
Short Term Outcomes of a Living Renal Transplant Program at a Private Hospital in Nairobi, Kenya
*Corresponding Author(s):
Hussein BaghaM.P Shah Hospital, Nairobi, Kenya
Email:baghahussein@yahoo.com
Abstract
Background
Kidney transplant is the preferred renal replacement therapy over dialysis as it improves quality of life and reduces mortality. In sub-Saharan Africa, kidney transplants are rare due to limited resources and high costs. We present a single-centre experience of a kidney transplant program at M.P Shah Hospital in Nairobi, Kenya.
Methods
This retrospective observational study reviewed all kidney transplants performed from 2018 to 2023. Data were obtained from patient files at M.P Shah Hospital and from private nephrologists following the patient's post-transplant. Recipient and donor demographics, immunological profiles, induction therapy use, hospital stay and serum creatinine levels were analysed.
Results
A total of 32 living kidney transplants were performed between June 2018 and December 2023. Recipients were predominantly male (81.3%), with 56.2% having HLA mismatches >3/6. Basilximab was used as induction in 68.7% of recipients; no patient received anti-thymocyte globulin or alemtuzumab. Average hospital stay was 10.7 days for recipients and 3.6 days for donors. Mean serum creatinine at one-month post-transplant was 123.9 µmol/L for recipients and 139 µmol/L at donor discharge. One recipient developed delayed graft function, which responded well to treatment.
Conclusion
Living donor kidney transplantation at M.P Shah Hospital shows excellent short-term outcomes without use of lymphocyte-depleting induction agents, even in high-risk recipients. Longer-term follow-up studies are recommended to assess graft survival and patient outcomes.
Introduction
Chronic Kidney Disease (CKD) represents a significant global health concern, with Sub-Saharan Africa (SSA) bearing a disproportionate burden despite under-resourced health systems. Kenya has a CKD prevalence of about 10% [1,2]. The rising incidence is largely attributed to increasing rates of hypertension, diabetes mellitus, chronic infections such as HIV [3]. Access to kidney care remains a major challenge across Kenya and SSA. Haemodialysis is the most widely available form of Kidney Replacement Therapy (KRT) for patients with End-Stage Kidney Disease (ESKD), yet it is largely confined to urban areas and remains unaffordable for many. Kidney transplant is the preferred modality of renal replacement therapy for patients with ESKD, offering better quality of life, lower long-term healthcare costs, significantly reduced mortality compared to dialysis [4,5]. Despite its proven benefits, kidney transplantation remains underutilized in SSA due to limited resources, high procedural costs, insufficient infrastructure [6]. High costs are driven by induction and maintenance immunosuppressive therapies and laboratory monitoring [7]. This study presents the short-term outcomes of a living donor kidney transplant program at M.P Shah Hospital, a 240-bed private tertiary facility in Nairobi. The hospital launched its transplant program in June 2018. These findings contribute valuable data from a region where transplant services are still emerging. Given the paucity of regional data, this study provides critical insight into short-term kidney transplant outcomes and highlights opportunities to strengthen transplant services in SSA.
Materials and Methods
This retrospective observational study was conducted over five years from January 2018 to December 2023 at M.P Shah Hospital, Nairobi, Kenya. All kidney transplant procedures performed during this period were included.
Data were collected from the hospital’s transplant registry, patient medical records and follow-up notes from both hospital and private nephrologists managing post-transplant care. Extracted recipient data included demographics (age, gender, blood group), transplant date, serum creatinine at transplant, discharge, one-month post-transplant, HLA mismatch, induction therapy use. Donor data included age, gender, blood group, serum creatinine on transplant day at discharge. Data were entered into a secure electronic database and analysed using descriptive statistics. Means, medians, ranges were calculated for serum creatinine values. Categorical variables such as gender, blood group, induction therapy and HLA mismatch were expressed as frequencies and percentages. This methodology enabled comprehensive assessment of short-term outcomes and early graft function in the transplant cohort.
Results
Between June 2018 and December 2023, 32 living donor kidney transplants were performed. Recipients were predominantly male (81.3%, n=26) and 69% (n=22) of donors were male (Table1). Recipient ages ranged from 21 to 72 years, with a mean of 42.25 years and median of 41.5 years. Donors had a mean age of 38.25 years (range 20–57 years). Most donors (67%, n=21) were aged 20–40 years; the rest were 41–60 years. No donors were under 20 or over 60 years. (Table 2) Most of the recipients were hypertensive (90.6%), followed by diabetes mellitus at 34.4%, ADPKD at 6.3%. Other co-morbids were equal in proportions and are shown in figure 1.
|
Characteristic |
Recipient, N = 32 |
Donor, N = 32 |
|
Gender, n (%) |
|
|
|
Female |
6 (18.8) |
10 (31.3) |
|
Male |
26 (81.3) |
22 (68.8) |
|
Age, Median (IQR)
|
41.5 (33.8 – 50.8) |
39.0 (31.5 – 43.8) |
Table 1: Demographic characteristics of recipients and donors.
|
Age Group (years) |
Recipients (n=32) |
% |
Donor (n=32) |
% |
|
20-40
|
15 |
46.9% |
21 |
67% |
|
41-60
|
16 |
50.0% |
11 |
33% |
|
>60
|
1 |
3.1% |
0 |
0 |
|
Mean Age
|
42.25 |
|
38.25 |
|
Table 2: Age Distribution of Kidney Transplant Recipient and Donors.
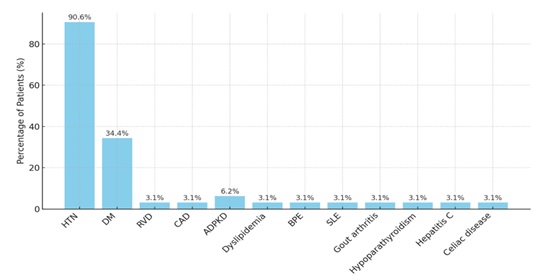 Figure 1: Cormorbidities presented among recipients. Comorbidities in kidney Transplant Recipients (N=32).
Figure 1: Cormorbidities presented among recipients. Comorbidities in kidney Transplant Recipients (N=32).
Eighteen recipients (56.2%) had HLA mismatches greater than 3 out of 6. Four recipients had prior multiple blood transfusions. Basiliximab was used as induction in 22 recipients (68.7%). No patients received anti-thymocyte globulin or alemtuzumab. (Figures 2&3) Blood group O positive predominated among recipients (53.13%) and donors (65.36%). One recipient was O negative, one was A negative. (Table 3) Average hospital stay was 10.7 days (median 11) for recipients and 3.6 days (median 4) for donors. Mean serum creatinine one-month post-transplant was 123.9 µmol/L (range 75–349) for recipients. Donor serum creatinine at discharge averaged 139 µmol/L (range 76–186) (Table 3). One recipient (3.1%) developed delayed graft function requiring three dialysis sessions. Renal biopsy confirmed acute cellular rejection, which responded well to high-dose methylprednisolone. Serum creatinine stabilized at 112 µmol/L at one month.
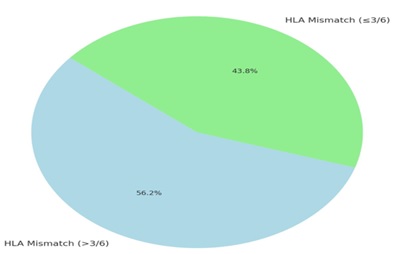 Figure 2: HLA Mismatch in recipient.
Figure 2: HLA Mismatch in recipient.
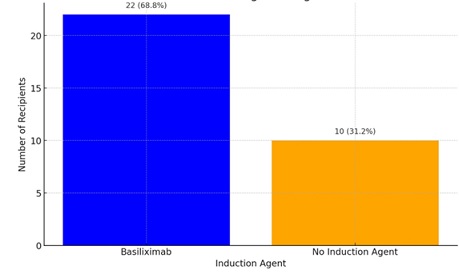 Figure 3: Induction agents used.
Figure 3: Induction agents used.
|
Characteristic |
Recipient, N=32 |
Donor, N=32 |
|
Blood Group, n (%) |
|
|
|
A negative |
1 (3.1) |
0 (0.0) |
|
A positive |
5 (5.6) |
6 (18.8) |
|
AB positive |
3 (9.4) |
0 (0.0) |
|
B positive |
5 (15) |
5 (15.6) |
|
O negative |
1 (3.1) |
0 (0.0) |
|
O positive |
17 (53.1) |
21 (65.6) |
|
Median at admission (IQR) |
749 (594.3-1,003.0) |
81.5 (64.8-95.0) |
|
Median at discharge (IQR) |
139 (114.8-173) |
124 (99.8-152.5) |
|
Median Cr at 1 month |
112mmol/L |
122mmol/L |
|
Mean Cr at one month |
123.9mmol/L |
139mmol/L |
|
Average length of stay (range : min- max) |
10.7 (8.0-24) |
3.9 (3.0-8.0) |
Table 3: Clinical characteristic of donors and recipients.
Discussion
This review of 32 living donor kidney transplants over five years provides insights into demographics, immunological profiles, early post-operative outcomes at our centre. Male predominance among recipients (81.3%) and donors (69%) aligns with reports from SSA where males often have greater access to transplant services due to socio-cultural and economic factors [8]. Recipients were relatively young (mean 42.25 years), consistent with earlier onset of ESRD in low- and middle-income countries due to poorly controlled hypertension and diabetes [8]. The study highlights the high burden of comorbidities among kidney transplant recipient, with hypertension (90.6%) and diabetes mellitus (34.4%) being the most common. These findings align with existing literature, as both conditions are major risk factors for adverse transplant outcomes [9,10].
Less common comorbidities include autosomal dominant polycystic kidney disease (6.2%), dyslipidemia, Coronary arterial disease, systemic lupus erythematosus others (each 3.1%). Although less frequent, these still contribute to early post-transplant complications, particularly cardiovascular risk [11].
Autoimmune and metabolic disorders such as SLE, gout and hypoparathyroidism require tailored immunosuppressive and metabolic management [12,13]. Overall, the comorbidity pattern in this private facility mirrors global trends and may reflect improved access to pre-transplant. Addressing these issues through integrated transplant care pathways could enhance outcomes and quality of life [14]. Despite over half of recipients having HLA mismatches >3/6, favourable short-term outcomes were observed, supporting evidence that living donor transplants with higher mismatches can have acceptable graft survival with appropriate immunosuppression. Basiliximab induction in 68.7% of recipients aligns with literature supporting its efficacy in reducing acute rejection in low to moderate immunologic risk patients [15]. The absence of anti-thymocyte globulin or alemtuzumab may reflect cost, availability, or institutional protocols. Blood group O positive predominance mirrors Kenyan population trends [16]. Hospital stays were comparable to centres using laparoscopic donor nephrectomy, despite open nephrectomy being performed [17]. Delayed graft function occurred in 3.1%, lower than typical rates of 4–10% in living donor transplants [18]. The rejection episode was effectively managed. Mean serum creatinine at one month (123.9 µmol/L) indicates satisfactory early graft function, predictive of better long-term survival [19].
Conclusion
Evaluating short-term transplant outcomes is crucial to assess program safety and efficacy, identify gaps in care and guide improvements. Our data suggest living donor kidney transplantation can be successfully implemented with favourable short-term results, even in high-risk recipients, using basiliximab induction without lymphocyte-depleting agents. The oldest recipient (73 years) had excellent graft function at one month, demonstrating safe transplantation in carefully selected older patients. Longer-term follow-up is essential to evaluate graft survival and overall patient outcomes.
References
- Stanifer JW, Jing B, Tolan S, Helmke N, Mukerjee R, et al. (2014) The epidemiology of chronic kidney disease in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Glob Health 2: e174-e181.
- Kenya Renal Association. Kenya Renal Association database.
- Wachira M, Otieno FC, Oyatsi D (2020) Prevalence of chronic kidney disease among patients admitted at the Kenyatta National Hospital medical wards. East Afr Med J 97: 245-252.
- Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, et al. (1999) Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730.
- Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, et al. (2011) Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 11: 2093–2109.
- Bukenya D, Marcellin F, Genberg B (2017) Barriers to kidney transplantation in sub-Saharan Africa: a systematic review.
- Naicker S, Luyckx VA (2016) Kidney transplantation in sub-Saharan Africa: challenges and opportunities. Transplantation 100: 230–234.
- Stanifer JW, Muiru A, Jafar TH, Patel UD (2016) Chronic kidney disease in low- and middle-income countries. Nephrol Dial Transplant 31: 868–874.
- Opelz G, Dohler B (2005) Improved long term outcomes after renal transplantation associated with blood pressure control. Am J Transplant 5: 2725-2731.
- Porrini E, Delgado P, Torres A (2010) Metabolic syndrome, insulin resistance and chronic allograft dysfunction. Kidney Int Suppl 78: S42-S46.
- Lentine KL, Costa SP, Weir MR, Robb JF, Fleisher LA, et al. (2012) Cardiac disease evaluation and management among kidney and liver transplantation candidates. Circulation 126: 617-663.
- Ponteceli C, Moroni G (2005) Renal transplantation in lupus nephritis. Lupus. Clin Transpl 14: 95-98.
- Evenepoel P, Bover J, Urena Torres P (2016) Parathyroid hormone metabolism and signalling in health and chronic kidney disease. Kidney Int 90: 1184-1190.
- Gonzalez FM, Gonzalez Cohen FDR (2024) Kidney transplantation outcomes: is it possible to improve when good results are falling down? World J Transplant 14: 91214
- Wang X (2016) Basiliximab induction in kidney transplantation with donation after cardiac death donors: a meta-analysis. Transplant Proc 48: 1904–1910.
- Omari-Furuya M, Lumdsen R, Katende S (1992) Blood-group systems ABO and RH in the Kenyan population. East Afr Med J 69: 366–369.
- Feng B, Sun KS, Liu J (2019) Comparison of surgical techniques in living donor nephrectomy: a meta-analysis. Ann Transplant 24: 476–483.
- Redfield RR, Scalea JR, Zens TJ, Muth B, Kaufman DB, et al. (2015) Predictors and outcomes of delayed graft function after living-donor kidney transplantation. Transplantation 99: e20–e21.
- Koh SM, Ju MK, Huh KH, Kim YS, Kim MS, et al. (2020) Serum creatinine level at 1-month posttransplant can independently predict long-term graft survival and functional status. Korean J Transplant 34: 93–100.
Citation: Bagha H, Farah S, Sharma A, Juma K, Twahir H (2025) Short Term Outcomes of a Living Renal Transplant Program at a Private Hospital in Nairobi, Kenya. J Nephrol Renal Ther 11: 103.
Copyright: © 2025 Hussein Bagha, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

