
Short Term Technology-Assisted-Aerobic Exercise (AlterGR, GlideTrakTM, Vasper) in a Community Fitness Center for Patients with Mild to Moderate Parkinson’s Disease: Subjective Perceptions and Motor Effects
*Corresponding Author(s):
Nancy BylDepartment Of Physical Therapy And Rehabilitation Science, School Of Medicine, University Of California, San Francisco, California, United States
Tel:+1 4155144816,
Email:Byln@ptrehab.ucsf.edu
Abstract
Background: Physical inactivity is a significant health risk, particularly in the growing population of elders with chronic neurodegenerative conditions like Parkinson’s Disease (PD).
Purpose: Determine if individuals with mild to moderate PD can achieve aerobic levels of exercise using novel rehabilitative technology (AlterGR, GlideTrakTM, Vasper) and if short term aerobic training is associated withmobility performance and subjective perceptions.
Methodology: Two quality assurance, pre-post test design studies were carried out with individuals with PD (Hoehn and Yahr I-III) involved in physical therapy in a health and wellness center. In study I, with a 3 month cross over delay, 12 participants were randomly assigned to daily training (5 days, 40 minutes/session) on two novel body weight supported treadmill systems (Alter-GR and GlideTrakTM). In study II, ten participants trained for 5 weeks (2x/week, 20 minutes/session) on the recumbent NuStepTMT5XR recumbent cross trainerwith cooling, compression and grounding by Vasper. Self reported signs, symptoms and training challenges were assessed before, during and after training complemented with mobility and balance assessments before and after training (ten meter walk, six minute walk, timed up and go and five times sit to stand).
Results: Twenty participants started and safely completed (three or four-omit) the assigned technology assisted-aerobic training sessions (each 200 minutes). All but two participants achieved a target heart rate of 60-80% of age relevant maximum with all reaching an exertion level ≥3/10). After each training protocol, participants achieved significant (p<0.025) gains in balance and gait, improving by 1-2 seconds on the TUG and FTSST and gaining 0.28 m/sec in walking speed to achieve a community level of participation. Walking endurance increased an average of 80 meters. During aerobic training, participants self reported mild to moderate discomfort, but noted improvement in energy, resilience and gait stability without exacerbation of PD signs and symptoms. Training gains varied by technology assisted exercise groups.
Summary: Novel rehabilitative-technology allowed participants with mild to moderate PD to exercise aerobically and improve mobility, balance and resilience without exacerbating pain, freezing or tremors. Participants recommend the incorporation of technology assisted aerobic equipment in community fitness centers and group exercise programs to enable individuals with PD to independently maintain health and wellness.
Keywords
ABBREVIATIONS
FMH: Fetomaternal Hemorrhage;
KB: Kleihauer-Betke
INTRODUCTION
The population is aging with problems of physical inactivity, Parkinson’s Disease (PD) and Parkinsonism becoming increasingly common [1,2]. PD is characterized by progressive impairments in motor function including a resting tremor, rigidity, bradykinesia, micrography, poor postural righting and reduced speech volume along with non-motor symptoms of inflammation, pain, depression, gastrointestinal dysfunction, sleep disturbances and decreased memory skills [3]. The most common conservative medical management for PD is based on dopamine replacement medication [4]. These medications may improve but not remediate problems of incoordination, dyskinesia, sensory dysfunction, balance, fall risk, depression, cognition or gastro-intestinal dysfunction. To maintain community independence and participation despite disease related impairments, exercise is recommended to complement medication management [5-13].
Physical immobility is the leading cause of disability and disease worldwide [14,15]. Physical activity facilitates cardiovascular fitness, mobility and musculoskeletal health. Further, aerobic exercise may uniquely maintain dopamine receptors as well as increase endorphins, Brain Derivative Neurotrophic Factors (BDNF), growth hormones, up-regulation of dopamine, motor control, postural righting responses, bone density, oxygen delivery and blood flow [16,17]. Recent animal and human studies of PD suggest intense, aerobic exercise and behavioral training may slow down aging (e.g., maintain telomere length) [18], improve memory [19-23], contribute to the reorganization of the brain and potentially be neuroprotective in patients with PD [24-28]. Adding learning elements to general exercise programs may further enhance memory and protect from Alzheimer’s disease in our aging population, with and without PD [29,30].
Unfortunately, neuromotor control problems associated with PD can challenge the ability to complete safe, vigorous aerobic exercises. Vigorous exercise programs like Tai Chi, Tango dancing, striding, race walking, boxing, cycling, running, hiking and tandem biking have been successfully completed by patients with PD [6,26,30]. While a treadmill can be used to force individuals with PD to move more rapidly, increased ground reaction forces can lead to increased joint and spine pain. Thus, harness systems have been created to protect from falling as well as un-weight individuals to minimize ground reaction forces and facilitate spinal pattern generators for walking/running (body weight supported treadmill training BWSTT) [31-34].
Unfortunately, when walking fast or running, small amounts of un-weighting with a harness (e.g., >20%) can be uncomfortable [35,]. Consequently creative un-weighting systems (e.g., (www.Alter-G.com) [36], or pelvic type suspension systems (www.GlideCycle.com) [37], have been developed to improve comfort, increase the degree of un-loading and allow free limb and trunk movements. Recumbent reciprocal, elliptical cross trainers and recumbent bicycles can also protect against falling and minimize weight bearing loads. More recently, in sports, cooling and compression appear to extend cardiopulmonary benefits, reduce muscle soreness, but increase strength and speed of movement through the release of human growth factors [38,39]. The vasper cooling system (www.vasper.com) provides this option.
Before a health care delivery system, a rehabilitation center, a fitness center or an individual can justify purchasing novel and expensive rehabilitation technology, it is necessary to demonstrate positive benefits without adverse events. In a Physical Therapy Health and Wellness program integrated into a community fitness center, two quality assurance studies (Figure 1) were carried out to determine if individuals with mild to moderate PD (Hoehn and Yahr I-III) could exercise with the AlterGR, the GlideTrakTM or the NuStep with Vasper to: 1) achieve aerobic levels of training; 2) maintain if not improve mobility and balance, and 3) perceive differences in procedural utilization, benefits or exacerbation of signs and symptoms of PD following aerobic training.
MATERIALS AND METHODS
Subjects
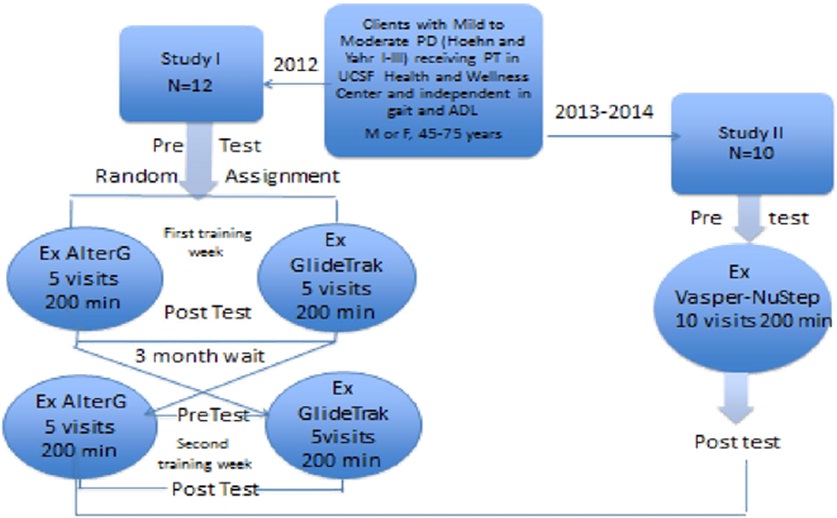
Figure 1: Design of Quality Assurance Studies.
For QA study I, 12 individuals were randomly assigned to daily Body Weight Supported Treadmill training (BWST) for five consecutive days (40 minutes/session; total 200 minutes), beginning either on the AlterGR (bodyweight supported by positive air pressure over a treadmill) or the GlideTrakTM (body weight supported by a suspended bicycle type seating system over a treadmill). The participants were crossed over after a 3 month delay. For quality assurance study II, 10 participants agreed to attend ten intense, aerobic interval training sessions on the recumbent elliptical cross trainer (NuStepTM) with compression and cooling by Vasper (20 minutes, twice a week for 5 weeks; total of 200 minutes). In both studies, 60-80% of age matched maximum heart rate was set as the target exercise heart rate. If individuals were on medications to reduce heart rate or had a pacemaker, physician approval was needed for participation and subjective exertion level (>3/10) served as the target performance in lieu of heart rate
Assessment
In both QA studies, before, during the workout and at the end of training, the participants were asked to self report signs and symptoms of pain, discomfort, fatigue, incoordination and tremor (ordinal scale from 0 [none or mild] to 10 [severe]).
Equipment

Figure 2: AlterGR Air Distributed Body Un-Weighting Treadmill (Standard M 300).
The AlterGR (www.Alter-G.com) [36], employs an air distribution system for un-weighting. This technology was developed to study the effects of gravity on bone health and physiology of astronauts in space. The technology was approved by the FDA for fitness and functional rehabilitation for patients with orthopedic and neurological impairments.
The individual dons a pair of polypropylene shorts which zip into a pressurized air bag chamber suspended over a treadmill. With the shorts zipped into the pressure chamber, and the individual standing on the treadmill, the machine “calibrates” the weight by generating an upward “lifting” force (140 to 300 pounds). After “weighing” the individual, the air is released and the calibrated weight is used as a reference for selected un-weighting during exercise (20-80%). There is some air left in the bag which underestimates the weight by about 6# [46]. The accuracy of un-weighting and re-weighting varies by approximately 5% [46].
The treadmill speed and the slope were controlled by the user or the therapist. The faster the speed, the greater the un-weighting needed to keep the ground reaction forces low [31]. The air distributed system allows more comfortable un-weighting than a harness system [35]. With greater un-weighting, individuals may achieve faster running speeds compared to over ground running [31]. For this study, the objective was to un-weight participants by 40-50% and jog with the treadmill speed 3.5 to 7 mph. GlideTrakTM(Figure 3).

Figure 3: GlideTrakTM Body Un-Weighting System.
The GlideTrakTM bodyweight support system blends un-weighted technology and low impact training indoors over a treadmill, (www.glidecycle.com) [37]. The unit un-weights the individual through support of the pelvis between a seat and a pelvic pad across the Anterior Superior IIiac Spines (ASIS). The unit has a posted seat suspended by two straps in the rear and two in the front. The pelvis is suspended for un-weighting with the angled seat supporting the ischium and a pelvic pad against both Anterior Superior Iliac Spines (ASIS) with no perineal pressure. The GlideTrakTM is adjusted to each individual with un-weighting created by tightening the straps (0-100%) [36]. The amount of un-weighting was estimated with the subject standing on a scale during tightening of the seating system. When un-weighted 40-50% by the seating system, the knee was flexed @10-20? [47]. For this study, striding rather than jogging was encouraged (e.g., upright trunk, good hip extension, forefoot roll off, heel rise with knee flexion as the weight bearing limb moved into the swing phase with hip, knee and ankle flexion until heel strike to begin the stance phase again). If necessary, the physical therapist could assist in the swing phase. The GlideTrakTM frame/seating system was placed over a Star Trek treadmill www.StarTrek.com [48]. The participant could hold on to the GlideTrak frame or swing the arms. The objective was to glide between 3 and 5 mph on a treadmill slope of 10%. The GlideTrakTMand the GlideCycle are approved by the FDA for fitness and rehabilitation.(NuStepTMwith Vasper Cooling and Compression (Figure 4).

Figure 4: Quality Assurance Study I: Participant Rating of Equipment Characteristics to Facilitate Mobility.
The NuStepTM[49], is a recumbent cross trainer which combines lower and upper extremity reciprocal body movements for a full body workout for users of virtually all ability levels.promotes independence, and invigorates users. It has user controlled step length and arm amplitude, low inertia startup, instant free coasting start and stop action for safety, a self-powered battery, quiet belt drive and a generator resistance range of 5-1400 watts. The seat swings out for easy transfer and there are leg stabilizers to keep the lower limb in neutral as needed. It is possible for the subject to use only the arms, only the legs or one arm or one leg if necessary due to pain, weakness or loss of motor control.
The Nu-StepTM is connected to a vasper cooling and compression unit [38,50,51]. This system cools both feet, the torso, the upper arms and the thighs (quadriceps and hamstrings) with an option for head cooling. Pressure is created by running cold water through the cuffs. For this quality assurance study, the pressure was adjusted primarily between 50-60 mmHg.
Intervention
Each subject warmed up over ground prior to treadmill training (e.g., walking with ankle and arm weights [2-5#], stepping over objects, integrating large arm swings, high stepping, rhythmical stepping to music and general stretching). An oximeter was used to record oxygen saturation and heart rate prior to, during and immediately after intensive exercise. An assistant or a physical therapist provided guidance for aggressive high stepping and reciprocal arm swinging.
A consistent physical therapist helped each subject on/off the GlideTrakTM and adjusted the un-weighting. A consistent research assistant helped each subject on/off the Alter-GR, zipped in the suit and calibrated the equipment. Each participant was un-weighted to approximately 50-60% of their body weight. On the Alter-GR, the amount of un-weighting, suit size, height of the air bag, running speed and time were documented each day. During the first GlideTrakTM session, the subject stood on a normal scale while the therapist tightened the straps to achieve 20o of knee flexion and approximately 40-50% of un-weighting.
On both of the BWST systems, the subject warmed up for 3-5 minutes, walking 1.0-2.4 mph. The speed was slowly increased (4.5 to 7.0 mph) depending on subject conditioning and tolerance. The subjects exercised at high intensity for 30 minutes and then cooled down by walking slowly for 3-4 minutes. Each individual was asked to stretch the heel cords before dismounting from the treadmill.
For Study II, on the NuStepTM-Vasper, each subject was scheduled to train for 10 sessions. Each session was 20 minutes of exercise (plus 5 minutes for set up and 5 minutes of post exercise cooling). One of two interval training programs was selected: “Super Six” or “Hummingbird”. Each participant trained at the low or medium level depending on their pre existing level of fitness. The hummingbird protocol included a warm up of 7 minutes at level 4 followed with 7 sprint intervals at level 5 or 6 (three 30 second sprints and four 15 second) followed by recovery intervals of 60 seconds) at 3 or 4 and a cooling period of 90 second at level 3. The super six protocol included a warm up of 9 minutes at level 4, with 6 sprint intervals at level 6, each for 30 seconds, followed by a recovery or cooling phase for 60 seconds at level 4 and a final cooling of 60 seconds. The 20 minute workout protocol ended with @10 minutes of cooling on a cooling mat.
Study design and data analysis
The primary mobility dependent variables included gait (10 Meter Walk, fast speed and Six Minute) and balance (FTSST and TUG). For each study, the primary dependent variables were summarized and described by mean (score or percentage), standard deviation and effect size [52]. The post-pre difference scores on the primary dependent variables were analyzed for significance using the nonparametric paired Wilcoxon test. Differences between the post-pre change scores for the different training groups were compared with the two sample Wilcoxon test (p<0.0125) [53].
For descriptive purposes, self reported signs and symptoms of PD and aging, ease, comfort, quality of the workout and likes and dislikes were monitored at the beginning and end of training. This data was summarized by frequency and qualitative summaries.
RESULTS
Study l
| Participant | Gender | Age (years) | Onset PD (years) | Hoehn & Yahr I-III | Pacemaker | Target HR 70-80% | Un-Weighting |
Training Speed (mph) |
| 1 | M | 77.2 | 77.2 | II | Yes | 100-104 | 50% | AG 7.0 GT 6.0 |
| 2 | M | 70 | 5 | II | No | 105-124 | 50% | AG 6.5 GT 5.5 |
| 3 | M | 66.9 | 10 | III | No | 107-126 | 50% | AG 4.8 GT4.5 |
| 4 | F | 64 | 8 | II | No | 104-124 | 50% | AG 6.0 GT 6.5 |
| 5 | M | 64.1 | 3 | III | No | 109-124 | 50% | AG 4.7 GT 6.8 |
| 6 | M | 66.7 | 5 | III | No | 107-122 | 50% | AG 5.8 GT 7.0 |
| 7 | F | 57.5 | 2 | II | No | 113-126 | 40% | AG 6.0 GT 5.5 |
| 8 | F | 73.1 | 3 | II | No | 110-118 | 50% | AG 5.0 GT 7.0 |
| 9 | M | 61.3 | 3 | II | No | 111-128 | 50% | AG 4.5 GT 5.5 |
| 10 | M | 71.5 | 3 | II | No | 104-118 | 50% | AG 5.5 GT 6.5 |
| 11 | M | 75.6 | 12 | III | Yes | 108-116 | 50% | AG 4.5 GT 5.5 |
*Pacemaker limited maximum heart rate
**Target heart rate set at 70-80% of maximum based on age [(220-age] x 70 % - [220-age] x 80 %)
***Speed of jogging/striding set by participant and therapist to achieve maximum heart rate
Note: Eleven participants completed the quality assurance study I. Seventy three percent of the participants were males. The participants had a mean age of 69.4 years of age with a diagnosis of mild to moderate PD for an average of 4.1 years. All were un-weighted to @ 50% of body weight to enable jogging/striding with reduced ground reaction forces. All but two participants achieved exercise heart rate. The two who did not achieve the desired heart rate were participants with pacemakers.
| Gait Speed (cm/sec) | 6-minute walk (m) | TUG* (sec) | FTSTS** (sec) | |
| AlterGR Group | ||||
| Pre Score Mean (SD) | 1.84 (0.23) | 419.1(84.6) | 6.86 (1.31) | 9.76 (2.05) |
| Post Score Mean (SD) | 2.06 (0.43) | 501.3(85.9) | 6.46 (1.52) | 8.11 (1.29) |
| Difference Score | 0.22 (0.36) | 82.2(40.95) | - 0.39 (1.24) | -1.65 (1.58) |
| % Difference Score | 12.00% | 19.80% | -5.70% | -16.90% |
| Effect Size | 0.61 | 2.01 | -0.31 | -1.04 |
|
Paired Wilcoxon p<0.025; Sum of Ranks: < 10 or > 45 |
Sum of ranks=6 Significant | Sum of ranks= 7 Significant | Sum of ranks =6 Significant | Sum of ranks =7 Significant |
| GlideTrakTM Group | ||||
| Pre Score Mean (SD) | 1.94 (0.32) | 460.2(87.8) | 6.53 (0.93) | 9.06 (1.75) |
| Post Score Mean (SD) | 2.08 (0.57) | 486.5(77.5) | 6.36 (1.48) | 7.94 (1.87) |
| Difference | 0.13 (0.44) | 26.5(72.1) | -0.16 (0.82) | -1.12 (1.27) |
| % Difference Score | 6.70% | 5.70% | -2.50% | -12.30% |
| Effect Size | 0.3 | 0.37 | -0.2 | -0.88 |
|
Paired Wilcoxon p<0.05; Sum of Ranks: 10 or >45) |
Sum of ranks=19 Not Significant | Sum of ranks=13.5 Not Significant |
Sum of ranks=14 Not Significant |
Sum of ranks=22 Not Significant |
| Alter-G compared to GlideTrakTM | ||||
| Mean Difference | 0.10 (0.65) | 58.4 (80.8) | -0.25 (1.75) | -0.64(1.75) |
| Effect Size | 0.14 | 0.72 | -0.21 | -0.36 |
| Two Sample Wilcoxin (Significant<0.025); sum < 65 >115) |
Sum=32.5 |
Sum= 43.0 significant (AG>GT) | Sum= 44.5 significant (AG>GT) | Sum= 17 significant (AG>GT) |
| Group | Pain | Pain | Pain | Incoordination | Balance | Fatigue | Tremor | Freezing |
| Back | Arms | Legs | ||||||
| AlterG (AG) | ||||||||
| Pre | 1.5 (1.5) | 1.2 (2.1) | 1.6 (23) | 2.5 (2.8) | 2.4 (3.0) | 2.1 (1.4) | 1.7 (1.8) | 0.9 (1.7) |
| Post | 1.6 (1.5) | 1.1 (1.8) | 1.6 (1.9) | 2.4 (1.9) | 2.5 (2.0) | 2.6 (2.1) | 1.3 (1.6) | 0.9 (1.6) |
| Difference | 0.7 (2.1) | -0.4 (0.7) | -0.7 (1.6) | -0.4 (2.5) | 0.1 (2.3) | 0.45 (1.6) | -0.6 (1.2) | -0.4 (1.3) |
| Effect Size | 0.33 | -0.57 | -0.44 | -0.16 | 0.04 | 0.28 | -0.5 | -0.31 |
| GlideTrak (Gk) | ||||||||
| Pre | 1.2 (1.5) | 0.6 (1.3) | 0.5 (0.8) | 2.9 (2.7) | 3.0 (2.6) | 2.2 (1.5) | 1.5 (1.5) | 0.6 (1.2) |
| Post | 1.2 (1.4) | 0.8 (0.8) | 1.3 (1.4) | 2.5 (2.5) | 2.4 (2.2) | 2.7 (2.1) | 1.0 (1.3) | 0.9 (1.6) |
| Difference | 0.03 (1.1) | -0.2 (1.6) | 0.14 (1.5) | -0.4 (1.7) | -0.5 (2.1) | 0.5 (1.8) | -0.6 (1.1) | 0.01 (1.5) |
| Effect Size | 0.03 | -0.13 | 0.09 | -0.24 | -0.24 | 0.28 | -0.55 | 0.01 |
Note: The participants reported mild signs and symptoms with minimal change before and after training except for tremor where there was a moderate reduction in both groups and a moderate reduction of arm and leg pain after training on the AlterGR.
Table 4, summarizes the participants’ subjective “likes” and “dislikes” about the technology. On the AlterGR, the subjects liked the feeling of a “good workout especially “without the fear of falling”. On the other hand, the participants disliked putting on the shorts and the feeling of bladder fullness or urgency when un-weighted to 50% of their body weight. On the GlideTrakTM, the subjects liked the feeling of standing tall, stretching the legs into a long stride, getting a good work out, challenging their balance, making a good heel strike and taking a long stride. However, the participants had trouble achieving a comfortable adjustment relative to the pelvic seating system.
| Liked | Disliked | General Comments |
| Alter-G R | ||
|
Freedom of running without fear of falling Gives me a glance back to my days as a runner but lost when diagnosed with PD |
Cumbersome to get into the equipment but easy to use |
Easy to maintain balance |
| GlideTrakTM | ||
|
Feel I could stretch my legs |
Never achieved anything close to comfortable |
If I could get comfortable, I think this could be a good challenge for my balance and a good workout |
| Liked | Disliked | General Comments |
| Characteristics of Work Out | Alter-GR | Glide TrakTM |
|
Ease of set up |
7.8 (2.0) |
5.6 (2.7) |
| Ease of making adjustments | 8.5 (1.2) | 4.8 (2.2) |
|
Comfort during workout |
8.3 (0.9) |
4.9 (2.7) |
| Quality of work out | 8.9 (0.9) | 7.7 (2.5) |
| Ability to heel strike | 7.9 (1.4) | 8.4 (1.2) |
| Length of stride | 8.0 (1.3) | 8.3 (1.1) |
| Post exercise soreness | 8.9 (0.9) | 7.7 (1.6) |
| Preference for Equipment | ||
| Want to purchase for home use | 64% | 36% |
| If cost =, preference to purchase for home | 82% | 18% |
| Recommend for a community fitness Center | 82% | 18%* |
Note: Participants were the most critical of the ease of set up, making adjustments and comfort during the workout on the GlideTrakTM but positive of the ability to work on improve gait parameters of heel strike and step length. The participants were the most satisfied with the quality of the work out on the AlterGR and the lack of post exercise soreness.
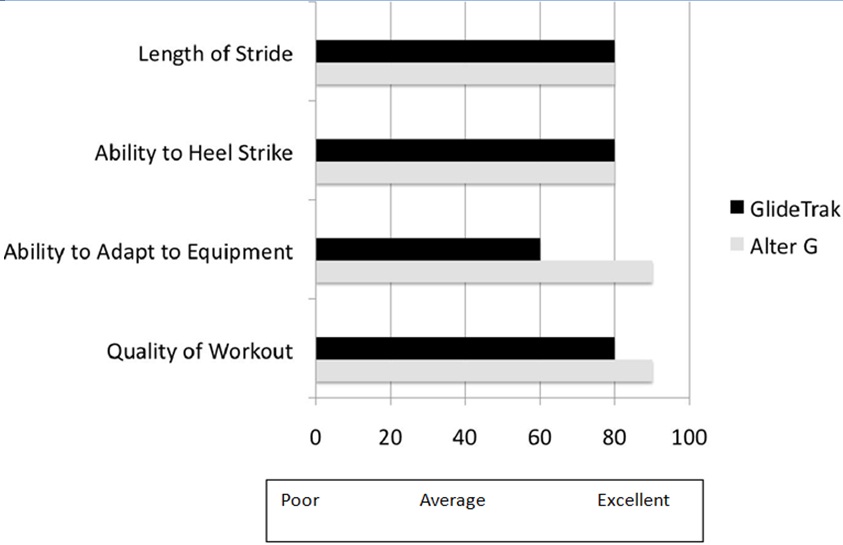 Figure 5: NuStepTM with VasperTM Cooling and Compression: Quality Assurance Study II.
Figure 5: NuStepTM with VasperTM Cooling and Compression: Quality Assurance Study II.Study II
| Participants | Gender | Age in years | Onset of PD (Years) | Hoehn and Yahr | Peak Watts Beginning/End | Target Aerobic HR 70-80% |
Exercise HR |
Sprint Level Beginning/End |
| 1 | M | 65 | 11 | II | 295/416 | 108-124 | 136 | 8/6 |
| 2 | M | 71 | 3 | II | 159/329 | 104-120 | 100 | 4/6 |
| 3 | F | 73 | 3 | II | 159/297 | 103-118 | 115 | 2/5 |
| 4 | F | 68 | 2 | II | 433/391 | 106-122 | 118 | 6/6 |
| 5 | M | 67 | 20 | III | 200/278 | 108-122 | 93 | 4/6 |
| 6 | M | 71 | 3 | II | 269/176 | 104-122 | 100 | 6/6 |
| 7 | M | 65 | 3 | III | 119/107 | 108-124 | 102 | 3/ 4 |
| 8 | F | 70 | 10 | III | 81/42 | 105-120 | 116 | 2/4 |
| 9 | M | 65 | 3 | III | 132/338 | 108-124 | 112 | 3/4 |
The participants significantly (p<0.025) increased gait speed (1.73 to 2.01 m/sec), endurance (440 to 471 meters) and balance performance (Table 7). Post training, the participants performed within age expected norms on the two balance tests, with a significant reduction in performance time (performed the tests 3 to 4 seconds faster; p<0.025). Effect sizes ranged from 0.39 to 0.82.
| 10 Minute Walk Speed (m/s) | 6 Minute Walk Distance (m) | Timed Up and Go (s) | 5 Times Sit to Stand (s) | |
| VasperTM with NuStep | ||||
| Pre Score Mean (SD) | 1.73 (0.36) | 439.9 (126.3) | 14.6 (23.6) | 12.5 (8.7) |
| Post Score Mean (SD) | 2.01 (0.40) | 470.7 (135.4) | 10.4 (13.1) | 9.6 (2.8) |
| Difference Score (SD) | 0.28 (0.34) | 30.8 (54.4) | -4.1 (10.6) | - 2.9 (6.5) |
| % Difference Score | 16.4% | 7.0% | -23.0% | -28.3% |
| Effect Size | 0.82 | 0.57 | -0.39 | - 0.45 |
|
Significance < 2 or> 26 |
Sum of ranks =7 pSum of ranks =6 pSum of ranks=6 pSum of ranks= 7 p |
Over the week of NuStep-Vasper training, participants reported a decrease in pain in both ankles, but 11-22% of the participants reported continued neck, low back or knee pain during training. Moderate pain persisted during intense training on the NuStepTM-Vasper from the initiation to the end of the training sessions. However, overall self reported signs and symptoms were in the mild range, with self reported symptoms of pain, sleeping, fatigue and freezing slightly improved (gains of 1.2-11.8%) with a moderate gain in resilience (effect size 0.55) (Table 8).
| (A) Pain* | (B)Freezing (%)** | (C)Resilience (%)** | (D) Fatigue (%)** | (E) Sleep (%)** | |
| (E) Sleep (%)** | (E) Sleep (%)** | 30.2 (22.4)% | 77.2 (12.0) | 43.4 (18.8) | 70.1 (15.5) |
| Post Score Mean (SD) | 1.61 (1.45) | 33.7 (23.4) | 85.8 (8.8) | 42.8 (18.6) | 67.1 (18.2) |
| Difference Score (SD) | 0.12 (1.36) | 3.56 (11.6) | 8.5 (15.4) | -0.54 (15.1) | -3.0 (7.0) |
| % Difference Score | 8.3% | 11.8% | 10.9% | -1.2% | -4.3% |
| Effect Size | 0.09 | 0.31 | 0.55 | -0.04 | -0.43 |
**(B-E) were reported as a percentage of the maximum score on standardized questionnaires: B) Freezing of Gait Questionnaire (FOG-Q); (C)The 14-Item Resilience Scale (RS-14); D) Fatigue Questionnaire; (E) Parkinson’s Disease Sleep Scale.
Eight of the nine participants in Study II trained on the NuStepTM/Vasper as well as the AlterGR. Table 9, summarizes the likes and dislikes as reported by the participants who trained on both pieces of technology. In general, the participants liked training on both the NuStepTM-Vasper and the AlterGR. Very few participants expressed dislikes about the NuStepTM-Vasper. The dislikes reported about the AlterGRrelated to putting on the shorts and a preference to run over ground rather than run on a treadmill. After training on the AlterGR, 62% of the participants reported an increase in energy level and 75% reported an improvement in balance and gait safety. After training on the NuStepTM-Vasper, 100% of the participants reported increased energy and 44% reported improved balance, gait safety and reduction in muscle tension.
| Liked | Disliked | General Comments |
| NuStepTM/Vasper | ||
|
Excellent supplement to AlterGR |
No negatives |
Good exercise w/o impact |
| AlterGR | ||
|
Positive health benefits |
Having to deal with putting on shorts and their leaks (2) |
At 50% un-weighting, I have a feeling of “really running” |
| Characteristics of Equipment/Work out* | NuStepTM-Vasper | AlterGR |
| Ease of using equipment | 8.2 (1.9) | 5.8(3.2) |
| Comfort during training | 8.1 (1.5) | 7.3(2.2) |
| Ease of making adjustments | 7.8 (1.6) | 7.9(1.4) |
| Getting used to equipment | 8.7 (1.0) | 7.8(1.5) |
| Ability to achieve intense workout | 8.7 (1.6) | 7.5(2.9) |
| Good challenge to balance | 7.0 (1.6) | 8.4(1.5) |
| Minimal post exercise soreness | 8.6 (0.9) | 8.1(1.5) |
| Receiving feedback re performance | 8.4 (1.3) | 8.0(1.4) |
| Recommendations/Preferences** | ||
| Preference for using VasperTM versus AlterGR | 75% | 25% |
| Would purchase VasperTM versus AlterGR for home use | 75% | 25% |
| If fitness center could only purchase one new piece of equipment, which would you recommend? | 63% | 37% |
**Proportion of participants indicating yes. Eight participants in Study II worked on the AlterGR.
Note: Eight of 9 participants would like to use NuStepTM-Vasper at home, recommend it to their friends and to community fitness centers.
The gains in mobility after training on the NuStepTM-Vasper in Study II compared to the participants training on the AlterGR and the Glide TrakTM from Study I are summarized in table 11. The gains in gait speed, endurance and balance were significantly greater following training on the NuStepTM-Vasper than training on the AlterGR. However, the gains in gait speed and performance on the TUG were not significantly greater after training on the NuStepTM-Vasper compared to the GlideTrakTM.
| Comparison by Training Groups | 10 Minute Walk Speed (m/s) | 6 Minute Walk Distance (m) | Timed Up and Go (s) | 5 Times Sit to Stand (s) |
| NuStepTM-Vasper and AlterGR | ||||
| Mean Difference | 0.50 (0.66) | -45.8 (62.2) | -4.10 (10.41) | -1.38 (5.62) |
| Effect Size | 0.76 | -0.74 | -0.39 | -0.25 |
| Significance <8 or >37 |
Sum of ranks = 73; pAG |
Sum of ranks = 52.5; pNV | Sum of ranks = 83; pNV | Sum of ranks = 63.5; pNV |
| NuStepTM-Vasper and GlideTrakTM | ||||
| Mean Difference | 0.12 (0.51) | 8.4 (122.6) | -4.04 (10.29) | -2.28 (6.65) |
| Effect Size | 0.23 | 0.07 | -0.39 | -0.34 |
| Significance < 65 or >115 | Sum of ranks = 70; p>0.05 NS | Sum of ranks = 53; pGT | Sum of ranks = 65; p>0.05 NS | Sum of ranks = 62; pGT |
DISCUSSION
Following these two QA studies, protocols for integrating rehabilitation technology into the clinic were more clearly defined. To facilitate improvement in walking speed, endurance and balance while minimizing the ground reaction forces on the lower limbs [31,35], the therapist could select either the AlterGR or the GlideTrakTM. A more complete history on urological problems was added to the medical history to minimize urinary complaints specifically on the AlterGR. All patients are asked to stop by the rest room before training on the AlterGR and each therapist discussed the potential feelings of urgency with each patient before training on the AlterGR, particularly when planning to unweight up to 50% of body weight. Patients with a history of occasional incontinence are now asked to purchase their own training shorts for purpose of cleanliness and those with severe incontinence are asked to train on the NuStepTM-Vasper or the GlideTrakTM instead of the AlterGR. The therapist also educates patients about short bouts of progressive training to achieve comfortable support on the GlideTrakTM. Before training, on the NuStepTM-Vasper, the therapist inquires about neck, back or knee pain. Where necessary, the patient may be asked to wear a back or knee support during training.
Following quality assurance Study I, participant enthusiasm for rigorous, technological assisted aerobic exercise led to the creation of an intense aerobic exercise class (90 minutes) for patients with mild to moderate PD. In this class, over ground gait, balance, strengthening and coordination training are complemented with interval type aerobic training on the NuStepTM-Vasper, the AlterGR or the GlideTrakTM. Seven of the 9 subjects participating in QA Study II joined this PD Exercise group.
While all participants noted some discomfort with intense aerobic exercise, the discomfort with un-weighting was more bothersome on the GlideTrakTM than the AlterGR. Although the participants were set up by the same therapist, a therapist who used the GlideTrakTM and the AlterGR regularly, the pelvic support was still not considered comfortable by some participants. To accommodate this adjustment, individuals start with a short training session (e.g., 5-10 minutes), with a slow increase to a session of 30-40 minutes. In addition, some individuals have elected to use the GlideTrakTM overground rather than over a treadmill. There is a bicycle model available for use outside (GlideCycle).
On the TUG, individual participants had variable performance. In Study I, the participants performed at a level similar to young controls (7.36±0.945 sec) suggesting maintenance was a more reasonable expectation than improvement [45,55,56]. In Study II, at baseline, performance was not as good as age expected norms. After training, participants significantly improved the TUG scores, performing better than the norms for individuals at risk for falling (18.14±4.6 sec) or individuals with an average age of 62.7 years (norm of 16.8 sec [±6.8]). A reduction of 2.3 seconds is considered a minimally significant improvement [55,56]. Also, initial performance on the FTSST was below normative values for the participants in Study II [56]. As in Study I, post training, mean scores were actually better than age matched normative performance. This was interesting given the aerobic workout was done in a sitting position on the NuStepTM-Vasper.
One unique difference in the outcomes between the different technologically assisted aerobic exercise protocols related to energy level and resilience. More than 75% of the subjects experienced an increase in energy and resilience after training on the NuStepTM-Vasper. To determine if this is a predictable outcome related to the features of the compression and cooling, additional research studies are needed. In terms of gait speed, the participants recruited for this QA study were independent in activities of daily living and active in the community. Their average gait speed fell into the community level of performance (>0.8 m/sec) [57-59].
A variety of community exercise programs have been established for patients with PD (tandem biking, “Delay the Disease” [Zid], Tango, Mark West Dance for PD, PWR!MovesTM, Rocksteady Boxing, Tai Chi) [60-65]. Our quality assurance studies reinforce the benefits of specific, short term aerobic exercise protocols using different rehabilitation technologies to improve mobility and balance without exacerbating signs and symptoms of PD (e.g., pain). However, longitudinal studies with a large heterogeneous group of participants with PD would be needed to clarify if exercise (intense, aerobic or moderate) is neuroprotective for PD.
With physical inactivity as a primary health problem in the elderly [14,60-68], exercise must be a standard part of health care services not only for those that are aging but also for those with impairments associated with chronic neurological disease like PD. To enhance opportunities for physiological and neuromusculoskeletal change, exercise protocols should follow the principles of “overload” (e.g., speed, performance time, frequency, progressive difficulty) with adaptation to individual signs and symptoms and individual preferences [66,67]. Unfortunately, the effects of exercise are transient unless continued [68-70] wireless monitoring of mobility (e.g., pedometer, sleep, medication management) with occasional face to face visits for a review of exercises, may help with compliance along with the convenience of the fitness center location, efficiency of performance, time of day, safety, potential group support and positive feedback [70-71].
Study limitations
These quality assurance studies have several potential confounding variables. All of the participants had previous training on the AlterGR but none had trained on the NuStepTM-Vasper nor the GlideTrakTM. However, this was not associated with a consistent preference for the AlterGR over the other two pieces of equipment. In QA Study I, a cross over repeated measures study design lends itself to the possibility of residual training effects even when the order of training is randomized and separated by 3 months. Finally, some of the self report questionnaires regarding feedback about the technology and change in signs and symptoms were based on ordinal scores (0-10) and not based on standardized measurement instruments.
Falls were not measured as outcomes in these studies. The subjective reporting of falls as an outcome variable is usually based on self report. Unfortunately, when the information is collected from the subject after one to three months, the information is even more unreliable and generally only remembered when a fall was associated with an injury. In this particular population, the majority of the patients were Hoehn and Yahr I and II. Of the 20 participants who completed QA I and II, 17 were not fallers. However, there was one patient in Study I (Hoehn and Yahr III) and one patient in Study II (Hoehn and Yahr III) who were regular, daily fallers (2-3x/day). This had been their history for at least a year, yet they were still living independently. During the study period, the two participants continued to have falls but were either falling approximately every other day and the falls were not associated with a serious injury. In a controlled randomized trial, falls should be tracked carefully.
Objective posturography data was not gathered in these preliminary, unfunded quality assurance studies to assure the safety of incorporating new technology into a Health and Wellness Center. The time based clinical tests of balance (TUG and FTSST) can be used to inexpensively and objectively monitor improved balance performance and predict individuals at risk for falls [44]. However, in a controlled randomized clinical trial, objective posturography as well as more detailed kinematics of gait and balance tests would potentially increase the sensitivity of measuring improvement in outcomes in patients with PD following controlled intervention strategies.
CONCLUSION
Novel rehabilitation technologies such as un-weighting systems (e.g., the GlideTrakTM, AlterGR) and exercise under conditions of compression and cooling (NuStepTM-Vasper) enable individuals with mild to moderate PD to safely exercise at aerobic levels. Post training, participants improved mobility and balance without exacerbating motor and non-motor signs and symptoms of aging and PD. Participants admitted there was some discomfort during the training, but perceived improvement in energy and resilience. New rehabilitation technology is more expensive than traditional fitness equipment; however with the increasing population of elders, it is important to create safe opportunities for all individuals to exercise aerobically, even those with neurological impairments associated with chronic disease such as PD. As the benefits of technologically enhanced aerobic exercise are documented and the demand for the technology increases, the cost of this technology should become more reasonable in price and more accessible in community sites.
SUMMARY OF KEY POINTS
1 QA study I
- Can achieve aerobic levels of training by jogging/running (HR60-80% of maximum).
- Can improve mobility and balance without exacerbation of pain, in coordination, fatigue, tremor or freezing.
- May be associated with discomfort during training (e.g., pelvic discomfort relative to the pelvic support on the GlideTrakTM and urinary urgency in the AlterGR when un-weighted by 50%.
- Find it easier to adjust the AlterGR than the GlideTrakTM.
- Recommend that community fitness centers integrate new rehabilitation technology to enable individuals with impairments to safely exercise aerobically.
QA study II
- Made significant gains in mobility, balance and resilience without exacerbating musculoskeletal pain, freezing or fatigue.
- Who trained on the AlterGR and the NuStepTM-Vasper reported the NuStepTM-Vasper to be easier to adjust, more comfortable to use and provided the opportunity for a more intense workout.
- Would recommend training on the VasperTM to their friends and for purchase by community fitness centers.
REFERENCES
- Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, et al. (2007) Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68: 384-386.
- Nussbaum RL, Ellis CE (2003) Alzheimer’s disease and Parkinson’s disease. N Engl J Med 348: 1356-1364.
- Suchowersky O, Reich S, Perlmutter J, Zesiewicz T, Gronseth G, et al. (2006) Practice Parameter: diagnosis and prognosis of new onset Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 66: 968-975.
- Hauser RA (2009) New considerations in the medical management of early Parkinson’s disease: impact of recent clinical trials on treatment strategy. Parkinsonism Relat Disord 15: 17- 21.
- Tunik E, Feldman AG, Poizner H (2007) Dopamine replacement therapy does not restore the ability of Parkinsonian patients to make rapid adjustments in motor strategies according to changing sensorimotor contexts. Parkinsonism Relat Disord 13: 425-33.
- Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL (2008) The effectiveness of exercise interventions for people with Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 23: 631-640.
- Keus SH, Munneke M, Nijkrake MJ, Kwakkel G, Bloem BR (2009) Physical therapy in Parkinson’s disease: evolution and future challenges. Mov Disord 24: 1-14.
- Hirsch MA, Farley BG (2009) Exercise and neuroplasticity in persons living with Parkinson’s disease. Eur J Phys Rehabil Med 45: 215-229.
- Gobbi LT, Oliveira-Ferreira MD, Caetano MJ, Lirani-Silva E, Barbieri FA, et al. (2009) Exercise programs improve mobility and balance in people with Parkinson’s disease. Parkinsonism Relat Disord 3: 49-52.
- King LA, Horak FB (2009) Delaying mobility disability in people with Parkinson disease using a sensorimotor agility exercise program. Phys Ther 89: 384-393.
- Allen NE, Canning CG, Sherrington C, Lord SR, Latt MD, et al. (2010) The effects of an exercise program on fall risk factors in people with Parkinson’s disease: a randomized controlled trial. Mov Disord 25: 1217-1225.
- Seppi K, Weintraub D, Coelho M, Perez-Lloret S, Fox SH, et al. (2011) The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson’s disease. Mov Disord 3: 42-80.
- Fox SH, Katzenschlager R, Lim SY, Ravina B, Seppi K, et al. (2011) The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the motor symptoms of Parkinson’s disease. Mov Disord 3: 2-41.
- Center for Disease Control and Prevention (2015) Division of Nutrition, Physical Activity, and Obesity. Center for Disease Control, Georgia, USA.
- Fletcher GF, Balady G, Blair SN, Blumenthal J, Caspersen C, et al. (1996) Statement on exercise: benefits and recommendations for physical activity programs for all Americans. A statement for health professionals by the Committee on Exercise and Cardiac Rehabilitation of the Council on Clinical Cardiology, American Heart Association. Circulation 94: 857-862.
- Ahlskog JE (2011) Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology 77: 288-294.
- 17. Perlmutter D, Loberg K (2014) Gran Brain: The Surprising Truth about Wheat, Carbs, and Sugar--Your Brain’s Silent Killers. (1stedn), Little Brown and Company, New York, USA. Pg no: 336.
- Ornish D, Lin J, Chan JM, Epel E, Kemp C, et al. (2013) Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol 14: 1112-1120.
- Green CS, Bavelier D (2008) Exercising your brain: a review of human brain plasticity and training-induced learning. Psychol Aging 23: 692-701.
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, et al. (2006) Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci 61: 1166-1170.
- Tillerson JL, Caudle WM, Reverón ME, Miller GW (2003) Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience 119: 899-911.
- Villar-Cheda B, Sousa-Ribeiro D, Rodriguez-Pallares J, Rodriguez-Perez AI, Guerra MJ, et al. (2009) Aging and sedentarism decrease vascularization and VEGF levels in the rat substantia nigra. Implications for Parkinson’s disease. J Cereb Blood Flow Metab 29: 230-234.
- Vu?kovi? MG, Li Q, Fisher B, Nacca A, Leahy RM, et al. (2010) Exercise elevates dopamine D2 receptor in a mouse model of Parkinson’s disease: in vivo imaging with [18F]fallypride. Mov Disord 25: 2777-2784.
- Zigmond MJ, Cameron JL, Leak RK, Mirnics K, Russell VA, et al. (2009) Triggering endogenous neuroprotective processes through exercise in models of dopamine deficiency. Parkinsonism Relat Disord 3: 42-45.
- Fisher BE, Wu AD, Salem GJ, Song J, Lin CH, et al. (2008) The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease. Arch Phys Med Rehabil 89: 1221-1229.
- Suchowersky O, Gronseth G, Perlmutter J, Reich S, Zesiewicz T, et al. (2006) Practice Parameters: Neuroprotective strategies and alternative therapies for Parkinson’s disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 66: 976-982.
- Ridgel AL, Vitek JL, Alberts JL (2009) Forced, not voluntary, exercise improves motor function in Parkinson’s disease patients. Neurorehabil Neural Repair 23: 600-608.
- Alberts JL, Linder SM, Penko AL, Lowe MJ, Phillips M (2011) It is not about the bike, it is about the pedaling: forced exercise and Parkinson’s disease. Exerc Sport Sci Rev 39: 177-186.
- Merzenich M (2013) Soft-Wired: How the New Science of Brain Plasticity Can Change Your Life. (2ndedn), Parnassus Publishing, San Francisco, USA.
- Werman R (2004) Living with an Aging Brain: A self-Help Guide for Your Senior Years. Freund Publishing House, Israel.
- Grabowski AM, Kram R (2008) Effects of velocity and weight support on ground reaction forces and metabolic power during running. J Appl Biomech 24: 288-297.
- Miyai I, Fujimoto Y, Ueda Y, Yamamoto H, Nozaki S, et al. (2000) Treadmill training with body weight support: its effect on Parkinson’s disease. Arch Phys Med Rehabil 81: 849-852.
- Miyai I, Fujimoto Y, Yamamoto H, Ueda Y, Saito T, et al. (2002) Long-term effect of body weight-supported treadmill training in Parkinson’s disease: a randomized controlled trial. Arch Phys Med Rehabil 83: 1370-1373.
- Earhart GM, Williams AJ (2012) Treadmill training for individuals with Parkinson disease. Phys Ther 92: 893-897.
- Ruckstuhl H, Kho J, Weed M, Wilkinson MW, Hargens AR (2009) Comparing two devices of suspended treadmill walking by varying body unloading and Froude number. Gait Posture 30: 446-451.
- www.Alter-G.com
- www.glidecycle.com
- www.vasper.com
- Manini TM, Yarrow JF, Buford TW, Clark BC, Conover CF, et al. (2012) Growth hormone responses to acute resistance exercise with vascular restriction in young and old men. Growth Horm IGF Res 22: 167-172.
- Fung S, Byl N, Melnick M, Callahan P, Selinger A (1997) Functional outcomes : The development of a new instrument to monitor the effectiveness of physical therapy. Eur J Phys Med Rehabi 7: 31-41.
- Tariq SH, Tumosa N, Chibnall JT, Perry MH 3rd, Morley JE (2006) Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder--a pilot study. Am J Geriatr Psychiatry14: 900-910.
- Bohannon RW (1997) Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing 26: 15-19.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories (2002) ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 166: 111-117.
- Bohannon RW (2006) Reference values for the five-repetition sit-to-stand test: a descriptive meta-analysis of data from elders. Percept Mot Skills 103: 215-222.
- Shumway-Cook A, Brauer S, Woollacott M (2000) Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. PhysTher 80: 896-903.
- Garcia M, Cohen S, Pang J, Reynolds S, Fedulow I, et al. (2012) Integration of lower body positive air pressure treadmill system for gait training into a PT Health and Wellness Setting: Recommendations for quality practice. Poster CPTA Annual Conference, Santa Clara, California, USA.
- Perry J, Johnson AW, Ridge ST (2013) GlideCycle mobility device reduces ground reaction forces compared to running in healthy individuals. Combined Sections Meeting, American Physical Therapy Association, USA.
- Kumari A, Catanzaro R, Marotta F (2011) Clinical importance of lactic acid bacteria: a short review. Acta Biomed 82: 177-180.
- www.NuStep.com
- Cook SB, Murphy BG, Labarbera KE (2013) Neuromuscular function after a bout of low-load blood flow-restricted exercise. Med Sci Sports Exerc 45: 67-74.
- Manini TM, Yarrow JF, Buford TW, Clark BC, Conover CF, et al. (2012) Growth hormone responses to acute resistance exercise with vascular restriction in young and old men. Growth Horm IGF Res 22: 167-172.
- Sackett D, Straus S, Richardson W, Rosenberg W, Haynes R (2000) Evidence-Based Medicine, (2nd edn), Churchill Livingstone, Elsevier, Philadelphia, USA.
- Marascuilo LA, McSweeny M (1977) Nonparametric and Distribution-free Methods for the Social Sciences. Brooks Cole. Pg no: 556.
- Steffen TM, Hacker TA, Mollinger L (2002) Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther 82: 128-137.
- Wall JC, Bell C, Campbell S, Davis J (2000) The Timed Get-up-and-Go test revisited: measurement of the component tasks. J Rehabil Res Dev 37: 109-113.
- Meretta BM, Whitney SL, Marchetti GF, Sparto PJ, Muirhead RJ (2006) The five times sit to stand test: responsiveness to change and concurrent validity in adults undergoing vestibular rehabilitation. J Vestib Res 16: 233-243.
- Bowden MG, Balasubramanian CK, Behrman AL, Kautz SA (2008) Validation of a speed-based classification system using quantitative measures of walking performance poststroke. Neurorehabil Neural Repair 22: 672-675.
- Bohannon RW, Williams Andrews A (2011) Normal walking speed: a descriptive meta-analysis. Physiotherapy 97: 182-189.
- Schmid A, Duncan PW, Studenski S, Lai SM, Richards L, et al. (2007) Improvements in speed-based gait classifications are meaningful. Stroke 38: 2096-2100.
- Zid D (2007) Delay the Disease: Exercise and Parkinson’s disease. Columbus Health Works Productions, Columbus, USA.
- KNGF Guidelines for physical therapy in patients with Parkinson’s disease (2004) Parkinson’s Disease. Dutch Journal of Physiotherapy 114: 1-84.
- Farley BG, Koshland GF (2005) Training BIG to move faster: the application of the speed-amplitude relation as a rehabilitation strategy for people with Parkinson’s disease. Exp Brain Res 167: 462-467.
- Duncan RP, Earhart GM (2012) Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair 26: 132-143.
- Hackney ME, Earhart GM (2009) Health-related quality of life and alternative forms of exercise in Parkinson disease. Parkinsonism Relat Disord 15: 644-648.
- Combs SA, Diehl MD, Staples WH, Conn L, Davis K, et al. (2011) Boxing training for patients with Parkinson disease: a case series. Phys Ther 91: 132-142.
- Department of Health and Human Services (1979) Surgeon General Report, Healthy People 2000. National Health Promotion and Disease Prevention Objectives. Centers for Disease Control and Prevention, Georgia, USA.
- Fair SE (2011) Wellness and Physical Therapy. Jones and Bartlett Publishers, Boston, USA.
- McArdle WD, Katch FL, Katch VL (2009) Exercise Physiology: Nutrition, Energy and Human Performance. (7thedn), Lippincott Williams & Wilkins, Philadelphia, USA. Pg no: 1136.
- Seiler S (2009) Principles of training: Revisited.
- Huberty JL, Ransdell LB, Sidman C, Flohr JA, Shultz B, et al. (2008) Explaining long-term exercise adherence in women who complete a structured exercise program. Res Q Exerc Sport 79: 374-384.
- Allen NE, Sherrington C, Suriyarachchi GD, Paul SS, Song J, et al. (2012) Exercise and Motor Training in People with Parkinson’s Disease: A Systematic Review of Participant Characteristics, Intervention Delivery, Retention Rates, Adherence, and Adverse Events in Clinical Trials. Parkinson’s Disease. Pg no: 15.
Citation: Byl N, Kretschmer J, Chung A, Thomas A, Fedulow I, et al. (2015) Short Term Technology-Assisted-Aerobic Exercise (AlterGR, GlideTrakTM, Vasper) in a Community Fitness Center for Patients with Mild to Moderate Parkinson’s Disease: Subjective Perceptions and Motor Effects. J Community Med Public Health Care 2: 009.
Copyright: © 2015 Nancy Byl, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

