresource Technology100: 6647-50.
- Mengome LE, Akue JP, Souza A, Tchoua GRF, Emvo EN (2010) In vitro activities of plant extracts on human Loa loa isolates and cytotoxicity for eukaryotic cells. Parasitology Research107: 643-650.
- Kim SI, Park C, Ohh MH, Cho HC, Ahn YJ (2003) Contact and fumigant activities of aromatic plant extracts and essential oils against Lasioderma serricorne (Coleoptera: Anobiidae). Journal of Stored Products Research39: 11-19.
- Zeng X, Li X, Wang X, Wen X, Jiang X (2019) The effect of Zanthoxylum bungeanum Maxim extract on crow's feet: a double?blind, split?face trial. Dermatologic therapy
- Liu YY, Wang DN, Liu YS, Yang Y, Zhao YW, et al. (2007) Preventive and therapeutical effect of the kernel of Zanthoxylum seed oil on experimental hyperlipidemia in rat. Journal of the Fourth Military Medical University 28: 411-413.
- Yang C, Yang Z, Zou M, Hu J, Den W (1994) Estimation of four plant products' repellent action to some stored insect pests. Journal Huazhong (Central China) Agricultural University6: 576-580.
- Chen SW, Lai MX (1985) Pharmacognostical study on the root of fourteen medicinal species in genus Zanthoxylum. Acta Pharmaceutica Sinica 20: 598-605.
- He W, Van Puyvelde L, De Kimpe N, Verbruggen L, Anthonissen K, et al. (2002) Chemical constituents and biological activities of Zanthoxylum usambarense. Phytotherapy Research 16: 66-70.
- Kawada H, Ohashi K, Dida GO, Sonye G, Njenga SM, et al. (2014) Insecticidal and repellent activities of pyrethroids to the three major pyrethroid-resistant malaria vectors in western Kenya. Parasites &Vectors 7: 208.
- World Health Organization (2006) Guidelines for testing mosquito adulticides for indoor residual spraying and treatment of mosquito nets (WHO/CDS/WHOPES/GCDPP/2006.3).WHO, Geneva, p.3-6.
- Robertson JL, Preisler HK, Russell (2003) M. PoloPlus. Probit and Logit Analysis. Users guide.LeOra Software, Petaluma, CA.
- Bisseleua HBD, Gbewonyo SWK, Obeng-Ofori D (2008) Toxicity, growth regulatory and repellent activities of medicinal plant extracts on Musca domestica(Diptera: Muscidea). African Journal of Biotechnology 7: 4635-4642.
- Trongtokit Y, Rongsriyam Y, Komalamisra N, Apiwathnasorn C (2005) Comparative repellency of 38 essential oils against mosquito bites. Phytotherapy Research 19: 303-309.
- Egunyomi A, Gbadamosi IT, Osiname KO (2010) Comparative effectiveness of ethnobotanical mosquito repellents used in Ibadan, Nigeria. Journal of Applied Biosciences 36: 2383-2388.
- Quiros DI, Emmen DA, Dominguez E, Heller MV, Coley PD, et al. (2006) A rapid, efficient method for the bioassay of extracts, fractions and compounds for activity against tropical aphids. International Journal of Pest Management 52: 333-342.
- Trongtokit Y, Rongsriyam Y, Komalamisra N, Krisadaphong P, Apiwathnasorn C (2004) Laboratory and field trial of developing medicinal local Thai plant products against four species of mosquito vectors. Southeast Asian Journal of Tropical Medicine and Public Health 35: 325-333.
- Pitasawat B, Champakaew D, Choochote W, Jitpakdi A, Chaithong U, et al. (2007) Aromatic plant-derived essential oil: an alternative larvicide for mosquito control. Fitoterapia 78: 205-210.
- Tiwary M, Naik SN, Tewary DK, Mittal PK, Yadav S (2007) Chemical composition and larvicidal activities of the essential oil of Zanthoxylum armatum DC (Rutaceae) against three mosquito vectors. Journal of Vector Borne Diseases 44: 198-204.
- Nissanka AP, Karunaratne V, Bandara BM, Kumar V, Nakanishi T, et al. (2001) Antimicrobial alkaloids from Zanthoxylum tetraspermum and caudatum. Phytochemistry 56: 857-861.
- Kubo I, Klocke JA, Matsumoto T, and Kamikawa T (1984) Insecticidal and molluscicidal activities of isobutylamides isolated from Fagara macrophylla and their synthetic analogs. ACS Symposium Series 255: 163-172.
- Ginesta E, Cunat P, Primo J, Primo-Yufera E (1994) Compounds with ovicidal effect isolated from Fagara xanthoxyloides Bioscience, Biotechnology, and Biochemistry 58: 936-937.
- Zirihi GN, Mambu L, Guédé-Guina F, Bodo B, Grellier P (2005) In vitro antiplasmodial activity and cytotoxicity of 33 West African plants used for treatment of malaria. Journal of Ethnopharmacology 98: 281-285.
- Japheth OO, Josphat MC, John VM (2014) Chemical composition and larvicidal activity of Zanthoxylum gillettii essential oil against Anopheles gambiae. African Journal of Biotechnology 13: 2175-2180.
- Ahmad S (1984) Flindersine from Fagara heitzii. Journal of Natural Products 47 : 391-392.
- Bongui JB, Blanckaert A, Elorari A, Seguin E (2005) Constituents of Zanthoxylum heitzii (Rutaceae). Biochemical Systematics and Ecology 33 : 845-847.
- Mbaze LM, Lado JA, Wansi JD, Shiao TC, Chiozem DD, et al. (2009) Oxidative burst inhibitory and cytotoxic amides and lignans from the stem bark of Fagara heitzii (Rutaceae). Phytochemistry 70 : 1442-1447.
- Ngouela S, Tsamo E, Connolly JD (1994) Lignans and other constituents of Zanthoxylum heitzii.Phytochemistry 37 : 867-869.
- Wangensteen H, Ho GTT, Tadesse M, Miles CO, Moussavi N, et al. (2016) A new benzophenanthridine alkaloid and other bioactive constituents from the stem bark of Zanthoxylum heitzii. Fitoterapia 109 : 196-200.
- Moussavi N, Malterud KE, Mikolo B, Dawes D, Chandre F, et al. (2015) Identification of chemical constituents of Zanthoxylum heitzii stem bark and their insecticidal activity against the malaria mosquito Anopheles gambiae. Parasites and Vectors 8 : 503.
- Goodman CD, Austarheim I, Mollard V, Mikolo B, Malterud KE, et al. (2016) Natural products from Zanthoxylum heitzii with potent activity against the malaria parasite. Malaria Journal 15 : 481.
Citation: Mikolo B, Overgaard HJ, Massamba D, Chandre F, Rossignol M, et al. (2019) Repellent Activity of Bark Extracts of Zanthoxylum heitzii Against the Malaria Vector Anopheles gambiae. J Plant Sci Curr Res 3: 009
Copyright: © 2019 Bertin Mikolo, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

