
Risk Factors Associated With Inguinal Hernia Diagnosis in Infants Admitted To the NICU: Evidence for Effect of Care Process
*Corresponding Author(s):
Dale R. GerstmannNeonatology (Pediatrix), Timpanogos Regional Hospital NICU, 750 W 800 N, Orem, Utah 84057, United States
Tel:801-714-6605,
Email:dale.gerstmann@pediatrix.com
Abstract
Objective
To explore inguinal hernia (IH) risk factors in NICU infants.
Study Design
Design was a retrospective review of de-identified data adjusted for gender, birth size, race/ethnicity, and gestational age. IH risk was defined as the case ratio, observed/expected (O/E).
Results
1433027 infant records were queried from 1/1/1997 to 12/31/2018, with IH reported in 1.49%. Male, Black, and small-for-dates infants had IH frequencies 3.9x, 1.9x, and 1.5x above baseline. IH prevalence increased with decreasing gestational age (10.3x at 25 weeks), and was 17 per 10000 admissions in term infants. O/E ranged 7.42 fold across sites. Infants with a congenital diaphragmatic hernia, omphalocele, gastroschisis, or suspected or medically treated necrotizing enterocolitis (NEC) had elevated IH risk (O/E 6.59, 6.12, 2.38, 1.40 and 1.26, respectively), but not those with surgical NEC (O/E 0.70) or those in one center where moderate feeding volumes were used (O/E 0.58).
Conclusion
We found that IH risk varied significantly across NICUs, suggesting that care process factors may influence IH risk. The authors hypothesize that increased intra-abdominal pressure contributes to IH development, as with certain neonatal abdominal surgical conditions or high volume feeds.
Keywords
Care process; Inguinal hernia; Newborn intensive care unit; Risk factors
Abbreviations
IH: Inguinal hernia
NICU: Neonatal intensive care unit
SGA: Small for gestational age
EGA: Estimated gestational age
O/E: Ratio of observed to expected cases
NEC: Necrotizing enterocolitis
Introduction
Inguinal hernia (IH) development in infants is a consequence of developmentally weak inguinal structures for which sufficient intra-abdominal pressure is present to push viscera into the process vaginalis [1]. The condition is reported to occur in up to 13% of preterm infants less than 33 weeks gestational age and 3-5% of term infants [2]. Repair of IH is the most common surgical procedure performed in infancy [3]. A recent study identified that 45% of NICU infants with IH would undergo surgical repair before hospital discharge [4]. Approximately 56% of these will be emergency surgeries with a subsequent complication rate of 27% and a recurrence rate of 14% [5].
The current study utilizes a 1.4 million patient multi-center database to define the influence of demographic factors on the risk of diagnosing IH in infants admitted to NICU care. Further, the study investigates the relationship of risk across centers and for certain neonatal abdominal surgical conditions. Finally, the authors discuss the role of abdominal pressure in neonatal IH etiology and the possible role of care process factors contributing to variation in IH risk.
Methods
Study type
This study is a retrospective review of de-identified neonatal intensive care patient data sets.
Patient data sets
Pediatrix Medical Group provides health care at 351 NICU in 34 states and Puerto Rico, including approximately 20% of all NICUs in the United States. Pediatrix Medical Group health care professionals (doctors and nurse practitioners) providing care to neonates admitted for intensive care use a proprietary software system to generate clinical admission, discharge, and daily progress notes. These data are stored in a consolidated data set and then de-identified for quality assurance and research. Previous publications detail our data set and population demographics [6,7]. The data warehouse is compliant with Health Insurance Portability and Accountability Act of 1996 regulations.
We performed a retrospective case series review of neonates with an IH diagnosis. Identification of cases occurred by searching the diagnosis table text field for the term “inguinal hernia.” We do not have data on whether the infant had only a unilateral hernia or bilateral hernias. The Western Institutional Review Board approved use of our de-identified data set with a waiver of consent. Additionally, the Timpanogos Regional Hospital (TRH) IRB approved the study project and the use of a local patient database (NeoData, Isoprime).
Patient population
Neonates were included in our study sample if they required admission to a NICU and survived beyond seven days. We included infants discharged between January 1, 1997, and December 31, 2018. The gestational age assignment was based on the best obstetrical estimate before delivery and recorded as completed weeks. Infant size (SGA, AGA, LGA) was determined based on Olsen growth charts [8].
Statistical methods
Our analytical approach to these data was descriptive. We calculated the prevalence of inguinal hernia as the number of inguinal hernia cases in the analysis group divided by the total number of infants. Statistical analysis for categorical variables employed 2-tailed chi-square tests. Multivariate analysis (using JMP 12, SAS Institute, Cary, NC) identified associations between multiple demographic factors and IH diagnosis. These demographic factors included: maternal age, gender, report of antenatal steroids, mode of delivery, gestational age less than 37 weeks, birth weight, being small for gestational age, race/ethnicity, 5 minute Apgar score, and use of assisted ventilation during the first three days after birth. Risk factor analysis did not include outborn infants, infants transferred to another hospital, or infants who died. We made these exclusions to ensure that demographic data were complete and avoid over-counting patients referred for IH surgical repair.
The multivariate analysis revealed that EGA week at birth (23-42 weeks), gender (male, female), birth size (SGA, AGA, LGA), and race/ethnicity (Asian, Black, Hispanic, White, other) had the highest Chi-Square scores (Table 1) and once identified, used as the significant factors in the risk analysis.
|
Parameter |
Chi-Square Value |
p-value |
|
Estimated Gestational Age (23-42 weeks) |
105037 |
<0.0001 |
|
Gender (Male, Female) |
6474 |
<0.0001 |
|
Size (SGA, AGA, LGA) |
2717 |
<0.0001 |
|
Race/Ethnicity (Asian, Black, Hispanic, Other, White |
1119 |
<0.0001 |
Table 1. Chi-Square Calculations for Major IH Risk Factors.
O/E ratios were determined by direct calculation using original software developed by one of the authors (DRG). The data set was large enough to calculate the individual non-zero cell frequencies for each combination of the categories for the four major risk factors, comprising 19•2•3•5 = 570 cells. For any chosen analysis group, calculation of the expected number of IH cases used these cell frequencies, then compared to the actual or observed number of cases using the ratio of observed to expected cases (O/E ratio).
Evaluation of site variation as a factor in IH risk used a subset analysis of 103 sites with 5000 or more patients per site in the Pediatrix dataset, expecting to have at least 75 patients per site diagnosed with IH. The O/E ratio was also calculated for patients with neonatal abdominal surgical conditions, including gastroschisis, omphalocele, congenital diaphragmatic hernia, and four categories of necrotizing enterocolitis: no NEC, suspected NEC, medically treated NEC, and surgically treated NEC.
For comparison, six years of patient data (2014-2020) comprising 1514 neonatal patients at one author’s site (TRH) underwent the same IH O/E risk analysis. These site data were independent of the Pediatrix data set.
Results
In the Pediatrix de-identified data set, there were 1433027 infants discharged between 1997 and 2018, of which 21421 had a diagnosis of IH, making the baseline database IH frequency 1.49%. The prevalence of IH by EGA for survivors beyond seven days was: 23-27wks, 1358 per 10000; 28-31wks, 473 per 10000; 32-36wks, 44 per 10000; >36wks, 17 per 10000.
The relative importance of risk factors is shown in Figure 1, displaying subgroup frequency as a ratio to baseline raw frequency. Presented are elevated frequency ratios (>1.0) for very low EGA (10.35 at 25wks), male gender (1.49), SGA size (1.91), and Black race (1.47). Significantly decreased frequency ratios (<1.0) were seen for EGA > 32wks (0.07 at 40wks), female gender (0.38), LGA size (0.42), and Hispanic ethnicity (0.76).
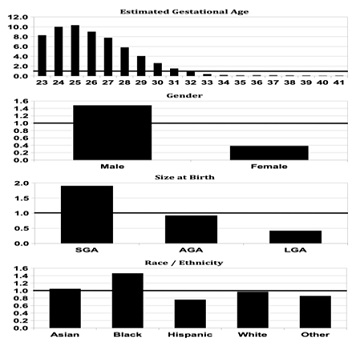 Figure 1: Inguinal hernia (IH) frequency as a ratio to base frequency (1.49%) for the four most significant demographic factors. Panel A: Estimated Gestational Age; Panel B: Gender; Panel C: Birthweight Percentile Size; Panel D: Race/Ethnicity. Extremely low gestational age SGA black male infants had the highest frequency of IH diagnosis.
Figure 1: Inguinal hernia (IH) frequency as a ratio to base frequency (1.49%) for the four most significant demographic factors. Panel A: Estimated Gestational Age; Panel B: Gender; Panel C: Birthweight Percentile Size; Panel D: Race/Ethnicity. Extremely low gestational age SGA black male infants had the highest frequency of IH diagnosis.
There was no clinically meaningful change in the O/E ratio for IH diagnosis year over year for the study period 1997-2018 (median 1.0, range 0.85-1.11, coefficient of variation 7%) (Figure 2).
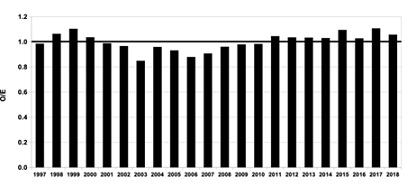 Figure 2: Inguinal hernia risk as ratio of Observed/Expected (O/E) by year 1997-2018 for neonates admitted to neonatal intensive care (Pediatrix Clinical Data Warehouse). The variation year-on-year is small (7% coefficient of variation).
Figure 2: Inguinal hernia risk as ratio of Observed/Expected (O/E) by year 1997-2018 for neonates admitted to neonatal intensive care (Pediatrix Clinical Data Warehouse). The variation year-on-year is small (7% coefficient of variation).
Figure 3 depicts site variation in the O/E ratio for 103 centers contributing 5000 or more patients to the data set, ordered from lowest O/E ratio (0.33) to highest O/E ratio (2.45). The percentage of these centers with O/E less than, equal to, and greater than 1.0 was 45%, 5%, 40%, respectively. The site with the maximum O/E ratio had a 7.42 times higher risk than the site with the lowest O/E ratio.
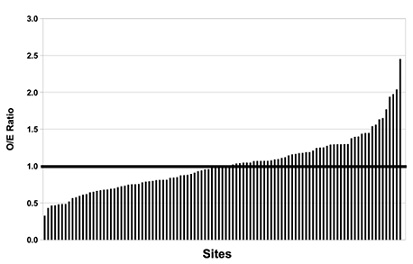 Figure 3: Inguinal hernia (IH) Risk as Observed/Expected (O/E) ratio for 103 Pediatrix NICUs with 5000 or more patients in the dataset, ordered from lowest IH risk to highest IH risk. There is an 7.42 fold difference in risk between the sites with lowest and highest IH O/E ratio.
Figure 3: Inguinal hernia (IH) Risk as Observed/Expected (O/E) ratio for 103 Pediatrix NICUs with 5000 or more patients in the dataset, ordered from lowest IH risk to highest IH risk. There is an 7.42 fold difference in risk between the sites with lowest and highest IH O/E ratio.
Figure 4 shows the IH risk analysis for neonates with certain neonatal abdominal surgical conditions. Illustrated are the O/E ratios for CDH (6.59), omphalocele (6.12), and gastroschisis (2.38). O/E ratios for the NEC categories of no NEC, suspected NEC, medical NEC, and surgical NEC were 0.98, 1.40, 1.26, and 0.70, respectively. Except for no NEC, Chi-square values for all conditions were highly significant (p < 0.0001).
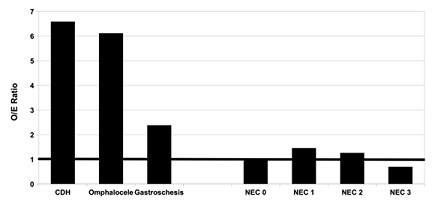 Figure 4: Inguinal hernia (IH) Risk as Observed/Expected (O/E) ratio for certain neonatal abdominal surgical conditions. Congenital diaphragmatic hernia (CDH), omphalocele and gastroschisis have significantly elevated risk for the diagnosis of IH (p<0.0001, each). Infants without necrotizing enterocolitis (NEC 0) have expected risk (O/E = 1). Infants with suspected NEC (NEC 1) and infants with medically treated NEC (NEC. 2) have significantly increased risk (p<0.001), whereas infants with surgically treated NEC (NEC 3) have significantly decreased risk (p < 0.001).
Figure 4: Inguinal hernia (IH) Risk as Observed/Expected (O/E) ratio for certain neonatal abdominal surgical conditions. Congenital diaphragmatic hernia (CDH), omphalocele and gastroschisis have significantly elevated risk for the diagnosis of IH (p<0.0001, each). Infants without necrotizing enterocolitis (NEC 0) have expected risk (O/E = 1). Infants with suspected NEC (NEC 1) and infants with medically treated NEC (NEC. 2) have significantly increased risk (p<0.001), whereas infants with surgically treated NEC (NEC 3) have significantly decreased risk (p < 0.001).
The same risk analysis was performed on a local (TRH) seven-year dataset of 1514 patients. The calculated O/E ratio (0.58) for TRH compared to the Pediatrix CDW was significantly < 1.0 (p < 0.0001). At this site, a standardized feeding protocol has been in place for many years, with an upper limit feeding volume for gavage feedings of 140 mL/kg/d (Figure 5). In the Pediatrix data warehouse dataset, 35.5% of neonates with IH underwent surgical repair before hospital discharge at a median age of 77 days (30-124, 10-90%tile).
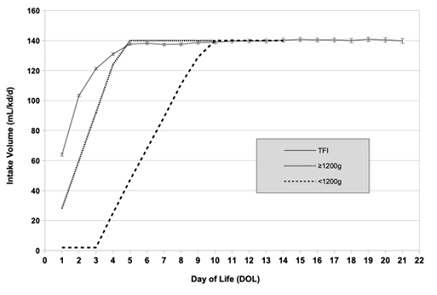 Figure 5: Volume intake at site TRH over the first 3 weeks of life, shown as Total Fluid Intake (TFI, solid line), and as feeding protocol volume for infants < 1200g (long-dash line) or ≥1200g (short-dash line). Maximum gavage feeding volume was 140 mL/kg/d.
Figure 5: Volume intake at site TRH over the first 3 weeks of life, shown as Total Fluid Intake (TFI, solid line), and as feeding protocol volume for infants < 1200g (long-dash line) or ≥1200g (short-dash line). Maximum gavage feeding volume was 140 mL/kg/d.
Discussion
Our study analysis of 22 years of data from the Pediatrix Clinical Data Warehouse confirms previous reports identifying increased IH frequency in the very preterm infant [9-11]. The current analysis provides more detail. We saw an almost linear increase in IH diagnosis frequency with decreasing EGA from 32 to 25 weeks, with a maximum at 25 weeks of 10.4 times above base frequency, or 15.5%. Contrary to the 2012 publication from the American Academy of Pediatrics Committee on Fetus and Newborn Section on Surgery [2] quoting a 3-5% IH frequency in term infants, our data set suggests a much lower IH frequency with a prevalence of 17 per 10000 in term infants (>36 weeks EGA). Although IH is not exclusive to the male gender in the NICU infant, male infants in our study were 3.9 times overall more likely than female infants to have a diagnosis of IH. The current study’s male/female ratio is slightly more than the male/female infant ratio of 3.4 reported by Unal et al [11] for VLBW infants or the ratio of 3.5 reported by Fu et al [12] for preterm infants. However, Fu et al [12] reported a male/female ratio of 3.9 for term infants, which is the same as for all patients in the current study. The medical literature has few references on whether intrauterine development influences the propensity to develop IH. One early report by Peevy et al [9]. suggests an association between neonatal inguinal hernia and intrauterine growth retardation. The current analysis identified an association between increased IH diagnosis frequency and SGA size at birth (<10th percentile for weight). The finding of increased IH frequency in SGA infants would support the IUGR association identified in 1986 by Peevy et al [9]. Our race/ethnicity analysis indicates an increased IH diagnosis frequency in black NICU infants. However, increased IH frequency in black NICU infants seems at odds with a report of reduced IH frequency in the US black adult male population, [13] presumably due to anatomic differences in inguinal canal dimensions [14] there is also the known confounding factor of increased SGA frequency in black infants [15]. However, in our multivariate analysis, both Black and SGA size at birth were independent risk factors for IH diagnosis in NICU infants.
There is good evidence for improved neonatal morbidity trends over the past two decades by applying quality methods, employment of best practices, and attention to decreasing process variation [16, 17]. Many neonatal conditions, particularly for the very premature infant, have benefitted [16,17]. Interestingly, we were unable to identify any trend for a longitudinal decrease in IH diagnosis frequency over the more than twenty-year time frame in the current data set. The O/E ratio was relatively stable over this period with a low degree of variation (7% coefficient of variation). One could conclude that the interventions affecting morbidity change, as noted above, have not influenced the propensity to develop IH or that IH frequency in the neonate is invariant. The latter is unlikely to be true. We also noted more than a seven-fold difference in IH risk as measured by the O/E ratio in the top 103 sites having the most patients in the Pediatrix data set. Care variation is an identifiable source of outcome differences between sites for many neonatal outcomes. So, what neonatal care differences across sites could modify the risk for IH diagnosis by a seven-fold extent?
Powell et al. [1] considered that non-anatomical factors could be important in determining which preterm infants developed IH in their sample of 995 infants, noting that infants with abdominal distention and increased abdominal pressure were more likely to develop IH. “Precipitation of an inguinal hernia must relate to increased abdominal pressure” [1]. Supporting this observation, patients in the current study with increased abdominal pressure due to surgically returning organs into the abdominal cavity (congenital diaphragmatic hernia, gastroschisis, and omphalocele) were 2.4-6.6 fold more likely to develop IH subsequently. Our analysis of NICU patients with NEC identified lower than expected risk for those with surgical NEC. A plausible explanation for lower O/E risk in infants surgically treated for NEC is that standard treatment options include peritoneal drainage and diverting ostomies; both procedures will release/decrease abdominal pressure. Overall, our data would support a role for abdominal pressure as a driving factor for IH risk.
Powell et al. also suggest that high volume feedings in susceptible neonates may increase abdominal pressure and promote IH development [1]. We queried the Pediatrix Clinical Data Warehouse to test whether feeding volume may influence IH risk. However, the Data Warehouse lacked detailed nutritional data to clarify any relationship. To further explore a relationship between feeding volume and IH risk, we sought to define IH O/E risk using a separate local database from a NICU using protocolized feedings. At this site, the IH O/E risk was reduced (0.58) and associated with a moderate maximum feeding volume of 140 mL/kg/d. Current NICU feeding standards recommend 150-180 mL/kg/d to achieve adequate caloric intake and somatic growth for preterm infants [18]. New studies propose even higher maximum feeding volumes for this patient population, up to 200 mL/kg/d [19]. The lower maximum feeding volume at the local site was adopted initially to help mitigate an observed effect of abdominal distention due to feedings on several common clinical problems, e.g., frequency of apnea and bradycardia, reflux symptoms, and basal oxygen requirements, to positive effect. Hyper-caloric human milk feedings were instituted at concentrations of 30-36 Cal/oz to compensate for the caloric decrease due to lower maximal feeding volume. Infants had excellent tolerance for hyper-caloric human milk feedings, which normalized growth rates.
Conclusion
The authors hypothesize that the suspected care variation noted between Pediatrix clinical sites in IH O/E risk may be due to differences in maximal feeding volumes, and thus indirectly, in abdominal pressure exposure. Recommendations to increase standard feeding volumes for the preterm infant by 30-40% may have the unwanted effect of increasing IH risk and increasing IH surgical repair frequency. Further studies to evaluate care process mechanisms that may reduce IH risk and decrease surgical exposure for NICU infants are warranted.
Funding Source
No funding was secured for this study.
Financial Disclosure
The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest
The authors have no conflict of interest to disclose
Declarations Of Interest
None Contributors’ statement: This work was supported by Pediatrix. No outside honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
Dr. Gerstmann developed the research plan and is the primary author of the manuscript.
Dr. Bickler assisted in detailed review of the research data and in manuscript preparation.
Dr. Clark provided editorial input to the writing of the final paper and approved the final manuscript as submitted.
References
- Powell TG, Hallows JA, Cooke RW, Pharoah PO (1986 ) Why do so many small infants develop an inguinal hernia? Arch Dis Child 61: 991-995.
- Wang KS (2012) Committee on Fetus and Newborn, American Academy of Pediatrics; Section on Surgery, American Academy of Pediatrics. Assessment and management of inguinal hernia in infants. Pediatrics 130: 768-773.
- Verhelst J, de Goede B, van Kempen BJ, Langeveld HR, Poley MJ, et al. (2016) Emergency repair of inguinal hernia in the premature infant is associated with high direct medical costs. Hernia 20: 571-577.
- Gulack BC, Greenberg R, Clark RH, Miranda ML, Blakely ML, et al. (2018) A multi-institution analysis of predictors of timing of inguinal hernia repair among premature infants. J Pediatr Surg 53: 784-788.
- de Goede B, Verhelst J, van Kempen BJ, Baartmans M, Langeveld HR, et al. (2015) Very low birth weight is an independent risk factor for emergency surgery in premature infants with inguinal hernia. J Am Coll Surg 220: 347-352.
- Clark RH, Bloom BT, Spitzer AR, Gerstmann DR (2006) Reported medication use in the neonatal intensive care unit: Data from a large national data set. Pediatrics 117: 1979-1987.
- Ellsbury DL, Clark RH, Ursprung R, Handler DL, Dodd ED, et al. (2016) A Multifaceted Approach to Improving Outcomes in the NICU: The Pediatrix 100 000 Babies Campaign. Pediatrics 137: e20150389.
- Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS (2010) New intrauterine growth curves based on United States data. Pediatrics 125: e214-e224.
- Peevy KJ, Speed FA, Hoff CJ (1986) Epidemiology of inguinal hernia in preterm neonates. Pediatrics 77: 246-247.
- Yeo CL, Gray PH (1994) Inguinal hernia in extremely preterm infants. J Paediatr Child Health 30: 412-413.
- Unal S, Isik DU, Bas AY, Arslan Z, Demirel N (2017) Inguinal Hernia Development in Very Low-Birth-Weight Infants: A Case-Control Study. Eur J Pediatr Surg 27: 341-345.
- Fu YW, Pan ML, Hsu YJ, Chin TW (2018) A nationwide survey of incidence rates and risk factors of inguinal hernia in preterm children. Pediatr Surg Int 34: 91-95.
- Ruhl CE, Everhart JE (2007) Risk factors for inguinal hernia among adults in the US population. Am J Epidemiol 165: 1154-1161.
- Mitura K, Koziel S, Pasierbek M (2018) Ethnicity-related differences in inguinal canal dimensions between African and Caucasian populations and their potential impact on the mesh size for open and laparoscopic groin hernia repair in low-resource countries in Africa. Wideochir Inne Tech Maloinwazyjne 13: 74-81.
- Kramer MS, Ananth CV, Platt RW, Joseph KS (2006) US Black vs White disparities in foetal growth: physiological or pathological? Int J Epidemiol 35: 1187-1195.
- Soll RF (2015) Progress in the Care of Extremely Preterm Infants. JAMA 314: 1007-1008.
- Kaempf J, Morris M, Steffen E, Wang L, Dunn M (2020) Continued improvement in morbidity reduction in extremely premature infants [published online ahead of print, 2020 Oct 27]. Arch Dis Child Fetal Neonatal Ed. 2021. fetalneonatal- 2020-319961.
- Dutta S, Singh B, Chessell L, Wilson J, Janes M, et al. (2015) Guidelines for feeding very low birth weight infants. Nutrients 7: 423-442.
- Travers CP, Wang T, Salas AA, Schofield E, Dills M, et al. (2020) Higher- or Usual-Volume Feedings in Infants Born Very Preterm: A Randomized Clinical Trial. J Pediatr 224: 66-71.
Citation: Gerstmann DR, Bickler SW, Clark RH (2022) Risk Factors Associated With Inguinal Hernia Diagnosis in Infants Admitted To the NICU: Evidence for Effect of Care Process. J Neonatol Clin Pediatr 9: 100.
Copyright: © 2022 Dale R. Gerstmann, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

