
Robic Socially Assistive Robot Enables Upper Extremity Endurance Training in Spinal Cord Injured Children
*Corresponding Author(s):
Elisa López-DoladoRehabilitation Department, Hospital Nacional De Parapléjicos (SESCAM), Finca La Peraleda S/n 45071, Toledo, Spain
Tel:+34 925 396827,
Email:elopez@sescam.jccm.es
Abstract
Background: Some sequelae of pediatric spinal cord injury (PedSCI) include loss of upper extremity and trunk balance, in addition to a tough and challenging cardiovascular training. Robic (NAO robot, Aldebaran Robotics) is a Class I medical device whose main goal is to provide assistance to human users through social interactions. In the present proof-of-concept study, supported by an assistant therapist, our objective is to evaluate the Robic’s usability, user experience and clinical feasibility in a PedSCI population.
Methods: This prospective observational study included 10 children with chronic SCI that underwent an upper extremities endurance programme (10 training sessions of 30 min each). Robic usability was measured through patient’s heart rate reserve percentage, hit rate, trunk deviation and shoulder and elbow range of motion during sessions. User experience was address with QUEST Spanish Version 2.0 and the Manikin Self-Assessment Scale. Adherence was evaluated using the Hopkins scale and manual dexterity using Leap Motion Controller.
Results and Conclusion: Robic's platform emerges as an innovative technological tool demonstrating adequate usability and also a good user experience in an upper limb training program for PedSCI patients. Future developments, incorporating eye-tracking strategies would help determine the engagement with the proposed task.
Keywords
Socially assisted robotic platforms; Robotic-based rehabilitation therapies; Pediatric spinal cord injury; Upper limbs endurance training; Usability; Feasibility; User experience
Introduction
Even though it is not a common condition in childhood and adolescence, managing growth and development after a Spinal Cord Injury (SCI) is a major challenge for the patient, their family, the medical team and healthcare system and, ultimately, their entire educational and social environment. The incidence rate of Paediatric Spinal Cord Injury (PedSCI) was estimated between 3.3 - 6.2 cases per million per year in Europe [1]. Some of their sequelae include loss or impaired of Upper Extremity (UE) and trunk balance [2] in addition to a tough and challenging cardiovascular training during exercise compared to healthy children [3]. These impairments affect not only independence in Activities of Daily Living (ADL), but also quality of motor performance, making social interactions a constant challenge.
Typically, children with PedSCI receive multiple rehabilitative interventions throughout childhood to maintain or improve their functional level and to facilitate somatic growth. The intensity of such therapies is usually moderate, aiming to recruit sufficient muscle mass to improve oxygen consumption and reduce Physical Strain (PS). Typically, a higher Physical Condition (PC) produces a lower PS [4]. The success in rehabilitation programs is based on two prerequisites: 1) adhering to the prescribed sessions [5,6]; 2) maintaining attention to the task to be trained during each session. Both are crucial in the analysis of therapeutic outcomes [7]. Several clinical studies in paediatric populations have shown that patient motivation is a key aspect of rehabilitation success and is directly related to patient adherence and their ability to understand and maintain the level of care during use [8,9]. Motivation and attention are important for adherence in SCI patients, as the subjective component of emotions decreases following the loss of peripheral body feedback [10]. On the other hand, it has been shown that variations in some peripheral physiological variables, particularly Heart Rate (HR), are strongly correlated with affective self-reports, even though emotions are mainly related to central mental processes, especially cognition and behavior [11].
To mitigate this, technological solutions like Socially Assistive Robotics (SAR), that is to say, robots whose main goal is to provide assistance to human users through social interactions, are being explored [12]. SAR is slowly being integrated into healthcare systems as new avenues to provide care for chronically ill and disabled patients. However, this process is proving to be complex, as it is based on human-robot interactions and this kind of communication faces multiple challenges, such as safety, usability or user experience, before its clinical relevance can be assessed [13].
A major advantage of using SAR as a motor rehabilitation tool in PedSCI is that they can measure the child-robot interaction in real time. In addition, they can be synchronized with other wearable devices like heart rate monitors or accelerometers, which makes it possible to control and individualize therapies, thus optimizing the resulting motor learning by improving peripheral body feedback [10,13]. One of these platforms, Robic's Inrobics Rehab Clinic (NAO robot, Aldebaran Robotics), has been shown to successfully guide motor training programmes in cardiac rehabilitation [14] and in children with cerebral palsy and obstetric brachial plexus palsy [15]. Using the same Robic humanoid robot, our group was able to analyze smoothness and efficiency metrics in a group of chronic SCI children following UE training, supporting the hypothesis that SAR can be used as a technique to guide and evaluate motor training therapies [16].
In the present proof-of-concept study supported by Robic platform as assistant therapist, our objective is to evaluate usability, user experience and clinical feasibility of Robic in a chronic PedSCI population. We followed SAR's AUSUS evaluation framework, where usability is defined as effectiveness, efficiency, learnability, flexibility, robustness and utility. User experience is understood as embodiment, emotion, human-oriented perception, sense of security and co-experience [13].
Materials And Methods
- Study design and participants
This prospective observational study included 10 children with chronic SCI, Five of them had cervical PedSCI (tetraplegic), with some degree of impaired motor function of the arms and trunk, and the other five had thoracolumbar PedSCI, (paraplegic), with preserved motor function of both arms, but varying degrees of impaired balance trunk control all of them attended in the Pediatric Unit of the Hospital Nacional de Parapléjicos of Toledo (Spain). It was approved by the Local Ethics Committee (Comité Ético de Investigación Clínica con Medicamentos, Complejo Hospitalario de Toledo; Approval number: 760; 29 September 2021) and was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Prior to enrolment, written informed consent was obtained from all participants, signed by parents or legal guardians.
Children with SCI who participated in this study met the following inclusion criteria (Table 1): 1) having a SCI of level C6 or below for complete motor injuries, classified according to the International Standards for the Neurological Classification of Spinal Cord Injuries (ASIA Impairment Scale [AIS]) [17] as AIS grades A or B, or any incomplete SCI of AIS level C or D that allows UE range of motion to be performed; 2) age between 7 and 14 years and scored 1 or 2 according to the Tanner Scale (prepuberal); 3) able to maintain a sitting position. Allowed to use their own wheelchair; 4) and signed the appropriate written informed consent (by parent or legal guardian). Exclusion criteria were: unstable orthopaedic injuries; moderate pain or joint stiffness; severe spasticity; severe bronchopneumopathy and/or heart disease requiring monitoring during exercise; visual impairment and cognitive impairment.
|
Variables |
Sample Analyzed |
|
|
Tetraplegic (n=5) |
Paraplegic (n=5) |
|
|
Sex(Male)* |
1.00 (20.00) |
3.00 (60.00) |
|
Age(Years)+ |
9.67±4.04 |
10.67±2.08 |
|
Weight (kg)+ |
39.66±7.50 |
36.03±12.22 |
|
Height (cm)+ |
138.66±11.06 |
140.33±16.92 |
|
Etiology Injury ( Traumatic)* |
1.00 (20.00) |
1.00(20.00) |
|
Time since injury (months)+ |
3.66±2.51 |
10.00±3.00 |
|
Injury Level* |
C1: 1.00 (20.00) |
T3: 1.00 (20.00) |
|
|
C2: 1.00 (20.00) |
T12: 1.00 (20.00) |
|
|
C5: 1.00 (20.00) |
L1: 1.00 (20.00) |
|
|
C6: 1.00 (20.00) |
L2: 1.00 (20.00) |
|
|
C7: 1.00 (20.00) |
L3: 1.00 (20.00) |
|
AIS Classification* |
|
|
|
A |
- |
- |
|
B |
3.00 (60.00) |
- |
|
C |
1.00 (20.00) |
3.00 (60.00) |
|
D |
1.00 (20.00) |
2.00(40.00) |
|
UEMS+ |
31.33 ± 15.27a |
50.00 ± 0.00a |
|
SCIM-III+ |
|
|
|
Self-Care |
6 ± 5.39 |
17.2 ± 2.05 |
|
Breathing |
18.2 ± 9.65 |
28.8 ± 8.41 |
|
Mobility |
13.4 ± 14.88a |
32.2 ± 5.22a |
|
Total+ |
37.6 ± 29.56a |
78.2 ± 14.85 |
Table 1: Demographic characteristics and International Standards for the Neurological Classification of SCI (ISNCSCI) of the sample analyzed. Significant statistically differences are expressed in bold font. a (p < 0.01); * categorical variables are expressed as frequency and percentage; + continuous variables are expressed as mean and standard deviation. American Spinal Association Impairment Scale (AIS Classification). Upper Extremity Motor Score (UEMS) Spinal cord measures (SCIM-III).
The Upper Extremity Motor Score (UEMS) and Spinal Cord Injury Independence Measure version III (SCIM III) were obtained prior to the start of UE robotic endurance training. UEMS is a rated score of the strength of 10 key muscle groups of both UE, 5 on each arm. Every one of them could be scored from 0 (no function) to 5 (normal function), with a maximum of 25 points for each UE, 50 points considering both arms [17] (Table 1). SCIM-III is the specific scale for assessing the level of functional independence for SCI subjects, taking into account aspects of self-care, breathing, sphincter control and mobility [18].
- Socially assistive, Robic platform description
Robic (NAO robot, Aldebaran Robotics) is a SAR platform registered by the AEMPS from 22nd March 2021 as a Class I medical device (registration number RPS/777/2021).
The Inrobics system is a platform for rehabilitation and patient monitoring based on the interaction between various hardware and software components (Figure 1).
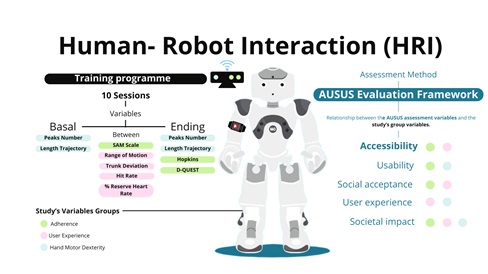 Figure 1: Human-Robot Interaction (HRI). Graphical diagram summarising the content of the training with Robic platform and the evaluation methodology.
Figure 1: Human-Robot Interaction (HRI). Graphical diagram summarising the content of the training with Robic platform and the evaluation methodology.
At the core of this system is the Orbbec Persee, featuring a Red-Green-Blue-Deep (RGB-D) sensor and a 3D sensor, which includes a compact computer running on the software architecture developed by Inrobics. This sensor enables accurate detection of the patient's movements, which is essential for the evaluation of the accuracy and effectiveness of rehabilitation exercises. The movements are recorded and measured with high accuracy in terms of active range of motion (maximum ROM achieved), trunk and neck deviation, amount of movement (hit rate) and attention span (visual connection to the platform, adherence). A core element in this ecosystem is the NAO V6 robot, designed to guide the rehabilitation sessions. NAO not only demonstrates and explains exercises to patients, but also acts as an interactive facilitator, enhancing the therapy experience. The patient's well-being is monitored by the Polar Verity Sense wristband, a heart rate sensor that records the heart rate during the sessions. This information is needed to monitor the patient's physical response to the exercises. The app installed on a tablet provides a user interface to set up exercises and monitor sessions. This app collects data on both the patient's heart rate and performance captured by the 3D camera, providing a comprehensive view of the patient's progress. The computer embedded in the system's 3D sensor manages the entire architecture, controlling the NAO robot in a fully autonomous way and receiving data from the 3D sensor. Its function is to ensure that patients perform the exercises correctly, providing real-time feedback to correct any errors. This ensures that each session is as effective as possible. Additionally, the system's architecture is responsible for, once receiving the configuration of the session, carrying out reasoning to execute the session. This reasoning is based on the transformation of low-level states into high-level PDDL (Planning Domain Definition Language) predicates that the Monitoring and Decision Support modules can process [19]. This allows for reasoning based on the state and actions of the different devices (robot, 3D sensor, and tablet), facilitating adaptation to the needs of the rehabilitation session. Finally, after the conclusion of each session, the data collected is stored in a cloud database. This information is used to query and calculate key metrics, allowing a detailed assessment of patient progress over time. This data-driven approach is essential to personalize and continuously improve the rehabilitation process.
- Experimental UE endurance training protocol
The design of this programme, developed at the Hospital Nacional de Parapléjicos of Toledo, is in line with the aerobic endurance recommendations of the International Spinal Cord Society (ISCoS): light intensity, 30% to 39% of reserve heart rate (%HRR), and moderate intensity (40% to 59% HRR) [20].
All recruited children underwent a UE endurance training programme consisting of 10 experimental sessions of 30 min each, divided into three parts: warm-up (10 min), main part (20 min) and cool-down (10 min). Before starting, an experimental session consisting of 3 exergames separated by 2 resting periods was performed. These sessions were scheduled for 2 or 3 days per week, with the aim of completing the endurance training in a maximum of 5 weeks. Patients performed the experimental sessions in their own wheelchairs, in a comfortable room with the same light and temperature conditions. Robic was positioned in front of the participant at the appropriate distance for real-time data recording. The therapist, who was close enough to ensure the patient's safety, had full view of the motion monitoring sensor at all times and could intervene during the session if necessary. To select the specific exercises included in the training protocol, it was taken into account that the muscles involved in each exercise should be the same as that of the ADLs (deltoids, shoulder rotators, elbow flexors and extensors, and wrist flexors and extensors), in order to meet the objective of reducing the energy cost of movement and improving the quality of life of children with SCI.
- Evaluation variables for hand motor control dexterity
The hand motor dexterity was recorded through the smoothness metric, which was calculated at the beginning and at the end of the training program using a non-immersive virtual application based on the Leap Motion Controller (LMC) available in the RehabHand software developed in our center [21,22]. This application proposed the execution of a functional task based on following a previously defined trajectory with an enveloping shape, passing only once through each node. With the aim of comparing the trajectories the order to reach the nodes is previously established. So the smoothness metric is obtained as the peaks number or movement units detected from the hand velocity profile during the execution of the task, and the efficiency metric was evaluated by calculating the length of the hand trajectory performed.
- Evaluation variables related to Robic interaction: usability and users experience
To address usability, Robic provided feedback on the patient's performance during the sessions in terms of heart rate reserve percentage (HRR%), hit rate, trunk deviation and shoulder and elbow range of motion (ROM). Mean values for each of these variables were calculated over the entire session. In addition, a graphical representation of the trend of the data was included, along with the corresponding linear regression curve and its characteristic equation. This facilitated the interpretation of changes in performance over time.
Heart Rate Reserve Percentage (HRR%) was estimated from continuous monitoring of the patient's heart rate throughout the session using the Polar Verity Sense, which was placed in the middle of the humerus of the dominant arm and previously connected to the Robic platform via Blue Tooth. Session PS [4] defines the intensity session and was estimated as percentage reserve HR based on the individual's HR:
HRR% = [(HRPeak - HRObserved)/(HRPeak -HRRest))*100,
Where HRPeak is the maximum HR (HRMax) value found during the execution of the session and HRRest is the lowest heart rate found during the entire session period. HRObserved is taken from the HR Range value observed.
HR Range= (HRMin- HRMax)
Hit rate, trunk deviation and kinematic goniometric data was registered with Robic using R software (version 4.3.1 for Ubuntu (R Core Team, 2020)). The kinematic variables were measured separately in elbows and shoulders. For the elbow joint, the first measure was the range of extension from 90° to full extension in the anatomical position. It should be noted that children with tetraplegic PedSCI generally do not reach full extension at 0°. Thereupon, elbow flexion was assessed, from 90° to 180°. Regarding to shoulder joint, shoulder flexion was recorded from 10° of flexion with the arm extended in anatomical position to 90°. Displacements of the shoulder between 90° and 180° were considered to be part of the shoulder elevation range.
To measure the user experience, we used two different questionnaires: the Spanish version of the Quebec User Satisfaction Evaluation with Assistive Technology (QUEST Spanish Version 2.0) (D-QUEST) and the Manikin Self-Assessment Scale (SAM), of which a record was taken at each of the sessions after completion. D-QUEST consists of a written questionnaire and has proven to be a reliable and valid instrument to assess their satisfaction with UE endurance training through SAR with respect to 8 differential aspects using a rating scale from 1 to 3, with 1 being the aspect with the highest relevance or importance, and 3 being the aspect with the lowest relevance or importance [23]. The SAM scale is a nonverbal pictorial assessment questionary that directly measures the pleasure, arousal, and dominance associated with a person's affective reaction to a wide variety of stimuli [24].
- Evaluation of adherence to the training programme
After the last training session, the Hopkins scale (HOPKINS) was used to measure the patient’s adherence to the endurance training protocol [25]. This scale measures the involvement in the UE endurance training program. The score reflects a summary impression of the participants' engagement during their respective therapy sessions. The scoring consisted of summing all scores, reversing item 2.
- Statistical analysis
All statistical analyses were conducted using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA). The clinical and demographic characteristics of the participants underwent descriptive statistical analysis, and the results were presented as mean and standard deviation.
For performance and training variables, including HRR%, hit rate, trunk deviation, goniometry (elbow flexion and extension; shoulder flexion and elevation), and dexterity (length and number of peaks), a normality analysis was performed using the Shapiro-Wilk test. Related-samples analyses were conducted to assess the means of variables in the baseline and Ending endurance EU training sessions for each group using the paired-samples t-test as a parametric test and the Wilcoxon signed-rank test as a non-parametric test. Additionally, independent-samples analyses were conducted in the first and last sessions to check for significant differences between the analyzed groups, utilizing the independent-samples t-test as a parametric test and the Mann-Whitney U test as a non-parametric test.
Pearson correlation was applied to assess the association between the UEMS scale and effectiveness and smoothness of movement variables, including peaks number and trajectory length.
Results
For the present study, 10 children with chronic SCI were recruited, all of them from the Paediatric Rehabilitation Unit of the Hospital Nacional de Parapléjicos of Toledo (Spain).
Demographic variables along with the International Standards for the Neurological Classification of SCI (ISNCSCI) are summarized in Table 1.
No statistically significant differences were found between tetraplegic versus paraplegic children in the demographic variables, but as expected, UEMS and SCIM scores were significantly lower in the former group. In fact, the UEMS score discriminated between the two experimental groups. Only the tetraplegic had UEMS scores below 50 (mean UEMS 31.33 ± 15.27 in tetrapelgic versus 50.00 ± 0.00 in paraplegic), since by definition, all levels of paraplegic score a maximum on this variable, which discriminates between tetraplegics and paraplegics, but not between different levels of paraplegia. Similarly, the total score of the SCIM III and its subscores are significantly lower in tetrpalegic (total 37.6 ± 29.56; selfcare 6 ± 5.39; breathing 18.2 ± 9.65; mobility 13.4 ± 14.88) than in paraplegic PedSCI (total 78.2 ± 14.85; selfcare 17.2 ± 2.05; breathing 28.8 ± 8.41; mobility 32.2 ± 5.22). However, and also as expected, in terms of the level of independence in performing ADLs, none of the subjects in the latter group achieved maximum scores, confirming the existence of infralesional motor sequelae in those less damaged subjects and the potential usefulness of training programmes such as that proposed for all levels and severities of SCI.
- Comparison related to dexterity and performance with Robic Platform
Regarding the analysis of hand motor control dexterity, tetraplegic children showed significantly less efficient movements than paraplegic children, as evidenced by the longer trajectories measured by the non-immersive virtual LMC application (286.01 ± 59.87 versus 123.61 ± 17.14; p=0.004) (Figure 2). However, no statistically significant differences were found between the number of spikes in the trajectories performed by the tetraplegic compared to paraplegic children. Nevertheless, the peaks number mean was higher in tetraplegic (81.67 ± 48.21 vs. 79.00 ± 31.34), which may indicate that they could have less smooth UE movements (Table 2, Figure 3), which will require larger sample sizes to investigate.
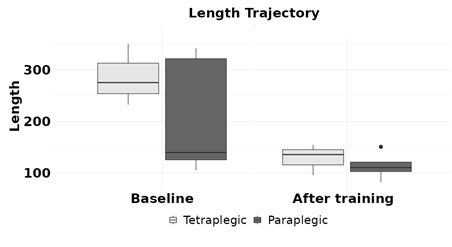 Figure 2: Length Trajectory. The columns represent the mean and the error bars represent the standard quadratic mean. * p < 0.05. Tetraplegic PedSCI are represented in grey, and paraplegic PedSCI are represented in black.
Figure 2: Length Trajectory. The columns represent the mean and the error bars represent the standard quadratic mean. * p < 0.05. Tetraplegic PedSCI are represented in grey, and paraplegic PedSCI are represented in black.
|
Variables |
Tetraplegic (n=5) |
Paraplegic ( n=5) |
||||
|
|
At baseline |
At ending |
Diff. |
At baseline |
At ending |
Diff. |
|
Reserve HR (Percentage) |
47.72 ± 11.25 |
42.56 ± 12.97 |
4.20 ± 7.25 |
52.92 ± 4.15 * |
47.64 ± 4.38 * |
6.60 ± 2.22 |
|
Trunk deviation (Degrees) |
3.00 ± 2.15 |
2.61 ± 1.57 * |
0.50 ± 1.30* |
4.19 ± 1.68 * |
9.18 ± 3.42 * |
-6.09 ±2.71* |
|
Tries successful (Attempts) |
35.20 ± 20.11* |
40.80 ± 18.18 |
0.33 ± 10.78 |
58.00 ± 7.58* |
54.60 ± 11.14 |
7.75 ± 6.55 |
|
Trajectory length (mm) |
286.01 ± 59.87** |
128.73 ± 30.07 |
157.27 ± 50.45 |
123.61 ± 17.14** |
114.13 ± 34.59 |
59.72 ± 101.59 |
|
Peaks number (units) |
81.67 ± 48.21 |
62.67 ± 6.65 |
19.00 ± 43.58 |
79.00 ± 31.34 |
44.25 ± 18.57 |
34.75 ± 39.12 |
|
Elbow flexion (Degrees) |
106.92 ± 10.83 |
112.48 ± 11.09 |
9.13 ± 5.22 |
108.26 ± 3.01 |
108.02 ± 7.65 |
1.11 ± 8.14 |
|
Elbow extension (Degrees) |
17.96 ± 5.62 |
14.70 ± 2.02 |
4.40 ± 7.70 |
16.79 ± 5.39 |
15.62 ± 5.48 |
7.09 ± 2.26 |
|
Shoulder Elevation (Degrees) |
117.65 ± 36.26 |
120.48 ± 26.61 |
0.66 ± 11.34 |
125.05 ± 15.02 |
113.20 ± 19.51 |
-13.54 ± 11.70 |
|
Shoulder Flexion (Degrees) |
40.45 ± 9.89 |
39.67 ± 9.48** |
1.89 ± 14.48 |
28.61 ± 7.17 |
25.81 ± 3.98** |
0.59 ± 6.09 |
Table 2: Performance and motor control variables at the beginning and end of endurance. UE training in tetraplegic and paraplegic children. The results are expressed as mean and standard deviation. Diff., difference between the Baseline and the Ending session of the mean and the standard deviation.*p < 0.05, **p < 0.01.
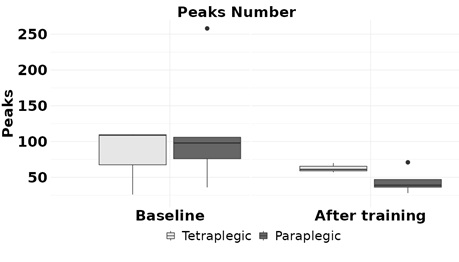 Figure 3: Peaks Number. The columns represent the mean and the error bars represent the standard quadratic mean. * p < 0.05. Tetraplegic PedSCI are represented in grey, and paraplegic PedSCI are represented in black.
Figure 3: Peaks Number. The columns represent the mean and the error bars represent the standard quadratic mean. * p < 0.05. Tetraplegic PedSCI are represented in grey, and paraplegic PedSCI are represented in black.
The dexterity and performance variables related to the use and interaction with Robic Platform (HRR%, trunk deviation and hit rate) obtained for both experimental groups were compared at the beginning and ending of UE endurance training (Table 2).
With respect to HRR%, there was a decrease in the mean HHR% at the end of the intervention, suggesting that the cardiovascular changes achieved were those intended by the programme implemented. But this decrease reached statistical significance in paraplegic (baseline 52.92 ± 4.15, ending value 47.64 ± 4.38; p=0.029), but not in tetraplegic (baseline 47.72 ± 11.25, ending value 42.56 ± 12.97; p>0.05). Further studies with larger sample sizes will need to analyze whether tetraplegic subjects need more sessions to achieve the same effect as paraplegics children, or whether a different therapeutic approach is needed (Table 2, Figure 4).
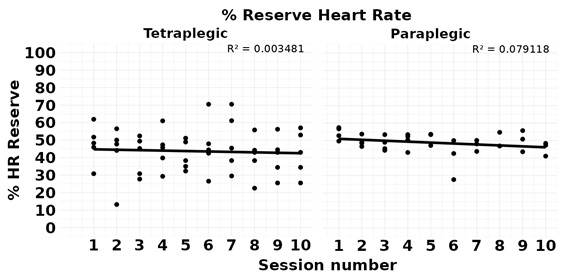 Figure 4: Percentage of heart rate reserve. Points represent the average of each of the endurance training program sessions and the linear regression line (R2).
Figure 4: Percentage of heart rate reserve. Points represent the average of each of the endurance training program sessions and the linear regression line (R2).
The hit rate in carrying out the training programme was significantly lower in tetraplegic than in paraplegic (35.20 ± 20.11 versus 58.00 ± 7.58; p=0.045) (Table 2, Figure 5). No statistically significant differences were found in the hit rate attributable to the training programme between the two groups studied, but tetraplegic tended to improve (baseline 35.20 ± 20.11; ending value 35.20 ± 20.11) and paraplegic tended to deteriorate (baseline 58.00 ± 7.58; ending value 54.60 ± 11.14).
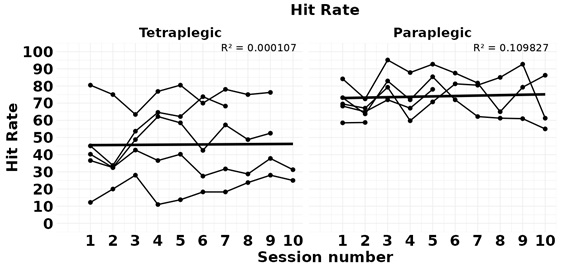 Figure 5: Hit Rate. Points represent the average of each of the endurance training program sessions and the linear regression line (R2).
Figure 5: Hit Rate. Points represent the average of each of the endurance training program sessions and the linear regression line (R2).
Paraplegic children significantly increased trunk deviation movements as a result of the endurance training programme (baseline 4.19 ± 1.68; ending value 9.18 ± 3.42; p=0.044), which was not the case for tetraplegic children (baseline 3.00 ± 2.15; 2.61 ± 1.57; p=0.028) (Table 2, Figure 6). This data highlights clear trunk deviation differences directly due to the level of SCI, with greater capability for trunk training in paraplegic than in tetraplegic subjects.
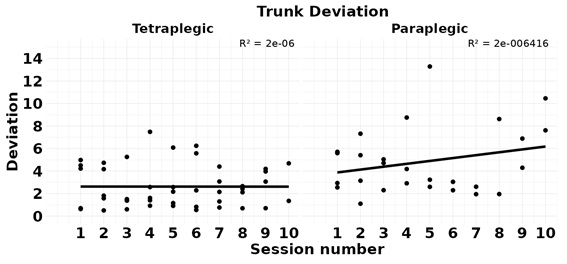 Figure 6: Trunk Deviation .Points represent the average of each of the endurance training program sessions and the linear regression line (R2).
Figure 6: Trunk Deviation .Points represent the average of each of the endurance training program sessions and the linear regression line (R2).
The kinematic analysis of the goniometric variables depicts the different impacts of the SCI on upper limb movement, revealing clear alterations in elbow flexion and shoulder movements in tetraplegic children. After completing all training sessions, a significantly greater increase in shoulder flexion was found in tetraplegic compared to paraplegic children (39.67 ± 9.48 versus 25.81 ± 3.98; p=0.028) (Table 2, Figure 7b). Regarding shoulder elevation, although paraplegic patients started the programme with a greater range of motion (125.05 ± 15.02 in tetraplegic subjects versus 117.65 ± 36.26 in paraplegic subjects), no statistically significant differences in the improvement due to the training programme were found in both experimental groups. Nor were there any changes in the maximum ranges of movement of elbow flexion and extension as a result of the training programme (Figure 7a).
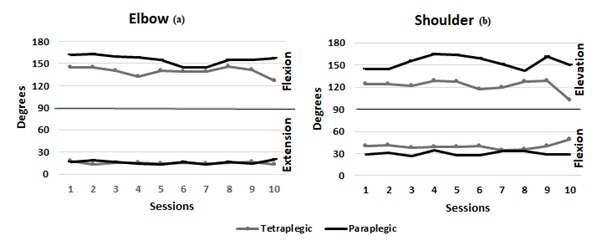 Figure 7: Goniometry. Tetraplegic PedSCI are represented in grey, and paraplegic PedSCI are represented in black. (a) Elbow goniometry: Flexion and extension; (b) Shoulder goniometry: Elevation and Flexion.
Figure 7: Goniometry. Tetraplegic PedSCI are represented in grey, and paraplegic PedSCI are represented in black. (a) Elbow goniometry: Flexion and extension; (b) Shoulder goniometry: Elevation and Flexion.
- Upper limb motor performance measures correlations:
The correlation between the UEMS score and upper extremity dexterity, measured by trajectory length (Figure 8) and number of velocity peaks (Figure 9). The analysis performed in tetraplegic children suggested a high correlation between UEMS and hand trajectory, both at baseline (r = -0.980, p=0.126) and also at ending timepoint (r= -0.915, p=0.394). On top of that, we found a positive correlation between the number of peaks and the UEMS score (Figure 9), although it only reached statistical significance upon completion of the training programme with Robic (r = 0.500, p=0.667 at baseline and r = 1, p < 0.01 at ending). As the UEMS score was always 50 points for all paraplegic subjects, no correlation can be made between this variable and the upper limb dexterity measures for this group (Figure 8, Figure 9).
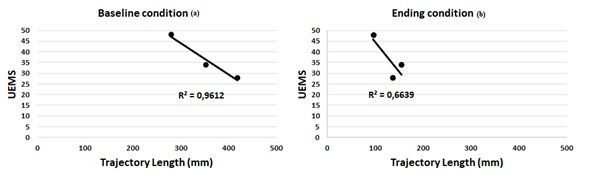 Figure 8: Correlation between UEMS and trajectory length: (a) in the first session of the training (Baseline timepoint); (b) in the last session (ending timepoint).
Figure 8: Correlation between UEMS and trajectory length: (a) in the first session of the training (Baseline timepoint); (b) in the last session (ending timepoint).
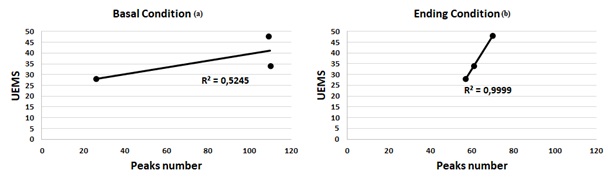 Figure 9: Correlation between UEMS and Peaks number: (a) in the first session (Baseline timepoint); (b) in the last session (ending timepoint).
Figure 9: Correlation between UEMS and Peaks number: (a) in the first session (Baseline timepoint); (b) in the last session (ending timepoint).
- SAR user experience and training program adherence
- D-QUEST
As explained above referring to the QUEST questionnaire, the experimental subjects described their user experience by means of quality attributes.
The qualities firstly chosen by tetraplegic PedSCI (Figure 10a) were “comfort” (60%) and “effectiveness” (20%). Secondly, “effectiveness” (40%), “safety” (20%), “ease of use” (20%), and “comfort” (20%), and thirdly, “safety” (40%), “ease of use” (20%), and “setting” (20%). Yet, the paraplegic PedSCI (Figure 10b) ranked “effectiveness” (40%), “safety” (20%) and “comfort” (20%) first; “ease of use” (40%), “effectiveness” (20%) and “comfort” (20%) second; and “ease of use” (40%), “safety” (20%) and “dimensions” (20%) third. The qualities “weight” and “durability” were not chosen by any patient, neither paraplegic nor tetraplegic. In addition, tetraplegic children ignored the quality "dimensions", while paraplegic children neglected the quality " setting".
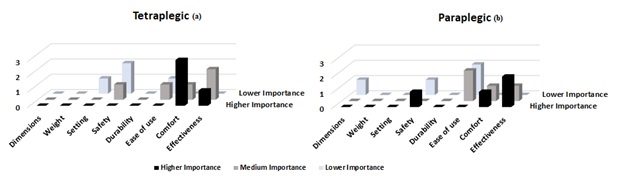 Figure 10: The Spanish version of the Quebec User Satisfaction Evaluation with Assistive Technology (D-QUEST Spanish Version 2.0). The qualities chosen with more importance or relevance are shown with darker colour bars. Qualities chosen with less importance or relevance are shown with lighter colour bars. (a) Outcomes Tetraplegic SCIPed. (b) Outcomes Paraplegic SCIPed.
Figure 10: The Spanish version of the Quebec User Satisfaction Evaluation with Assistive Technology (D-QUEST Spanish Version 2.0). The qualities chosen with more importance or relevance are shown with darker colour bars. Qualities chosen with less importance or relevance are shown with lighter colour bars. (a) Outcomes Tetraplegic SCIPed. (b) Outcomes Paraplegic SCIPed.
- Sam Scale
For each of the three direct rating questions “pleasure”, “arousal”, and “dominance”, responses from both groups across 10 sessions were analyzed (Figure 11). The maximum possible score for each question was 9 points. For “pleasure”, there was no change in scores between baseline and final sessions, but scores were high for both groups (tetraplegic 9.00±0.89; paraplegic 8.00±1.15). For “arousal”, the scores differed from the baseline session (tetraplegic 7.00±0.89; paraplegic 6.00±1.91) to the final session, where both groups scored lower (tetraplegic 1.00±1.09; paraplegic 3.00±1.00). Scores for “dominance” behave just the opposite, were lower for both groups in the baseline session (tetraplegic 5.00±1.41; paraplegic 6.00±1.91) and increased in the final session (tetraplegic 7.00±1.41; paraplegic 8.00±2.82).
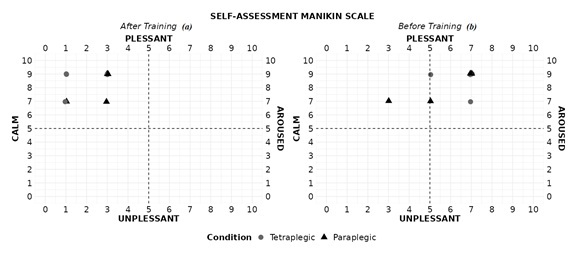 Figure 11: Manikin Self-Assessment Scale (SAM). The drawings with a light grey round shape represent the responses of the children with tretraplegia and the drawings with a triangular shape represent the responses of the children with paraplegia. (a) First session programme Training, (b) Last session programme training.
Figure 11: Manikin Self-Assessment Scale (SAM). The drawings with a light grey round shape represent the responses of the children with tretraplegia and the drawings with a triangular shape represent the responses of the children with paraplegia. (a) First session programme Training, (b) Last session programme training.
- Hopkins Scale
Involvement of each patient in their training was evaluated by the therapist using Hopkins scale, that assigned a value from 5 to 30. Tetraplegic children scored significantly lower than paraplegic PedSCI (27.00±0.54 versus 29.50±0.95; p=0,013). However, the high level of engagement of both groups with the training provided by Robic platform was a very positive aspect (Figure 12).
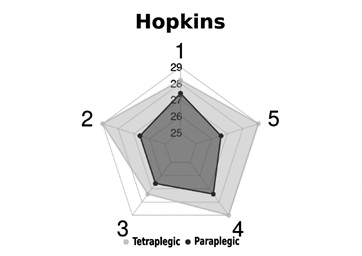 Figure 12: Hopkins scores obtained by both experimental groups. Tetraplegic PedSCI group is represented in grey and paraplegic PedSCI group in black.
Figure 12: Hopkins scores obtained by both experimental groups. Tetraplegic PedSCI group is represented in grey and paraplegic PedSCI group in black.
Discussion
This study aims to evaluate the capacity of Robic's Inrobics Rehab Clinic (NAO robot, Aldebaran Robotics) to act as a therapeutic assistant for a sample of SCI children, assisting them to perform an upper limb endurance training programme. For this purpose, we carried out a proof-of-concept study and analyzed the usability of Robic, the user experience of the children in their interaction with it, to elucidate the feasibility of using it as a therapeutic aid in rehabilitation programs designed for PedSCI population.
Usability is a parameter that describes the effectiveness, efficiency, ease of learning, flexibility, robustness, and utility applicable to devices provided by new robotic technologies that can be used in upper limb training, such as the ARMEO Spring [22], or new robotic power wheelchair [26]. For the purpose of proving the Robic platform usability, our results allow us to describe the behavior of two training variables: on one hand, physical capacity and physical strain, and on the other, dexterity movements. As expected, and as already described in other previous studies, with the same aerobic training program applied to tetraplegics and paraplegics, while paraplegic patients manage to train and thus modify the parameters of physical capacity such as isometric strength, peak power output (POpeak) and peak oxygen uptake or PS as HRR%, tetraplegic patients do not [26,27] , likely, this can be explained by the neurological severity of the SCI in tetraplegic patients, which does not allow them to reach the desired training intensity [28]. This could explain why our results indicate that despite the decrease in HRR% at the end of training in both groups, it is only statistically significant in the paraplegic group. They seem to be the group capable of achieving the desired cardiovascular training, with the use of this robotic tool. Regarding dexterity movements, the results show that Robic can detect that tetraplegic patients exhibit a higher deviation peaks number in the trajectories performed by the upper limbs, as demonstrated in other studies [16,22]. This suggests that with this training program assisted by the robot, tetraplegic patients achieve upper limb dexterity training. On the other hand, it is the paraplegic patients who manage to increase trunk deviation, while tetraplegics do not. This aligns with their level of injury, where their potential target for dexterity training will be trunk balance work. Tetraplegic patients may not be able to train this function due to the severity of the injury level. Another result that indicates the positive capability of the robot for dexterity training is that, as one would logically expect, the accuracy rate for any task will be lower in tetraplegic patients than in paraplegics. This is what the robotic platform detects when comparing both groups. Additionally, the fact that although there are no statistically significant differences when comparing this accuracy rate at the beginning versus the end of training in each group, there is a trend towards improvement in tetraplegic patients at the end of training. This could further highlight that while tetraplegic patients are able to train motor dexterity, paraplegic patients may not do so because the task is not stimulating for them or because it is not impaired in them, and therefore, they do not train it. Moreover, it is expected that in tetraplegic patients, the muscle groups that show the most improvement are those closest to the level of injury. Thus, in tetraplegic patients who already face difficulty in proximal movements of the upper limbs due to their level of injury, it is the proximal muscle groups that show more improvement compared to the distal musculature [28]. After training with the robot, statistically significant improvement is observed in tetraplegics in shoulder flexion but not in elevation (as the latter is not expected to depend on the training program but on the neurological recovery capacity allowed by the severity of the injury). Paraplegic patients do not show improvement as this group starts from a baseline of normality.
In terms of user experience, our results show that SCI children were successful in interacting with the Robic platform, as evidenced by their choice of the most important qualities through the QUEST questionnaire: "comfort", "effectiveness", "safety" and "ease of use". Interestingly, the effectiveness perceived by the users corresponds to the positive evolution of the usability variables recorded by Robic. The qualities “weight” and “durability” were not chosen by any patient, neither paraplegic nor tetraplegic, possibly because the children did not perceive Robic as a device to be worn, but as a tool to interact with the environment in order to be trained. The strong perception of child-Robic interaction as "safe" is a strength, but at least in part it could be related not to the Robic platform itself, but to the environment in which the therapist, the Robic platform and SCI children were located to conduct the present study. Robic platform was recognized as an effective tool for upper limb training for both paraplegic children, with upper limbs without neuromuscular restrictions, and tetraplegic children, whose arms have varying degrees of paralysis, making training more difficult but more necessary.
It is well established in human-robot interactions that SAR are perceived as social actors because they are able to evoke some patterns typical of human-human interaction [29]. Because of that, an emotional user experience evaluation through the SAM scale was included. SAM scale responses showed that the interaction with Robic was described always and by all SCI children as pleasant, increasingly calm and also with a growing sense of control (dominance) by the child over the training programme. These results are very encouraging for future developments and could be related to the human-like morphology of the Robic platform, the Robic’s direct gaze on the experimental subjects and, in the case of our paediatric sample, perhaps its paediatric dimensions. These results are in line with recent studies that have shown that the perception of the direct gaze of a humanoid robot can have similar effects to the perception of the direct gaze of another human [30].
Apart from the limitations in the analysis of the variables due to the small size of our sample, which is adequate for a usability proof-of-concept study but not for drawing precise conclusions on the effectiveness of the intervention applied, the main limitation of this study is the impossibility of evaluating the engagement with the Robic platform, as it lacks sensors for visual tracking of the user. Eye-tracking-based measurements have been described as reliable tools for accurately predicting autism diagnoses in the paediatric population [31,32], so future developments of the Robic platform will need to incorporate eye-tracking sensors and support real-time learning of the user's engagement with the proposed task.
Conclusion
Robic's Inrobics Rehab Clinic (NAO robot, Aldebaran Robotics) emerges as an innovative technological tool demonstrating adequate usability in measuring cardiovascular training and movement dexterity in an upper limb training program for pediatric patients with SCI in the study performed as proof-of-concept. Although all patients who participated in the study completed the training and tolerated it satisfactorily in terms of the variables measured, very high deviation values were obtained in some of them.
Therefore, the next step in this research is to design and conduct a clinical trial that includes, in addition to the experimental group of patients, a control group to act as a comparator. Furthermore, in order to obtain more conclusive results, the experimental groups will be further homogenized by classifying patients with cervical and thoracic SCI into high and low lesions.
Author’s Contribution
Conceptualization, A.dlR.-G, A.G.-A, J.-C.P.-P, R.M.-M and E.L.-D.; methodology, M.S.-M, V.C.-H, R.M.-M, Y.P.-B, F.G.-M, J.-C.P.-P, E.L.-D. and A.dlR.-G; software, V.C.-H, F.G.-M A.dlR.-G and J.-C.P.-P ; validation, A.dlR.-G, A.G.-A, J.-C.P.-P and E.L.-D.; formal analysis, M.S.-M, V.C.-H, R.M.-M, A.dlR.-G, Y.P.-B and E.L.-D.; investigation, M.S.-M, V.C.-H, R.M.-M, A.dlR.-G, Y.P.-B and E.L.-D.; resources, A.dlR.-G, A.G.-A, F.G.-M, J.-C.P.-P , J.-F.J.-D and E.L.-D.; data curation, M.S.-M, V.C.-H, R.M.-M, A.dlR.-G and E.L.-D.; writing—original draft preparation, M.S.-M, R.M.-M, A.dlR.-G and E.L.-D.; writing—review and editing, M.S.-M, V.C.-H, R.M.-M, A.dlR.-G, Y.P.-B, F.G.-M, A.G.-A, J.-C.P.-P, J.-F.J.-D and E.L.-D.; visualization, A.dlR.-G, A.G.-A, J.-C.P.-P, J.-F.J.-D and E.L.-D.; supervision, A.dlR.-G, J.-C.P.-P and E.L.-D.; project administration, A.dlR.-G, J.-C.P.-P. and E.L.-D. All authors have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Complejo Hospitalario de Toledo (protocol code 760 and date of approval 29th September 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study (by parents or legal guardians).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
Grant PID2020-117361RB-C22 funded by MCIN/AEI/10.13039/501100011033.
Conflicts of Interest
The authors declare no conflict of interest.
References
- New PW, Lee BB, Cripps R, Vogel LC, Scheinberg A, et al. (2009) Global mapping for the epidemiology of paediatric spinal cord damage: towards a living data repository. Spinal Cord 57: 183-197.
- Singh G, Aslan S, Ugiliweneza B, Behrman A (2020) Contribution of trunk muscles to upright sitting with segmental support in children with spinal cord injury. Children 7: 278.
- Wecht JM, Harel NY, Guest J, Kirshblum SC, Forrest GF, et al. (2020) Cardiovascular autonomic dysfunction in spinal cord injury: epidemiology, diagnosis, and management. Semin Neurol 40: 550-559.
- Janssen TW, van Oers CA, Veeger HE, Hollander AP, van der Woude LH, et al. (1994) Relationship between physical strain during standardised ADL tasks and physical capacity in men with spinal cord injuries. Spinal Cord 32: 844-859.
- Foley NC, Bhogal SK, Teasell RW, Bureau Y, Speechley MR (2006) Estimates of quality and reliability with the physiotherapy evidence-based database scale to assess the methodology of randomized controlled trials of pharmacological and nonpharmacological interventions. Phys Ther 86: 817-824.
- Sabaté E (2003) Adherence to long-term therapies: evidence for action. World Health Organization.
- Silvestri J (2017) Effects of chronic shoulder pain on quality of life and occupational engagement in the population with chronic spinal cord injury: preparing for the best outcomes with occupational therapy. Disabil Rehabil 39: 82-90.
- dos Santos AN, Pavão SL, de Campos AC, Rocha NA (2012) International classification of functioning, disability and health in children with cerebral palsy. Disabil Rehabil 34: 1053-1058.
- Wulf G, Lewthwaite R (2016) Optimizing performance through intrinsic motivation and attention for learning: The OPTIMAL theory of motor learning. Psychon Bull Rev 23: 1382-1414.
- Cannon WB (1927) The James-Lange theory of emotions: A critical examination and an alternative theory. Am J Psychol 39: 106-124.
- Cobos P, Sánchez MB, Vila J (2009) Algunos efectos de la lesión medular en el componente subjetivo de las emociones. Revista de Psicología de la Salud 14: 29-48.
- Colombo R, Pisano F, Mazzone A, Delconte C, Micera S, et al. (2007) Design strategies to improve patient motivation during robot-aided rehabilitation. J Neuroeng Rehabil 4: 3.
- Iglesias A, García J, García-Olaya Á, Fuentetaja R, Fernández F, et al. (2021) Extending the evaluation of social assistive robots with accessibility indicators: The AUSUS evaluation framework. IEEE Trans Hum Mach Syst 51: 601-612.
- Casas J, Irfan B, Senft E, Gutiérrez L, Rincon-Roncancio M, et al. (2018) Social assistive robot for cardiac rehabilitation: A pilot study with patients with angioplasty. In: Companion of the 2018 ACM/IEEE International Conference on Human-Robot Interaction 2018: 79-80.
- Pulido JC, Suarez-Mejias C, Gonzalez JC, Ruiz AD, Ferri PF, et al. (2019) A socially assistive robotic platform for upper-limb rehabilitation: a longitudinal study with pediatric patients. IEEE Robot Autom Mag 26: 24-39.
- Salas-Monedero M, Cereijo-Herranz V, DelosReyes-Guzmán A, Pérez-Borrego Y, Gil-Agudo A, et al. (2023) Smoothness and Efficiency Metrics Behavior after an Upper Extremity Training with Robic Humanoid Robot in Paediatric Spinal Cord Injured Patients. Applied Sciences 13: 4979.
- Kirshblum S, Waring W 3rd (2014) Updates for the International Standards for Neurological Classification of Spinal Cord Injury. Phys Med Rehabil Clin N Am 25: 505-517.
- Catz A, Itzkovich M, Tesio L, Biering-Sorensen F, Weeks C, et al. (2007) A multicenter international study on the Spinal Cord Independence Measure, version III: Rasch psychometric validation. Spinal Cord 45: 275-291.
- Pulido JC, González JC, Suárez-Mejías C, Bandera A, Bustos P, et al. (2017) Evaluating the child–robot interaction of the NAOTherapist platform in pediatric rehabilitation. Int J Soc Robot 9: 343-358.
- Martin Ginis KA, van der Scheer JW, Latimer-Cheung AE, Barrow A, Bourne C, et al. Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord 56: 308-321.
- Leap Motion Controller (2023) Leap Motion Controller.
- de Los Reyes-Guzmán A, Lozano-Berrio V, Alvarez-Rodríguez M, López-Dolado E, Ceruelo-Abajo S, et al. (2021) RehabHand: Oriented-tasks serious games for upper limb rehabilitation by using Leap Motion Controller and target population in spinal cord injury. NeuroRehabilitation 48: 365-373.
- Wessels RD, De Witte LP (2003) Reliability and validity of the Dutch version of QUEST 2.0 with users of various types of assistive devices. Disabil Rehabil 25: 267–272.
- Bradley MM, Lang PJ (1994) Measuring emotion: the self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry 25: 49-59.
- Kortte KB, Falk LD, Castillo RC, Johnson-Greene D, Wegener ST (2007) The Hopkins rehabilitation engagement rating scale: development and psychometric properties. Arch Phys Med Rehabil 88: 877-884.
- de Crignis AC, Ruhnau ST, Hösl M, Lefint J, Amberger T, et al. (2023) Robotic arm training in neurorehabilitation enhanced by augmented reality–a usability and feasibility study. J Neuroeng Rehabil 20: 105.
- Dallmeijer AJ, Hopman MT, van As HH, van der Woude LH (1996) Physical capacity and physical strain in persons with tetraplegia; the role of sport activity. Spinal Cord 34: 729-735.
- Valent LJ, Dallmeijer AJ, Houdijk H, Slootman HJ, Post MW, et al. (2008) Influence of hand cycling on physical capacity in the rehabilitation of persons with a spinal cord injury: a longitudinal cohort study. Arch Phys Med Rehabil 89: 1016-1022.
- Janssen TW, Dallmeijer AJ, Veeger DJ, van der Woude LH (2002) Normative values and determinants of physical capacity in individuals with spinal cord injury. J Rehabil Res Dev 39: 29-39.
- Spooren AI, Janssen-Potten YJ, Kerckhofs E, Seelen HA (2009) Outcome of motor training programmes on arm and hand functioning in patients with cervical spinal cord injury according to different levels of the ICF: a systematic review. J Rehabil Med 41: 497-505.
- Kiilavuori H, Peltola MJ, Sariola V, Hietanen JK (2022) Being watched by a humanoid robot and a human: Effects on affect-related psychophysiological responses. Biol Psychol 175: 108451.
- D’Onofrio G, Fiorini L, Sorrentino A, Russo S, Ciccone F, et al. (2022) Emotion recognizing by a robotic solution initiative (EMOTIVE project). Sensors 22: 2861.
Citation: Salas-Monedero M, Cereijo-Herranz V, Madroñero-Mariscal R, Pérez-Borrego Y, Gil-Agudo A, et al. (2024) Robic Socially Assistive Robot Enables Upper Extremity Endurance Training in Spinal Cord Injured Children. J Phys Med Rehabil Disabil 10: 091.
Copyright: © 2024 Miriam Salas-Monedero, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

