
Risk Factors for Relapse in Juvenile Nasopharyngeal Angiofibroma: 4 Year Experience In A Referral Hospital
*Corresponding Author(s):
María Fernanda Galindo TapiaInstituto Mexicano Del Seguro Social. Centro Médico Nacional La Raza. Departamento De Otorrinolaringología. Paseo De Las Jacarandas S/N, La Raza, Azcapotzalco, 02990 Ciudad De México, Mexico
Email:galindotapiamafer@gmail.com
Abstract
Introduction: Juvenile nasopharyngeal angiofibroma (JNA) is a rare benign neoplasm, it presents mainly in adolescent males as nasal obstruction and ipsilateral epistaxis, its diagnosis is based on diagnostic suspicion, imaging studies and angiography, surgical management preceded by embolization and in some cases, radiotherapy is also used. The aim of the present study was to describe the experience in the management of patients with JNA in our unit, as well as to perform a detailed analysis to correlate age at diagnosis, diagnostic stage, time of evolution, relapse, time of pre-surgical embolization and surgical procedure to determine whether any of these factors present a risk for relapse.
Material and methods: prospective, analytical, longitudinal clinical study in which 12 patients with diagnosis of JNA captured in the period from April 2017 to November 2020 were evaluated. All patients underwent a complete clinical history, nasal endoscopy and simple and contrasted CT of the nose and paranasal sinuses, staged using the Radkowski system. Surgical resection was performed with diagnostic angiography and pre-surgical embolization with prior informed consent for each of the procedures. Post-surgical CT was performed and followed up with imaging studies. Statistical analysis was performed with SPSS Statistics version 22.0.
Results: The average age at diagnosis was 16 years, 5 patients presented with stage IIIA, 2 in IIA and IIC and 1 in IA, IIB and IIIB. Angiography showed predominant irrigation of the internal maxillary artery. Embolization was performed between 24 and 120 hours before surgery. The surgical approach was performed depending on the diagnostic stage, open technique was used in 7 patients, endoscopic technique in 3 and combined in 2. During the follow-up, relapse occurred in 6 patients, 4 were residual tissue and 2 were de novo growths. The average number of months for relapse was 11 + 6.7 months. Relapse was managed by isolated surgical revision in 2 patients, single radiotherapy in 2 others and with both treatments in the remaining 2. When performing cross analysis of data between relapse and tumor irrigation, the presence of residual tissue and age at diagnosis, statistically significant differences were found with p-values <0.050. In the Kaplan Meier freedom analysis for preoperative embolization patients who underwent late embolization (>72 hr) tended to present greater and early recurrence within the first 100 days of follow-up.
Conclusions: there is a higher risk of relapse in patients with earlier age of onset, those with irrigation of the internal carotid system, pre-surgical embolization greater than 72 hours and those who after the surgical procedure present residual tissue, even if minimal. Relapse occurred in a higher percentage within the first year after resection.
Keywords
Angiography; Embolization; Juvenile nasopharyngeal angiofibroma; Radkowski scale
Introduction
Juvenile nasopharyngeal angiofibroma (JNA) is a highly vascularized benign neoplasm of the nose and nasopharynx. The incidence ranges from 0.05% to 0.5% of head and neck tumors [1-5] and corresponds to the most common nasopharyngeal neoplasm in adolescent males with an incidence of 1:150,000 [2,3]. The site of origin is considered to be the superior margin of the sphenopalatine foramen at the junction of the pterygoid process to the sphenoid [6-8] and along the course of the sphenoid artery [1,2,6]. Histologically, contain a mesenchymal and vascular pattern, lacking smooth muscle wrapped in fibrous stroma [9,10]; immunohistochemistry and electron microscopy give the appearance of vascular malformation with hamartomatous cells [10] and usually express alpha2 laminin, a marker of early angiogenesis, as well as the presence of androgenic receptors [11]; the latter explains its almost exclusive prevalence in adolescent males with cessation of growth associated with sexual maturity [9,12].
The JNA has a concentric growth pattern following natural paths through pre-existing foramina such as the sphenopalatine, the vidian nerve canal, the pterygomaxillary fissure and the orbital fissure; it can extend to the cavernous sinus, the optic chiasm and the pituitary gland [7,2]; in addition, its growth conditions the displacement of the posterior wall of the maxillary sinus anteriorly known radiologically as Holman-Miller sign [8].
It is usually asymptomatic during the first years of growth, manifesting initially with unilateral nasal obstruction and recurrent ipsilateral epistaxis [2,3], in more advanced cases it may present facial deformity with proptosis, headache, diplopia, rhinolalia, otalgia and cranial nerve involvement [1,11].
Diagnosis is based on a detailed clinical history, physical examination with nasal endoscopy, imaging studies such as simple and contrasted CT of the nose and paranasal sinuses and magnetic resonance imaging that allow establishing the tumor extension, its dissemination pattern and the planning of surgical approaches [13].
There are several classification systems described. The Radkowski system stages according to CT findings, taking into account that the preoperative tumor stage corresponds to the first factor for recurrence, allowing selection of the best surgical approach [7].
The treatment of choice is surgical excision. The approach generally depends on the characteristics of the tumor and the surgeon's preference [1,3,13,14]. Diagnostic-therapeutic angiography with preoperative embolization of the nutritional vessels is almost routinely used [1,2,11,13]. Other treatment alternatives are radiotherapy, the use of hormones (estrogen, testosterone), chemotherapy, radiofrequency ablation or laser resection assisted by video-navigation and microdebrider [10].
The aim of the present study was to describe the experience in the management of patients with juvenile nasoangiofibroma in our unit, as well as to analyze and determine if there was any correlation between the age at diagnosis, the stage at which they were detected, the time of evolution until diagnosis and the overall time of evolution until resolution and/or relapse, the time between embolization and the surgical procedure, as well as to determine if any of the factors studied could affect the course of the disease as a risk factor for relapse.
Material And Methods
This is a prospective, analytical, longitudinal clinical study in which 12 patients with a diagnosis of JNA were evaluated at the Otorhinolaryngology and head and neck surgery service of the High Specialty Medical Unit (UMAE) No 1 in Leon, Guanajuato in the period from April 2017 to November 2020.
The inclusion criteria were to be UMAE patients with a diagnosis of JNA captured in the aforementioned period. Those who were operated in another unit and who due to age or family condition lost affiliation to the medical service were excluded. All patients were referred from 2nd level units. Upon reception, a complete clinical history and nasal endoscopy were taken, in addition to a simple and contrasted CT scan of the nose and paranasal sinuses. The variables included were age, time of evolution, tumor stage at diagnosis, embolization technique, time between embolization and surgery, surgical approach, intraoperative bleeding volume, presence of immediate or late complications, relapse and need for reintervention and/or radiotherapy.
For all procedures, the consent form was read under information and signed by the patient if he/she was of legal age or by his/her parents or guardians if he/she was a minor. Descriptive statistics were used for the variables. For the age averages and standard deviation were obtained. Ranges were obtained for the time between embolization and surgery: 72 hrs; for intraoperative bleeding, it was classified as <500cc, 501 to 1000cc and >1000cc. In the case of evolution time, the range was made in years and average. The staging was performed using the Radkowski system (Table 1).
|
Ia. |
Limited to nose and/or nasopharynx. |
|
Ib. |
Extension to one or more breasts. |
|
IIa. |
Minimal extension into the pterygopalatine fossa. |
|
IIb. |
Complete occupation of the pterygopalatine fossa with or without erosion of orbital bones. |
|
IIc. |
Posterior invasion of sphenoid wings and/or infratemporal fossa. |
|
IIIa. |
Skull base erosion with minimal intracranial extension in FCM or basisphenoid. |
|
IIIb. |
Skull base erosion with wide intracranial extension with invasion of cavernous sinus. |
Table 1: Radkowski staging for juvenile nasopharyngeal angiofibroma.
Prior to surgery, all patients underwent diagnostic angiography and embolization by the hemodynamics service. Based on the angiographic findings, a subdivision was made according to the main irrigation of the tumor, depending on whether it was exclusive of the external carotid system or external and internal. The preoperative embolization was made by transarterial technique in 10 patients and percutaneous in 2 of them. In all cases of embolization, the patients underwent general anesthesia. In patients with the transarterial technique, a right femoral catheter was placed through which the embolization material was introduced to the feeding arteries. In cases of percutaneous embolization, the embolization material was introduced through the tumor until it reached the nutrient vessel. The embolization material was microspheres in all patients.
Based on the tomographic and angiographic findings, the type of approach to be given to each case was decided, being grouped in 3: endoscopic approach, open approach (maxillectomy by facial curettage, lateral rhinotomy, Weber-Ferguson and/or craniotomy in cases with intracranial involvement) and combined approach, endoscopic with any of the aforementioned open approaches (figure 1). In all cases, post-surgical CT was performed to determine the presence of residual tissue and follow-up was continued in the office; in cases without clinical data of tumor activity, imaging studies were requested at 6, 12 and 24 months. Patients with post-surgical tumor, either due to the presence of residual or de novo tissue, were determined as relapse, in which cases the patients were reevaluated each 3 months, and in these cases, the decision was made to reoperate, send for radiotherapy, or both procedures, depending on the rate of growth and the area of tumor invasion.
 Figure 1: Relation between clinical stage and surgical methods performed.
Figure 1: Relation between clinical stage and surgical methods performed.
The statistical analysis was run with SPSS Statistics version 22.0. Finally, they were grouped in two, those without relapse disease and those with relapsed, taking into account for the last group only the first recurrence.
Qualitative variables were compared using chi-square and quantitative variables were compared using the Mann Whitney U test because they did not follow a normal distribution, although the results are expressed as mean and standard deviation.
Results
Twelve male patients were studied with an average age of 16 years, with a maximum age of 24 years and a minimum age of 9 years at the time of diagnosis. The average time of evolution was 2.5 + 1.3 years (Table 2). The initial stage with Radkowski scale varied for each patient, with stage IIIA predominating in 5 patients, followed by stages IIA and IIC and finally stages IA, IIB and IIIB (Figure 2).
|
Variable |
No relapse |
With relapse |
Report of total patients |
Value of p |
|
Embolization (hr) |
68 + 31.8 |
72.6 + 40.3 |
70.333333 |
0.79 |
|
Bleeding (ml) |
483.33 + 292 |
1316.6 + 1340.7 |
970.0206 |
0.18 |
|
Age (years) |
18.3 + 5.8 |
13.6 + 3.9 |
16 + 4.02 |
0.038 |
|
Time of evolution (years) |
3.16 + 1.6 |
2 + 0.63 |
2.5 + 1.31 |
0.128 |
Table 2: Demographic characteristics.
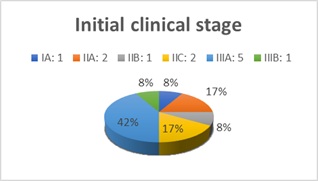 Figure 2: Clinical stage according to Radkowski scale.
Figure 2: Clinical stage according to Radkowski scale.
Diagnostic angiography denoted main supply from the ipsilateral internal maxillary artery, followed by supply from both carotid systems, then from other branches of the ipsilateral external carotid system and finally from the bilateral external carotid system (Figure 3).
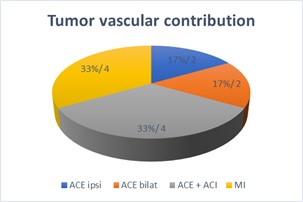 Figure 3: Tumor vascular contribution based on presurgical angiographic findings.
Figure 3: Tumor vascular contribution based on presurgical angiographic findings.
All patients underwent a surgical procedure in the first 4 months after diagnosis with preoperative embolization, between 24 and 120 hours before surgery, 50% at 72 hours.
Regarding the surgical approach, open technique was used in 7 patients, endoscopic technique in 3 and combined in two. An average trans-surgical bleeding of 900 ml was reported; however, it was grouped in bleeding >500 ml (7 patients), 501-1000 ml (3 patients) and > 1000 ml (2 patients). Regarding complications, trans-surgical bleeding occurred in 2 patients requiring transfusion and in post-surgical complications, diplopia and decrease in visual acuity occurred in one patient and rejection of the osteosynthesis material in another. In the post-surgical imaging study, five patients were reported with minimal residual tissue; four of them intracranial, attached to the ICA and 1 extracranial attached to the periorbita.
During follow-up, relapse occurred in 6 patients, of which 4 resulted from residual tissue and two of them were de novo growths. The average number of months for relapse was 11+6.7 months. Regarding the management of relapse, those patients with extracranial tumor were managed with open surgery, two patients with intracranial component were managed with radiotherapy and finally the 2 patients with intra and extracranial tumor component were managed with open surgery and radiotherapy. However, in these cases of relapse, follow-up was no longer continuous due to the fact that the study period was cut.
When cross analysis of data was performed to correlate relapse with the initial stage, the approach used, the hours of pre-surgical embolization, trans-surgical bleeding and the time of evolution to diagnosis, no statistically significant differences were found. However, in the correlation between relapse and tumor irrigation, the presence of residual tissue and age at diagnosis, statistically significant differences were found, with p-values < 0.050 (Table 3).
|
Variable |
No relapse (n=6) |
With relapse (n=6) |
Value of p |
Total patients |
|
|
Residual tissue: |
|||||
|
Attached to periorbite |
1 |
0 |
0.27 |
1 |
|
|
Attached to ACI |
0 |
1 |
|
1 |
|
|
Intracranial minimal temporal |
0 |
1 |
|
1 |
|
|
FCM floor left |
0 |
1 |
|
1 |
|
|
Lateral recess surrounding ACI. |
0 |
1 |
|
1 |
|
|
No residual tissue |
5 |
2 |
|
7 |
|
|
Approach: |
|||||
|
Open |
2 |
5 |
0.109 |
7 |
|
|
Combined |
2 |
0 |
|
2 |
|
|
Endoscopic |
2 |
1 |
|
3 |
|
|
Stadium |
|||||
|
IA |
1 |
0 |
0.66 |
1 |
|
|
II A |
1 |
2 |
|
3 |
|
|
II B |
1 |
0 |
|
1 |
|
|
II C |
1 |
1 |
|
2 |
|
|
III A |
2 |
2 |
|
4 |
|
|
III B |
0 |
1 |
|
1 |
|
|
Irrigation |
|||||
|
ACE |
5 |
1 |
0.021 |
6 |
|
|
ACE + ACI |
1 |
5 |
|
6 |
|
Table 3: Comparative analysis of patients with and without recurrence.
Kaplan Meier event freedom analysis was performed for residual tissue (Figure 4) in which, although there were no significant statistical differences, the patients who were left with residual tissue tended to present greater recurrence and this occurred within the first 100 days of follow-up (approximately two and a half months). In the Kaplan Meier freedom analysis for preoperative embolization (Figure 5), no significant differences were found; however, patients who underwent late embolization (>72 hr) tended to present greater recurrence and this occurred early, within the first 100 days of follow-up.
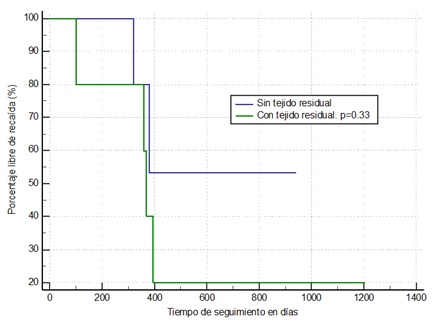 Figure 4: Kaplan Meier event freedom analysis in patients with and without postsurgical residual tissue.
Figure 4: Kaplan Meier event freedom analysis in patients with and without postsurgical residual tissue.
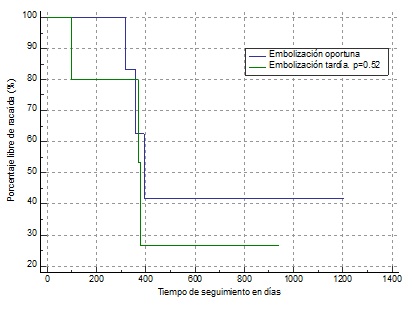 Figure 5: Kaplan Meier event freedom analysis. Timely embolization within 72 hrs and late embolization > 72 hrs.
Figure 5: Kaplan Meier event freedom analysis. Timely embolization within 72 hrs and late embolization > 72 hrs.
Discussion
Juvenile nasopharyngeal angiofibroma (JNA) usually presents a low incidence and is almost exclusive of adolescent males. Francisco and Mello-filho FV mention in their studies an age prevalence between 14 and 25 years of age (2.5) however in our experience there was a case of a 9-year-old boy. Regarding tumor growth, Fahmy SA mentions that in older patients the vascular cellularity disappears and the tumor contains more fibrosis (9); on the other hand, Doody J. mentions that the growth of JNA ceases when young people reach sexual maturity, around 25 years of age (12), both scenarios would explain the regression or tumor stabilization at older age seen in our patients, as well as a more aggressive behavior at younger age of presentation, with a value of p= 0.038 when comparing relapse and age of presentation.
Alshaikh mentions in his review study that the growth pattern is also determinant for management, since due to its concentric expansion through natural foramina it extends towards the pterygomaxillary and infratemporal fossae, orbit and intracranial [7]. Peraza-McLiberty reported that it invades the infratemporal fossa, ethmoid, orbital region and sphenoid in 90% of the cases, data similar to our sample with 91% (11 patients) and intracranial extension up to 10% [13], however our sample had a higher intracranial behavior with 33% (4 patients).
Regarding the clinical picture, our data coincide with that reported by Ore Acevedo where he mentions that the initial and most frequent symptoms are nasal obstruction in 80 to 91% and ipsilateral epistaxis in 60-63%, in our case they occurred in 91% of patients together and two patients (16%) presented with facial deformity. Once the diagnosis was made based on the clinical and imaging studies, the tumor extension was established and with it the planning of surgical approaches as mentioned by Peraza-McLiberty in her study [13].
There are several classification systems described, among which the Chandler system proposed in 1984 stands out; however, it focuses on the extension in the nasal cavity and nasopharynx, leaving aside the intracranial extension, which is why it was not chosen as our staging system. The Andrews-Fisch classification established in 1989 takes into account the growth pattern of the tumor from its origin to the extra and intradural intracranial extension, however, Alshaikh mentions that it is not applicable to new surgical techniques or radiological advances, which makes it difficult to predict healing rates and complications; On the other hand, the Radkowski system proposed in 1996, staged according to radiological findings by CT, taking into account that the preoperative tumor stage corresponds to the first factor for recurrence, allowing the selection of the best surgical approach [7] based on this, for stages I and IIA an endoscopic approach was performed, for IIb, IIc and IIIa combined approaches and in stages IIIb a combined approach with craniotomy.
In 2008, Carrillo et al., as a part of the National Cancer Institute in México, proposed a modified system (Table 4) of the Radkowski and Andrews-Fisch scales with a greater impact on the prediction of recurrence and disease-free survival, according to which stages I and IIA can be managed only with endoscopic approach, stages IIB and III, combined approach preferably with midfacial stripping and stage IV with combined anterolateral or skull base approach [15], it would be worthwhile to apply this system in the approach of patients with JNA in our unit.
|
I |
Limited to nose, nasopharynx, maxillary antrum, anterior ethmoid and sphenoid. |
|
IIa. |
Pterygomaxillary or infratemporal fossa invasion anterior to pterygoid processes with diameter <6 cm. |
|
IIb. |
Invasion of pterygomaxillary or infratemporal fossa anterior to pterygoid processes with diameter >6 cm. |
|
III. |
Occupation of pterygomaxillary fossa posterior to pterygoid processes or posterior ethmoid cells. |
|
IV. |
Skull base erosion > 2cm or intracranial extension. |
Table 4: INCan Staging for Juvenile Nasopharyngeal angiofibroma.
Diagnostic-therapeutic angiography with preoperative embolization was used in all patients. Chavolla-Magaña documented that the most commonly found afferent vessel is the ipsilateral internal maxillary artery in 71.4% of the cases [11], on the other hand Wu reports up to 40% of bilateral contribution [16] and Nicolai reports contribution from the internal carotid system in 35.6%, associated to extension to the cavernous sinus and the skull base and bilateral contribution in up to 30.08% of the cases [8]. In our casuistry, pre-surgical angiography showed that the main contribution was from the ipsilateral internal maxillary artery in 33% and from the internal carotid system associated to the external system in 33%.
Diaz Cardenas recommends embolization 24 to 48 hours before surgery to reduce the risk of revascularization [10], while other authors such as Nicolai P do not recommend pre-surgical embolization, since blocking the supply favors a process of vascular neoformation favoring the persistence of residual non-visible tissue that contributes to recurrence [8]. Based on these observations, we were able to prove that patients with pre-surgical embolization greater than 72 hrs presented greater recurrence within the first 100 days of follow-up.
Follow-up must be performed for at least 3 years after surgical resection, by means of control CT and endoscopic exploration [2], since in spite of ample resection, the recurrence percentages are high. Herrera mention that recurrence is reported in up to 50%, Mena refer them in periods between 5 and 66 months, mostly in the first year, with a median of 7 months [14], on the other hand Nicolai P., refers an incidence reported between 6 and 39% diagnosed in the first year [8] data that coincide with what was observed in our patients in which recurrence was observed in 50% with an average detection time of 11 months.
Regarding surgical management, authors such as Ore Acevedo and Díaz Cárdenas recommend the trans palatal approach for tumors limited to the nasopharynx, nasal cavity and sphenoidal sinus, trans maxillary approach by lateral rhinotomy and/or midfacial degloving for tumors of great extension that involve the pterygopalatine and infratemporal fossa, paranasal sinuses, orbit, ethmoidal sinus and medial part of the cavernous sinus [2,10], due to the fact that most of the patients under study presented in advanced stages, the latter was used as open approach.
It is important to keep in mind other treatment alternatives, Nicolai recommends radiotherapy at low doses of 30 to 36 Gy [8] especially in recurrent lesions or in advanced lesions where it is foreseen that complete resection cannot be performed due to inaccessible sites. In our sample, radiotherapy was indicated only in cases of intracranial recurrence, achieving stabilization of the lesion; however, at the time of closing the study, no remission was obtained in these cases.
Other treatment alternatives that have been proposed are the use of hormones (estrogen, testosterone), chemotherapy based on doxorubicin and dacarbazine [13], radiofrequency ablation or laser resection assisted by video-navigation and microdebrider [10], treatments to be considered for the management of patients, especially those who do not respond adequately to surgery and radiotherapy.
Conclusion
The present study showed that the younger the age of onset, the more aggressive the disease was, the higher the risk of relapse, as well as in cases with an intracranial component, in which vascular contribution of both external and internal carotid systems was demonstrated, mainly located in the cavernous sinus attached to the internal carotid artery, which made complete resection difficult. Regarding residual tissue, we consider that it should be treated immediately once found, due to the higher incidence of relapse shown.
According to the data obtained, the longer the pre-surgical embolization time, the higher the risk of recurrence, so it is suggested that there should be no more than 72 hours difference between the approach and the embolization. Open surgical techniques combined with endoscopy and/or craniotomy were more useful in complex cases. Follow-up should be performed more closely with imaging studies at least at 6 and12 months after resection because relapse in our cases occurred during this period.
Declaration of Interests
The authors declare that they have no conflicts of interest.
Sources of Financing
This research has not received specific support from public sector agencies, commercial sector or non-profit entities.
References
- Herrero M, de Leyva P, Sagüillo K, Villegas D, Picón M, et al. (2015) Juvenile nasopharyngeal angiofibroma: Presentation of a case. Rev española cirugía oral y Maxilofac 37: 119-121.
- Oré Acevedo JF, La Torre Caballero LM, Urteaga Quiroga RJ (2019) Surgical treatment of juvenile nasopharyngeal angiofibroma in pediatric patients. Acta Otorrinolaringológica Española 70: 279-285.
- Ramirez Merlano SA (2018) Juvenile Nasoangiofibroma vs arteriovenous hemangioma, diagnostic challenge. Case report. Arch Med 18: 201-207.
- Pamuk AE, Özer S, Süslü AE, Akgöz A, Önerci M (2018) Juvenile nasopharyngeal angiofibroma: A single centre's 11-year experience. J Laryngol Otol 132: 978-983.
- De Mello-Filho FV, Araujo FCF, Marques Netto PB, Pereira-Filho FJF, et al. (2015) Resection of a juvenile nasoangiofibroma by le Fort i osteotomy: Experience with 40 cases. J Cranio-Maxillofacial Surg 43: 1501-1514.
- Camilon PR, Rahbar R, Cunningham MJ, Adil EA (2019) Juvenile nasopharyngeal angiofibroma in prepubertal males: A diagnostic dilemma. Laryngoscope 129: 1777-1783.
- Alshaikh NA, Eleftheriadou A (2015) Juvenile nasopharyngeal angiofibroma staging: An overview. Ear, Nose Throat J 94: 12-22.
- Nicolai P (2020) Benign Tumors of the Sinonasal Tract. In: Flint PW, Francis HW, Haughey BH, Lesperance MM, Lund VJ, et.al. Cummings Otolaryngology Head and Neck Surgery pp. 2968-3024.
- Fahmy SA (1973) Nasopharyngeal fibroma: Its histopathological nature. J Laryngol Otol 87: 1107-1123.
- Díaz Cárdenas A (2018) Current status of the treatment of juvenile nasal angiofibroma. Rev Medica Hered 29: 52-57.
- Chavolla-Magaña R, Peraza-McLiberty RA, Penagos-Noriega S, Guerrero-Avendaño GML (2019) Preoperative embolization of nasopharyngeal angiofibromas: angiographic findings and most frequent vascular contribution. Second part. Rev An Radiol Mexico 18: 18-27.
- Doody J, Adil EA, Trenor CC, Cunningham MJ (2019) The Genetic and Molecular Determinants of Juvenile Nasopharyngeal Angiofibroma: A Systematic Review. Ann Otol Rhinol Laryngol 128: 1061-1072.
- Peraza-McLiberty RA, Cortés-Benavides MC, Guerrero-Avendaño GML, Enríquez-García R, Graniel-Palafox LE (2019) Interdisciplinary management of juvenile nasoangiofibroma: presurgical embolization, surgical approach and literature analysis. Rev An Radiol Mexico 17: 20-29.
- Mena C C, Bogado R G, Klassen ZC (2009) Juvenile nasoangiofibroma: Our experience in the last 10 years and review of the literature. J Otorhinolaryngol Head Neck Surg 69: 243-248.
- Dabholkar JP (2008) Juvenile nasopharyngeal angiofibroma: Clinical factors associated with recurrence, and proposal of a staging system. J Surg Oncol 98:75-80.
- Wu AW, Mowry SE, Vinuela F, Abemayor E, Wang MB (2011) Bilateral vascular supply in juvenile nasopharyngeal angiofibromas. Laryngoscope. 121: 639-643.
Citation: Tapia MFG, González MAH (2022) Risk Factors for Relapse in Juvenile Nasopharyngeal Angiofibroma: 4 Year Experience In A Referral Hospital. J Otolaryng Head Neck Surg 8: 67
Copyright: © 2022 María Fernanda Galindo Tapia, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

