
Safe Delivery of High-Dose Radiation Therapy to an Aggressive Head and Neck Malignancy in a Patient with Deep Brain Stimula
*Corresponding Author(s):
Lyndell E KellyDepartment Of Oncology, Dunedin Public Hospital, Dunedin, New Zealand
Tel:+64 3 474 0999,
Fax:+64 34709689
Email:Lyndell.kelly@southerndhb.govt.nz
Abstract
Head and neck radiotherapy in people with deep brain stimulation is going to be an increasing concurrence. There is very little guidance as to how to manage this.
A man with severe Parkinson’s Disease dependent on deep brain stimulators required high dose radiotherapy for a pleomorphic dermal sarcoma or malignant fibrous histiocytoma. The contra-lateral Implantable Pulse Generator (IPG) received a low dose (2.93Gy) from divergence and the uni-lateral lead the full dose (60Gy superiorly and 54Gy inferiorly). We received advice from the manufacturer and the very limited literature.
The device was safely turned off for each of 30 fractions. The patient has remained free of cancer for 4 years and the deep brain stimulator leads and IPGs remain functional.
It is possible to give a low dose of radiation to a deep brain stimulator IPG and a high dose to a lead with minimal effect.
Keywords
INTRODUCTION
In the USA 63,000 cases of head and neck cancer will be diagnosed annually (excluding skin cancers) [1]. Of these, 78% are likely to have radiotherapy as part of their treatment [2]. Coinciding with this are an estimated five million artificial cardiac pacemakers worldwide and over 70,000 people with Deep Brain Stimulators (DBS) for a variety of reasons [3,4].
Case reports and in vitro studies have highlighted potential clinical consequences of delivery of ionising radiation to these devices. Limited evidence exists in the literature to guide safety assessments when prescribing radiotherapy that may cause electromagnetic disturbance to these devices.
A previous case reported a patient with severe Parkinson’s disease and DBS requiring radiotherapy for larynx squamous cell carcinoma with bilateral lymphadenopathy and extracapsular extension. To control the tremor, device settings were switched to maximal during each fraction. There were no deleterious effects on the device from scattered dose of 7.5Gy over 33 fractions [5].
Guy et al., reported a case of palliative radiotherapy to metastatic frontal cerebral lesions. A hypofractionated scheme delivered 21Gy in 3 fractions over one week using dynamic intensity-modulated radiotherapy [6]. Less than 1Gy and less than 0.01Gy were observed on the electrodes and on the generator respectively [6]. No changes were made to the devices during delivery of radiotherapy.
More recently successful delivery of whole brain radiotherapy in a patient with deep brain stimulators was reported [7]. In this case the first three fractions were delivered whilst the DBS remained turned on and the subsequent seven fractions with the device switched off after manufacturers advice. No difference in patient symptoms was reported.
CASE REPORT
A 52-year-old man presented with a malignant atypical fibroxanthoma, since renamed dermal pleomorphic sarcoma, on his right forehead. Histology revealed a high mitotic rate and perineural invasion. Subsequent palpable masses in the parotid were resected by superficial parotidectomy and modified right neck dissection. Histology confirmed metastases with positive margins in the parotid, strongly indicating radiotherapy.
The DBS electrodes had been implanted in the sub-thalamic nuclei bilaterally as seen in figure 1a three years prior to this cancer diagnosis, along with bilateral leads going down the neck to Implanted Pulse Generators (IPGs) in the chest. Advice from DBS manufacturers Medtronic was to switch the device off before each fraction using a Medtronic DBS control unit (Table 1). Voltage was slowly reduced to zero bilaterally. A physician was on hand during each treatment, after which the bilateral DBS were switched on and voltage increased gradually back to usual parameters.
|
MEDTRONIC additional guidance Electromagnetic radiation has the potential to:
Guidelines recommend that the neurostimulator should be turned off to reduce patient discomfort, overstimulation effects and tissue damage (MEDTRONIC unpublished guidelines correspondence 2012). |
Table 1: MEDTRONIC information on using radiation therapy and possible risks with deep brain stimulators.
In the post-operative period before beginning radiotherapy, our patient began to have increased symptoms from his Parkinson’s disease such as dysphagia and dysphonia, motor incoordination and rigidity. During radiotherapy symptoms continued to worsen, prompting a recommendation for the voltage to be increased slightly. Increased voltage from 3.6 to 3.7mV in the last few days of radiotherapy doubled the current, while impedance was unaffected (Table 2). His Parkinson’s symptoms improved considerably.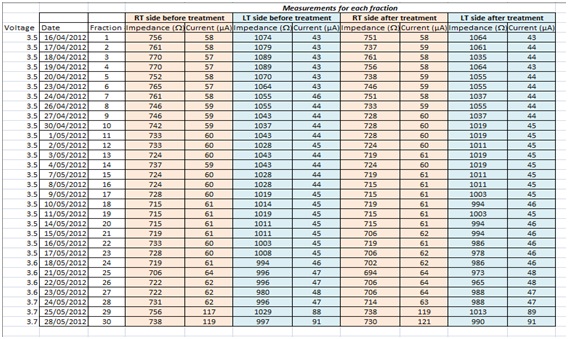
Table 2: Impedance and current before and after each fraction of radiotherapy.
Radiotherapy delivered 60Gy in 30 fractions to the 95% volume covering the right frontal, temple and parotid areas and 54Gy to the right lower neck (Figure 1a and 1b). It was planned using IMRT with a 6MV beam. The deep brain stimulator casing was protected with lead shielding over the chest. The patient was monitored via CCTV during the treatments. The treatment was completed without any immediate complications although has expected the usual radiation effects occurred requiring daily dressings.
The left chest IPG received 2.93Gy from divergence. The right neck lead received 60Gy superiorly and from level 3 inferiorly 54Gy as it was within the Planning Tumour Volume (PTV) (Figure 1b). The electrodes in the sub-thalamic nuclei received virtually no dose.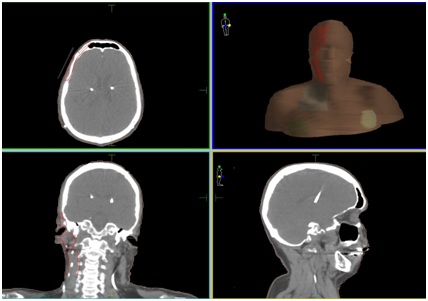
Figure 1a: Planning (CT) images showing part of (PTV) (red for 60Gy and light blue for 54Gy) and deep brain stimulator devices in chest and leads in neck and brain.
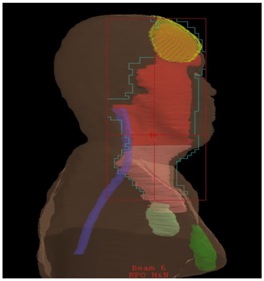
Figure 1b: Lateral view of radiotherapy plan showing.
yellow=primary site with wax bolus on surface
red= high-dose volume 60Gy
pink= moderate dose volume 54Gy
light green= left sided stimulator unit
dark green= right sided stimulator unit
purple=spinal cord
Our patient remains free of cancer 4years post-radiotherapy. Two years after radiotherapy, the electrodes moved and were replaced in the sub-thalamic nuclei, however the IPGs and leads have lasted at least 4 years without problems. His Parkinson’s symptoms have not changed over the last 10 years. He is still able to live independently in his own home.
DISCUSSION
Described as a rare primary cutaneous sarcoma, pleomorphic dermal sarcoma locoregional cutaneous metastases are reported to occur in up to 10% of cases [8]. However, the reporting of radiotherapy and post-operative radiotherapy is sparse despite the reported rate of loco regional disease. This was clearly a malignant disease with metastases to the right parotid and a positive margin at resection, therefore post-operative radiotherapy was recommended.
Planning envisaged two problems. Radiation-induced IPG damage and cerebral side effects on ON/OFF switching of the DBS device.
Radiation damage to DBS may be due to similar processes that occur in pacemakers. Titanium casing offers minor protection [3]. Pacemaker malfunction during radiotherapy is a consequence of damage to hardware and software [3]. Susceptibility to radiation is either due to destruction of electrical components (often with direct irradiation) or effects on the random access memory (secondary to scatter radiation or electromagnetic interference) [6]. Abnormal current flows, changes in threshold voltage and alteration of stimulation threshold due to thermal damage at electrode tips can occur [3]. The metal oxide semiconductor circuitry used in cardiac pacemakers is sensitive to radiation effects and therapeutic stimulation thresholds can be altered due to thermal damage at the stimulating surfaces of pacemaker electrodes [9].
The potential damage to DBS systems and leads by Magnetic Resonance Imaging has been explored in greater detail than radiotherapy induced damage. This has been particularly so with CNS MRI imaging which is used to confirm placement post implantation. MRI heating of the electrodes has been shown to induce significant temperature changes at the electrode tip and local tissue damage. This damage has been described as oedema and local haemorrhage [10]. Although a single case report, delivery of whole-brain radiotherapy by Kotecha et al., did not report side effects within their patient with no discernible damage to leads or electrodes. It could be expected that local effects of radiation on the lead in our patient would occur but the dose to electrodes was negligible.
Little exists in the literature regarding turning the DBS device ON/OFF so frequently. Our patient had his device switched ON/OFF thirty times over a period of 6weeks. Daily recordings of the parameters showed no change in current or impedance until the voltage was increased. Alteration of DBS can lead to clinical manifestations such as pathological crying, transient apathy, depression, anxiety and involuntary laughter [11,12]. Deep brain stimulation can worsen speech and gait in some patients, requiring stimulation parameters to be adjusted [13].
Patients with DBS have been reported to have an increased rate of suicide14but this is disputed as reviewed by Faggiani and Benazzouza et al., [14,15]. Our patient felt happiness when DBS was switched on again and the voltage was increased. It is unclear if the temporary worsening of Parkinsonian symptoms in our patient was due to frequent switching ON/OFF, but his symptoms improved when voltage was slightly increased. Extensive and high-dose head and neck radiotherapy can be expected to cause temporary worsening of mobility and strength even without Parkinson’s disease.
CONCLUSION
This case highlights the limited understanding of the hazards of radiation therapy in patients with DBS. The published literature now sets out the delivery of various dose fractionation schedules and dose exposures to the DBS device that have resulted in limited consequences for the patient. However, in some cases this may require direct radiotherapy to the implanted device and have greater potential for device malfunctions than have been documented in our and previous case reports. This may even require position changes of IPGs. A clear outline of the risks, potential clinical problems and a pre-determined management plan need to be formulated before commencement of radiation therapy (Table 3). Communication of the risks with the patient and colleagues is key but unfortunately in most cases the seriousness of the underlying cancer is warrant enough to continue with radiation treatment.
|
S No |
Factors to consider before starting therapy |
|
1 |
Availability on daily basis of Nursing staff, Physicist, and Oncologist |
|
2 |
Direct irradiation to the casing and scatter radiation |
|
3 |
Severity of symptoms with device turned off |
|
4 |
Access to equipment to turn DBS On/OFF |
|
5 |
Setup of radiation room and availability of CCTV to monitor patient |
|
6 |
Pre-existing symptoms |
|
7 |
Previous radiotherapy and exposure of DBS |
|
8 |
Specialist Neurology input |
|
9 |
Access to Psychiatric support |
Table 3: Planning considerations to address prior to beginning therapy for patients with DBS.
REFERENCES
- Stenson K (1994) Epidemiology and Risk Factors for Head and Neck Cancer. Semin Oncol 21: 281-288.
- Delaney G, Jacob S, Featherstone C, Barton M (2005) The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 104: 1129-1137.
- Sundar S, Symonds RP, Deehan C (2005) Radiotherapy to patients with artificial cardiac pacemakers. Cancer Treat Rev 31: 474-486.
- Bronstein JM, Tagliti M, Alterman RL (2011) Deep Brain Stimulation for Parkinson Disease. Arch Neurol 68: 165-171.
- Mazdai G, Stewart DP, Hounsell AR (2006) Radical Radiation Therapy in a Patient with Head and Neck Cancer and Severe Parkinson’s Disease. Clin Oncol (R Coll Radiol) 18: 82-84.
- Guy JB, Levy A, Malkoun N, Chargari C, Bigot L, et al. (2014) Preventing radiotherapy-induced side effects on deep brain stimulators: the need for a multidisciplinary management. British Journal of Neurosurgery 28: 107-109.
- Kotecha R, Berriochoa CA, Murphy ES, Machado AG, Chao ST, et al. (2016) Report of whole-brain radiation therapy in a patient with an implanted deep brain stimulator: Important neurosurgical considerations and radiotherapy practice principles. J Neurosurg 124: 966-970.
- Kohlmeyer J, Steimle-Grauer S, Hein R (2017) Cutaneous Sarcomas. J Dtsch Dermatol Ges 15: 630-648.
- Wadasadawala T, Pandey A, Agarwal JP, Jalali R, Laskar SG, et al. (2011) Radiation Therapy with Implanted Cardiac Pacemaker Devices: A Clinical and Dosimetric Analysis of Patient and Proposed Precautions. Clinical Oncology 23: 79-85.
- Erhardt J, Fuhrer E, Gruschke O, Leupold J, Wapler MC, et al. (2018) Should patients with brain implants undergo MRI? J Neural Eng 15: 041002.
- Larson PS, Martin AJ (2011) Safety Concerns and Limitations. In: Arle JE, Shils JL (eds.). Essential neuromodulation, (1st edn). Elsevier, London, UK. Pg no: 397-412.
- Wojtecki L, Nickel J, Timmerman L, Maarouf M, Südmeyer M, et al. (2007) Pathological Crying Induced by Deep Brain Stimulation. Mov Disord 22: 1314-1361.
- Shipton EA (2012) Movement disorders and neuromodulation. Neuro Res Int 2012: 309431.
- Schneider F, Habel U, Volkmann J, Regel S, Kornischka J, et al. (2003) Deep Brain Stimulation of the Subthalamic Nucleus Enhances Emotional Processing in Parkinson Disease. Arch Gen Psychiatry 60: 296-302.
- Faggiani E, Benazzouza A (2017) Deep brain stimulation of the subthalamic nucleus in Parkinson’s disease: From history to the interaction with the monoaminergic systems. Prog Neurobiol 151: 139-156.
Citation: Page SM, Paris MI, Kelly LE (2019) Safe Delivery of High-Dose Radiation Therapy to an Aggressive Head and Neck Malignancy in a Patient with Deep Brain Stimulators J Clin Stud Med Case Rep 6: 060.
Copyright: © 2019 Simon M Page, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

