
Reverse Aging- A Boon to Longevity
*Corresponding Author(s):
Heena TabassumDr. D. Y. Patil Biotechnology And Bioinformatics Institute, Dr. D. Y. Patil Vidyapeeth, Pune, Maharashtra 411033, India
Email:heena.tabassum@dpu.edu.in
Abstract
Aging is defined as the gradual physical and biological changes in a living thing that leads to senescence or a downturn in biological functions and the organism's capacity to respond to metabolic stress. Reverse aging is a procedure that reverses the body's natural ageing process. Using human cells and simple organisms, some scientific studies have shown that it is possible to reverse the ageing process, but little has been added to the field. Research into lengthening life expectancy has historically been met with scepticism and worries that it might increase the number of elderly people and the prevalence of aging-related diseases. This review summarizes our current knowledge of reverse ageing. Several substances, including rapamycin and metformin, are crucial to the process of reversing ageing. Recent studies have found that metformin-based drug therapy, vitamin D3 supplementation, and calorie restriction can all slow down or reverse the effects of ageing. This manuscript discusses the mechanism of reverse ageing, the role of various molecules in it, and studies done in vitro, in vivo and in silico.
Keywords
Metformin; Polyphenols; Rapamycin; Reverse aging
Introduction
Early adulthood is the beginning of a gradual, ongoing process of natural change called aging. Many bodily processes start to gradually deteriorate in the early middle years. The According to the Danaid hypothesis, taxon-specific limitations on an organism’s capacity to sustain itself for an indefinite period frequently results from the fact that living things are innately highly complex systems. Although it doesn't rely on it, this is comparable to a natural selection force that weakens with ageing [1]. At the molecular, cellular, tissue, and organismal levels, ageing is marked by a progressive decline in functional capacity. An organism's susceptibility to illness rises as it ages, making it more frail and increasing the likelihood that it will pass away. Age is the main risk factor for a wide range of illnesses in people, including osteoporosis, cancer, cardiovascular disease, diabetes and neurodegeneration [2].
Research into lengthening longevity has historically been met with scepticism and worries that it might increase the number of elderly people and the prevalence of aging-related diseases. However, research on a variety of organisms has shown that major lifespan extensions typically go hand in hand with reduced or delayed morbidity [3]. By preventing and/or delaying physical and biological decline and regaining lost functional abilities, reasoned anti-aging techniques based on scientific data seek to slow down the ageing process. Hormone supplements, such as the growth hormone Dehydroepiandrosterone (DHEA), melatonin, and oestrogen, as well as dietary supplements containing synthetic and natural antioxidants in purified form or in plant extracts are some methods. Even though some of these treatments have shown various clinical advantages in the care of the elderly, none of them really slows down the ageing process [4]. Numerous theories contend that different ageing phenotypes result from the age-dependent buildup of damage [5,6]. Senescence, defined as irreversible cell-cycle arrest brought on by a number of mechanisms such as telomere shortening, genotoxic stress, mitogens, inflammatory cytokines, and activation of the p53 tumour suppressor and/or the cyclin-dependent kinase inhibitor p16, may eventually result from this. It is believed that cellular senescence plays a role in the dysfunction of ageing tissues and organs. Senescence and ageing both affect metabolic pathways improperly and may be brought on by internal or external signalling. On the other hand, both protect the organism from the effects of the spread of damaged cells by limiting the proliferation of potentially damaged cells (due to exposure to harmful conditions) [7].
Current Research on Reverse Aging
In mice, cellular rejuvenation therapy effectively reverses the effects of ageing. Wang and colleagues first revealed in 2016 that they could use the Yamanaka factors to slow down the ageing process and lengthen the lifespan of mice with a disease that causes premature ageing. More recently, the team discovered that the Yamanaka factors can speed up muscular renewal in young mice. Following these initial findings, additional researchers have applied the same methodology to enhance the performance of various tissues, including the heart, brain, and the vision-related optic nerve [8]. First indication that a person's biological age can be changed: The body's epigenetic clock, which calculates a person’s biological age, has been suggested for the first time in a small clinical study in California that it might be possible to turn it backward. Nine healthy volunteers were given a cocktail of three common drugs over the course of a year: growth hormone, two diabetes medications, and they lost, on average, 2.5 years from their biological ages, which were determined by analysing genetic markers. Immune systems of the participants also displayed signs of renewal. Even the trial’s organisers were surprised by the results, but because the trial was small and lacked a control arm, researchers warn that the results are preliminary. The body’s epigenome, which consists of methyl groups that tag DNA, is what powers the epigenetic clock. These tags follow a person's biological age, which can be older or younger than chronological age and changes over the course of a person’s life. By choosing groups of DNA-methylation sites throughout the genome, scientists can create epigenetic clocks [9]. Lu and colleagues found that old mice are regenerating into young ones. Sinclair and his team have restored ageing cells in mice to younger iterations of themselves by using proteins that can transform an adult cell into a stem cell. Old mice with poor eyesight and damaged retinas were miraculously able to see again in his team’s first discovery, which was published in late 2020, with vision that occasionally even matched that of their progeny [10]. Using growth hormone and DHEA to treat 10 healthy men for a month, Fahy and his team discovered some regeneration of his own thymus. During the treatment phase of the TRIIM trial, participants’ blood was drawn by the researchers. Tests revealed that each participant's blood-cell count had improved. Magnetic Resonance Imaging (MRI) was also used by the researchers to compare the thymus composition between the beginning and end of the study. They discovered that in seven of the participants, regenerated thymus tissue had taken the place of accumulated fat [11] (Table 1).
|
Bioactive Compounds
|
Role |
References |
|
Korean Panax ginseng C A Meyer |
Korean red ginseng was discovered to be useful in extending life in test animals when taken over an extended period. Specifically found the presence of phenol chemicals such as maltol has longevity properties by inhibiting oxidation of lipid molecules. |
Choi [12] |
|
Berry supplementation |
Berries high in antioxidants can improve memory and cognition in older animals. This influence is likely to be by polyphenols' direct interactions with aging neurons, which decrease the effects of tension-induced cellular signals and boosts neurons' ability to retain appropriate activities throughout lifespan. |
Willis et al., [13] |
|
Rapamycin
|
Lengthening of lifespan is achieved via mTOR suppression. Although the function of rapamycin in diet in preventing age-related vascular dysfunction is unknown, it is believed that it has a positive impact through lowering cell damage, stimulating AMPK, and enhancing the production of proteins that regulate the cell cycle. |
Lesniewski et al., [14] |
|
Vitamin D3 |
Reverses the age-related elevation in microglial stimulation and the corresponding rise in IL-1 levels while acting as an anti-inflammatory molecule. |
Moore et al., [15] |
|
Metformin |
In humans, it may slow down ageing and postpone the onset of age-related illnesses by reducing ER damage and peroxidation. Metformin also affects AMPK, to reduce type II programmed cell death linked to ageing, oxidative stress, and inflammatory processes. |
Wang et al., [16] |
|
Dietary supplementation |
Diet. Suppl-containing vegetables and/or extracts rich in antioxidants can reduce the susceptibility to oxidative stress and, as a result, can be a crucial part of a healthy lifestyle plan to promote improved neural and cognitive functionality in later stages of life. |
Galli et al., [17] |
|
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA); omega-3 fatty acids |
In the ageing brain, DHA and EPA have therapeutic potential. DHA itself causes a substantial restoration of the alterations brought on by ageing, indicating a significant role in brain ageing. |
Dyall et al., [18] |
Table 1: Role of various molecules in reverse aging.
Abbreviations: mTor- mammalian target of rapamycin, AMPK-5′-AMP-activated protein kinase, Suppl- Supplementation, ER- Endoplasmic Reticulum, IL-1- Interleukin-1, mTorC-mammalian target of rapamycin complex
Mechanisms Involved In Reverse Aging
There are several pathways that have been identified for reversing the effects of aging. In this review we have focused on 3 such pathways:
Metformin and rapamycin in reverse aging
Rapamycin: Mammal longevity is increased, however the mechanism of action is uncertain, but it might entail changes in cell growth, proliferation, increase in type II programmed cell death, or reductions in mitochondrial dysfunction. The mTOR signaling system in mammals is inhibited by rapamycin [19].
There are two complexes that makeup mTOR: mTORC1 and mTORC2. mTOR plays a crucial role in the regulation of growth factor recognition, insulin/Insulin Growth Factor/Phosphoinositide-3-Kinase/AKT, ribosome efficiency, AMPK, lysosomal amino acid detecting, caloric restriction, translation, type II programmed cell death, mitochondrial biogenesis, and metabolic processes as well as stem cell activity [20]. By reducing autophagy and proteasomal function, mTORC1 inhibition is thought to have pleiotropic effects that enhance protein and organelle balance. In toxin-induced variants of Parkinson’s controls, rapamycin therapy has been demonstrated to minimize the death of neurons and reverses amyloid β - accumulation [21].
Metformin: It is a biguanide analog and the primary medication recommended for the clinical management of adult-onset diabetes (Type 2). Despite the fact that metformin has a weaker hypoglycemic impact than other biguanides, doctors frequently recommend it because of its safety. AMPK is a crucial component in the process of its anti-glycemic impact [22]. Forkhead box O-class (FOXO) transcription factors that stimulate genes involved in cancer suppression and thermoregulation are activated by AMPK, which also phosphorylates and promotes Unc-51 Like autophagy activating Kinase 1 (ULK1) of the ULK complex. Metformin reduces ER oxidation, ROS, and aging-related oxidative stress which in turn, fuels the ongoing inflammatory activity. By encouraging the synthesis of mitochondrial Uncoupling Protein (UCP-2), AMPK reduces Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) and lowers ER and lipid peroxidation. UCP-2 actively suppresses the generation of ROS through decreasing activity of NAD(P)H oxidase and induces the production of thioredoxin by activating the transcription factor FOXO3 [16]. Studies in multiple model organisms and human cell lines have elucidated metformin’s role in targeting multiple mechanisms of aging. It inhibits mitochondrial complex I and thereby oxidative phosphorylation leading to an increased AMP: ATP ratio, causing a direct activation of AMPK. AMPK-dependent mechanisms contribute to the downstream inhibition of mTORC1, improved nutrient sensing and type II programmed cell death; thereby reducing the occurrence of diseases associated with aging (Figure 1) [23].
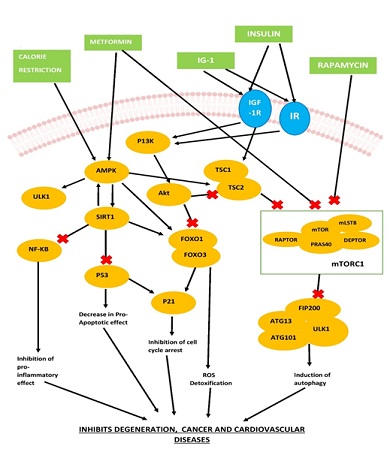 Figure 1: Explains how metformin and rapamycin help individuals prolong their lives by preventing heart disease, cancer, and cellular ageing. Both rapamycin and metformin target different signalling pathways. Rapamycin inhibits the mTOR signalling system in mammals, which is assumed to have pleiotropic effects that strengthen protein and organelle stability. Metformin, on the other hand, imposes its impact on AMPK, AMPK-dependent pathways contribute to the downstream inhibition of mTORC1, improved nutrient sensing, and type II programmed cell death. Adapted from Wang et al., [16].
Figure 1: Explains how metformin and rapamycin help individuals prolong their lives by preventing heart disease, cancer, and cellular ageing. Both rapamycin and metformin target different signalling pathways. Rapamycin inhibits the mTOR signalling system in mammals, which is assumed to have pleiotropic effects that strengthen protein and organelle stability. Metformin, on the other hand, imposes its impact on AMPK, AMPK-dependent pathways contribute to the downstream inhibition of mTORC1, improved nutrient sensing, and type II programmed cell death. Adapted from Wang et al., [16].
Abbreviations: P13K- Phosphoinositide 3-kinases, TSC 1-Tuberous sclerosis 1, TSC 2-Tuberous sclerosis 2, RAPTOR- Regulatory-associated protein of mTOR, mLST8-mammalian lethal with SEC13 protein 8, DEPTOR- DEP domain containing MTOR interacting protein, PRAS40- Proline-rich Akt substrate of 40 kDa, FIP200- PTK2/FAK family-interacting protein of 200 kDa, ATG13- Autophagy Related-13, ATG101- Autophagy Related-101, IGF-1R- Insulin Growth Factor- 1-Receptor, IR- Insulin Receptor.
Role of Sirtuins in Longevity
Age-related disorders now have sirtuins, which are epigenetic regulators as a targeted therapy. The cellular distribution and functioning of the seven human sirtuins (SIRT1-7) vary. There is mounting data that indicates small-molecule SIRT1 enhancers could prevent age-related diseases including type 2 diabetes [24]. In liver, SIRT1 levels decline with ageing, perhaps as a result of decreased NAD+ availability and concurrently rising build-up of damaged DNA. SIRT1 level was shown to decline with age in both arteries, indicating that it may play a role in cardiovascular disorders associated with aging [25]. Through the deacetylation of crucial substrates like p53, Peroxisome Proliferator Activated Receptor (PPAR) co-activator 1 (PGC-1) and NF-κB which are tightly associated to some age-related ailments, SIRT1 protein regulates various cellular functions. The most well-known SIRT1 substrate is p53, a tumour suppressor that regulates programmed cell death and the cell cycle in response to numerous biological cues. It deacetylates and links to p53, inhibiting p53-dependent programmed cell death [26]. It has been demonstrated that SIRT1 and SIRT2 are able to block Nuclear Factor-κB signaling by deacetylating p65, which alters the protein’s ability to interact with DNA and activates the transcriptional activity of inflammatory protein genes [25]. Overall, sirunins support the longer lifespan induced by calorie restriction (Figure 2).
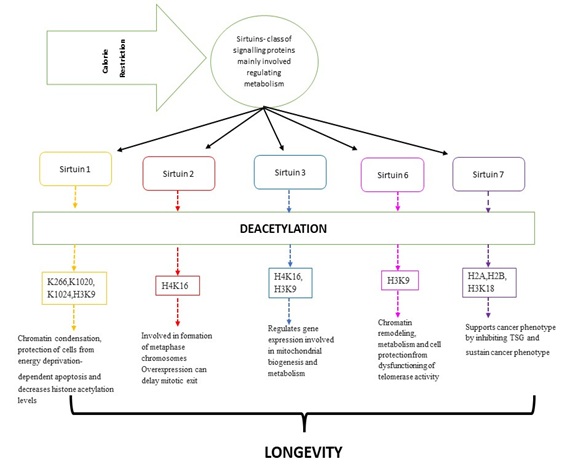 Figure 2: Explains the role of sirtuins in longevity. Sirtuins belongs to the class of signalling molecules. Calorie Restriction causes the levels of sirtuins to increase, different types of sirtuins exert different functions which overall contribute in promoting prolonged lifespan. Adapted from Grabowska et al., [25].
Figure 2: Explains the role of sirtuins in longevity. Sirtuins belongs to the class of signalling molecules. Calorie Restriction causes the levels of sirtuins to increase, different types of sirtuins exert different functions which overall contribute in promoting prolonged lifespan. Adapted from Grabowska et al., [25].
Abbreviations: H4K16- Histone H4 lysine K16, H3K9- Histone H3 lysine K 9,H2A- Histone H2A, H2B- Histone H2B, H3K18- Histone H3 lysine K18
Plant polyphenols in reverse aging
Organic molecules called polyphenols, which are abundantly present in plants, have drawn the attention of researchers from around the world because of their unique actions [27]. Due to its anti-oxidant qualities and abilities to reduce inflammation, it has sparked significant amount of interest in enhancing health, minimizing chronic illnesses, and prolonging lifespan. Several polyphenols, such as kaempferol, ellagic acid, curcumin, catechins and quercetin, have indeed been found to extend survival in a variety of animal studies [28]. A growing body of studies has shown that organic polyphenols can treat age-related neurological diseases. Grapes are a glaring indication of fruits that are rich in polyphenolic amounts. In a mouse model of Parkinson’s, the addition of grape polyphenol to the diet greatly improved memory reconsolidation; a process by which fresh learning events are converted into long-term memory [27]. The free radical theory is the most widely accepted explanation of the mechanism of ageing. ROS and Reactive Nitrogen Species (RNS) are primarily produced in mitochondria during terminal oxidation or commonly termed as oxidative phosphorylation. Although a complex of both exogenous and endogenous antioxidants neutralises ROS/RNS, some of it nevertheless manages to evade these protective mechanisms and harm biological molecules like peptides, nucleic acids and lipids. The free radical ageing theory postulates that ROS/RNS cause reactive damage, which leads to cell malfunctioning and functional decline, ageing, and emergence of progressive illnesses. This suggests that antioxidants that were effective in eliminating ROS/RNS are also capable of lowering the rate at which people age [29]. The location, functional patterns of the hydroxyl groups, amount and polyphenols’ ability to remove ROS are all influenced by the glycosylation of phytochemical compounds. They can control the generation and functioning of endogenous antioxidant and oxidase enzymes and may increase cellular antioxidant capacity by controlling the nuclear factor erythroid 2-related factor 2(Nrf2)-regulated route [28]. When polyphenols like resveratrol and quercetin were originally observed in yeast, numerous other experimental models, including C. elegans, Drosophila melanogaster, and mice, were used to corroborate the findings [29] (Figure 3 and Table 1).
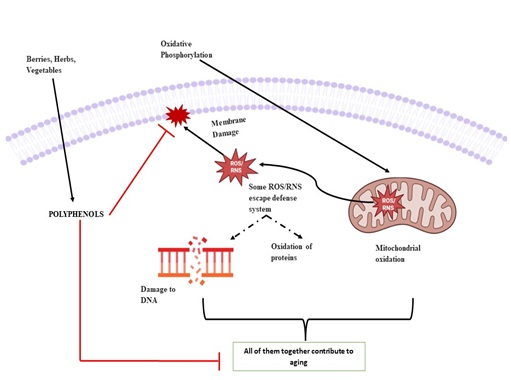 Figure 3: Explains the possible role of plant polyphenols in reducing the detrimental effects of aging and contributing towards expanding the lifespan due to its anti-oxidant qualities, abilities to reduce inflammation and minimizes the risk of chronic illnesses. Adapted from Hano & Tungmunnithum [29].
Figure 3: Explains the possible role of plant polyphenols in reducing the detrimental effects of aging and contributing towards expanding the lifespan due to its anti-oxidant qualities, abilities to reduce inflammation and minimizes the risk of chronic illnesses. Adapted from Hano & Tungmunnithum [29].
|
Type of Study |
Objective |
Cell line/ Animal model |
Compound |
Results |
Ref |
|
In vitro |
The goal of this study was to determine whether exogenous antioxidants have the ability to counteract the effects of oxidative stress and GSH depletion associated with ageing. |
1. Human Umbilical Vein Endothelial Cells (HUVEC). 2. Human Skin Fibroblast (HSF) cell models |
Quercetin |
The results showed that GSH depletion increased the sensitivity of vascular endothelial cells and fibroblasts to oxidative stress-related inflammatory stimuli. In summary, dietary antioxidants may be crucial in lowering inflammatory reactions. |
[30]
|
|
In vivo |
Determining the differences between the effects of Rapamycin (RP) and subacute Calorie Restriction (CR) on the homeostasis of the liver proteome in mice and the ability to slow the effects of ageing. |
C57BL/6 female mice |
Rapamycin |
The data provides a thorough analysis of proteome dynamics with ageing, subacute CR, and RP, and they highlight a functional relationship between protein homeostasis and longevity. |
[31] |
|
In silico |
Analyzing the human Genotype-Tissue Expression (GTEx) transcriptomic data combined with eight different expression profile datasets to define the aging matreotype across tissues. |
- |
Chodroitin Sulphate Supplementations |
These supplements are linked to reduced rates of mortality and contribute to enhanced lifespans in model species. Possible mechanisms involve improving extracellular matrix balance and lowering prolonged age associated inflammatory activity. |
[32] |
|
In vivo |
According to the study, a surprising amount of plasticity exists in ageing, with many molecular characteristics of mammalian ageing potentially reversible by the focal genetic blockade of NFκB. |
ΔSP-p50-ER transgenic mice |
4-hydroxytamoxifen |
Genetic blockade of NFκB in the skin of chronologically aged mice reversed the global gene expression program and tissue characteristics to those of young mice, demonstrating for the first time that disruption of a single gene is sufficient to reverse features of aging, at least for the short-term. |
[33] |
Table 2: Studies on age reversal that were conducted in silico, in vivo and in vitro.
Abbreviations: ΔSP-p50-ER -Dominant negative p50 fused to mutant estrogen receptor, GSH- Glutathione
Future Prospective and Conclusion
Till date, only lower organisms have been able to exhibit age reversal. Complex higher organisms can produce side effects that could increase the risk of cancer. Since Embryonic Stem Cells (ESCs) can specialize into any form of adult cell, the main emphasis is on discovering ways to convert adult cells back to ESCs [34]. Due to the intrinsic biological properties of Stem Cells (SCs), including capacity for self-renewal, and ability for multidirectional specialization, SC therapies offer a wide range of future prospects in the area of regenerative medicine and hence could slow down or possibly stop ageing [35]. One such research looked at caffeine, a quasi adenosine receptor inhibitor, as a potential medication to prevent cognitive deficits due to important alterations in adenosinergic neuronal activity associated with age [36].
Another study demonstrated that the GABA(B) blocker CGP55845 completely reversed the acquisition impairments in olfactory discrimination in cognitive impairments in older Fischer 344 rats, restoring functionality. These findings indicated the value of olfactory discrimination learning as a preclinical model for evaluating innovative therapeutics to enhance cognitive abilities in ageing and emphasised the possibilities of attacking GABA(B) receptors to alleviate age-related cognitive impairments [37]. Researchers from all over the world are working to create approaches to comprehend the aging process so that technology can be developed to do so at the cellular level. Hopefully, in the future scientists might be able to successfully carry out reverse aging in higher complex animals as well.
Acknowledgment
The authors are gratified to the Director, Dr. D. Y. Patil Biotechnology and Bioinformatics Institute, Dr. D. Y. Patil Vidyapeeth, Pune for providing with the necessary research facilities.
Declaration of Conflict of Interest
The authors declare no conflicts of interest with respect to publication of paper.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
References
- Wensink MJ, Cohen AA (2022) The Danaid Theory of Aging. Front Cell Dev Biol 9: 671208.
- Booth LN, Brunet A (2016) The Aging Epigenome. Mol Cell 62: 728-744.
- Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, et al. (2015) Interventions to Slow Aging in Humans: Are We Ready?. Aging cell 14: 497-510.
- Rattan SI (2005) Anti-ageing strategies: Prevention or therapy? EMBO reports 6: 25-29.
- Ferrucci L, Gonzalez-Freire M, Fabbri E, Simonsick E, Tanaka T, et al. (2020) Measuring biological aging in humans: A quest. Aging cell 19: 13080.
- Maynard S, Fang EF, Scheibye-Knudsen M, Croteau DL, Bohr VA (2015) DNA Damage, DNA Repair, Aging, and Neurodegeneration. Cold Spring Harb Perspect Med 5: 025130.
- Jacczak B, Rubis B, Toton E (2021) Potential of Naturally Derived Compounds in Telomerase and Telomere Modulation in Skin Senescence and Aging. Int J Mol Sci 22: 6381.
- Wang C, Rabadan Ros R, Martinez-Redondo P, Ma Z, Shi L, et al. (2021) In vivo partial reprogramming of myofibers promotes muscle regeneration by remodeling the stem cell niche. Nat Commun 12: 3094.
- Abbott A (2019) First hint that body's 'biological age' can be reversed. Nature 573: 173.
- Lu Y, Brommer B, Tian X, Krishnan A, Meer M, et al. (2020) Reprogramming to recover youthful epigenetic information and restore vision. Nature 588: 124-129.
- Fahy GM, Brooke RT, Watson JP, Good Z, Vasanawala SS, et al. (2019) Reversal of epigenetic aging and immunosenescent trends in humans. Aging cell 18: 13028.
- Choi K-T (2008) Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin 29: 1109-1118.
- Willis LM, Shukitt-Hale B, Joseph JA (2009) Recent advances in berry supplementation and age-related cognitive decline. Curr Opin Clin Nutr Metab Care 12: 91-94.
- Lesniewski LA, Seals DR, Walker AE, Henson GD, Blimline MW, et al. (2017) Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging cell 16: 17-26.
- Moore ME, Piazza A, McCartney Y, Lynch MA (2005) Evidence that vitamin D3 reverses age-related inflammatory changes in the rat hippocampus. Biochem Soc Trans 33: 573-577.
- Wang C, Chen B, Feng Q, Nie C, Li T (2020) Clinical perspectives and concerns of metformin as an anti-aging drug. Aging Med (Milton) 3: 266-275.
- Galli RL, Shukitt-Hale B, Youdim KA, Joseph JA (2002) Fruit polyphenolics and brain aging: nutritional interventions targeting age-related neuronal and behavioral deficits. Ann N Y Acad Sci 959: 128-132.
- Dyall SC, Michael GJ, Michael-Titus AT (2010) Omega-3 fatty acids reverse age-related decreases in nuclear receptors and increase neurogenesis in old rats. J Neurosci Res 88: 2091-2102.
- Martínez-Cisuelo V, Gómez J, García-Junceda I, Naudí A, Cabré R, et al. (2016) Rapamycin reverses age-related increases in mitochondrial ROS production at complex I, oxidative stress, accumulation of mtDNA fragments inside nuclear DNA, and lipofuscin level, and increases autophagy, in the liver of middle-aged mice. Exp Gerontol 83: 130-138.
- Zhang Y, Zhang J, Wang S (2021) The Role of Rapamycin in Healthspan Extension via the Delay of Organ Aging. Ageing Res Rev 70: 101376.
- Mallikarjun V, Swift J (2016) Therapeutic manipulation of ageing: Repurposing old dogs and discovering new tricks. EBioMedicine 14: 24-31.
- Hu D, Xie F, Xiao Y, Lu C, Zhong J, et al. (2021) Metformin: A Potential Candidate for Targeting Aging Mechanisms. Aging Dis 12: 480-493.
- Kulkarni AS, Gubbi S, Barzilai N (2020) Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab 32: 15-30.
- Milne JC, Denu JM (2008) The Sirtuin family: Therapeutic targets to treat diseases of aging. Curr Opin Chem Biol 12: 11-17.
- Grabowska W, Sikora E, Bielak-Zmijewska A (2017) Sirtuins, a promising target in slowing down the ageing process. Biogerontology 18: 447-476.
- Zeng L, Chen R, Liang F, Tsuchiya H, Murai H, et al. (2009) Silent information regulator, Sirtuin 1, and age-related diseases. Geriatr Gerontol Int 9: 7-15.
- Meccariello R, D'Angelo S (2021) Impact of Polyphenolic-Food on Longevity: An Elixir of Life. An Overview. Antioxidants (Basel) 10: 507.
- Luo J, Si H, Jia Z, Liu D (2021) Dietary Anti-Aging Polyphenols and Potential Mechanisms. Antioxidants (Basel) 10: 283.
- Hano C, Tungmunnithum D (2020) Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines (Basel) 7: 26.
- Hu HL, Forsey RJ, Blades TJ, Barratt ME, Parmar P, et al. (2000) Antioxidants may contribute in the fight against ageing: An in vitro Mech Ageing Dev 121: 217-230.
- Karunadharma PP, Basisty N, Dai DF, Chiao YA, Quarles EK, et al. (2015) Subacute calorie restriction and rapamycin discordantly alter mouse liver proteome homeostasis and reverse aging effects. Aging cell 14: 547-557.
- Ewald CY (2021) Drug Screening Implicates Chondroitin Sulfate as a Potential Longevity Pill. Front Aging 2: 741843.
- Adler AS, Kawahara TL, Segal E, Chang HY (2008) Reversal of aging by NFkappaB blockade. Cell Cycle 7: 556-559.
- Poston L (2022) Future anti-aging technology: Is age reversal really possible? Invigor Medical, Washington, USA.
- Chang L, Fan W, Pan X, Zhu X (2022) Stem cells to reverse aging. Chin Med J 135: 901-910.
- Prediger RD, Batista LC, Takahashi RN (2005) Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol Aging 26: 957-964.
- Lasarge CL, Bañuelos C, Mayse JD, Bizon JL (2009) Blockade of GABA(B) receptors completely reverses age-related learning impairment. Neuroscience 164: 941-947.
Citation: Tabassum H, Mathur S, Gawas CG (2023) Reverse Aging- A Boon to Longevity. J Gerontol Geriatr Med 9: 160.
Copyright: © 2023 Heena Tabassum, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

