
Single Center Evaluation of Restorative Neck Complex with TriHex Technology® in Aging Skin of the Neck and Décolleté
*Corresponding Author(s):
Alan WidgerowDepartment Of Plastic Surgery, Director Center For Tissue Engineering, University Of California, Irvine, United States
Email:alan.widgerow@galderma.com
Abstract
Introduction: A Restorative Neck Complex with TriHex Technology®-RNC (Alastin Skincare, Inc. Carlsbad, CA) has been shown to reduce wrinkles and improve the overall texture of the neck, which is related to the clearance of the extracellular matrix, increased hydration, neocollagenesis, and neoelastogenesis.
Aim: This study evaluated the efficacy of RNC on aging skin of the neck and décolleté.
Subjects/Methods: In this open-label, non-randomized study, eligible subjects underwent 4 study visits including one screening/baseline visit and 3 follow-up visits.The subjects used a cleanser, sunscreen (morning), and RNC (morning and evening), and returned for follow-up visits at 4, 8, and 12 weeks. At each follow-up visit, standardized photos were taken of the neck and décolleté, and the questionnaires were completed. At week 12, the investigator’s assessment was completed.
Results: Twenty female subjects aged 35-80 years were enrolled in the study. Investigator assessments revealed statistically significant reductions in severity from baseline to week 12 in both neck and décolleté, in all parameters, including coarseness, crepiness, dryness, wrinkles, laxity, redness, texture, uneven skin, and global appearance.Participants consistently reported ‘softer/smoother skin’ in over 95% of the subjectsfrom weeks 4 to 8 and 12. At the 12-week point, 100% of participants expressed overall satisfaction.
Conclusion: The use of RNC topical products formulated with TriHex appears to significantly improve the overall appearance of neck skin and decolorization, particularly related to crepiness, dryness, wrinkles, laxity, redness, and texture, when used as a daily regimen.
Background
Neck skin laxity and Décolleté thinning involve alterations at the microscopic level, similar to those observed during general aging. Fragmented collagen and elastin create a situation in which long spindle-shaped fibroblasts are no longer able to attach comfortably to these fragmented fibers. This results in rounded senescent fibroblasts that no longer efficiently produce collagen and elastin, resulting in skin laxity and surface changes [1].
However, the neck and the décolleté have site-specific nuances. The chief concerns of patients with neck and décolleté complaints are secondary to these surface changes, including crepiness, pigmentation, submental fullness during skin thinning, and poor skin tone. Extrinsic factors (mainly photo damage) and intrinsic factors contribute to skin aging and Extracellular Matrix (ECM) alterations. The neck and décolleté are certainly no exception to this aging phenomenon; in fact, neck skin changes can be exaggerated owing to the constant dynamic forces that result from variations in movements related to daily activities. In addition, common exposure to ultraviolet light and other exposomes and these areas are highly susceptible to age-related changes. Both photoaging and chronological aging are progressive. Both processes release reactive oxygen species and ECM-degrading enzymes, causing matrix breakdown and accumulation of fragmented collagen, elastin, and metabolic waste products, which create dysfunctional proteins, resulting in aged skin features [2,3]. The neck can manifest as loose skin, which is particularly problematic in older patients, but more subtle changes start with loss of texture, crepiness, wrinkles, and dryness [2]. This dryness is particularly relevant in the décolleté, where thin skin with relatively few sebaceous glands, constantly exposed to photodamage, creates an area in constant need of hydration [4].
A Restorative Neck Complex with TriHexTechnology®RNC (Alastin Skincare, Inc. Carlsbad, CA) have been shown to reduce wrinkles and improve the overall texture of the neck. Histologically, RNC has demonstrated regeneration of elastin and improvement of the epidermis [5]. This is particularly relevant considering the aging nuances of the anatomical areas described above. Thus, replenishing ECM, removing waste products, and replacing freshened collagen and elastin are critical for skin health in this region. This is achieved with the tripeptide hexapeptide combination, with further attention to elastin replenishment contributed by various peptides and active agents stimulating tropoelastin and lysyl oxide-like enzyme 1 (LOXL1) [5,6]. This study evaluated the efficacy of RNC on aging skin of the neck and décolleté. In particular, the skin changes anticipated in the context of these anatomical areas were assessed (dryness, redness, texture, and laxity) to gauge the effect of a topical formulation on the skin after 12 weeks.
Materials and Methods
The aim of this study is to evaluate the efficacy (change from baseline) of RNC with TriHex Technology® through Investigator assessments, subject assessments and photography.
Twenty participants were enrolled in this study. Eligible subjects selected to participate included women between the ages of 35 and 80 years with no known medical conditions that could impact the study; mild to moderate fine lines, wrinkles, and laxity on their neck and décolleté; no skincare products other than the study products provided; avoidance of excessive sun exposure and tanning beds during the study; and no procedures to the treatment area during the study period.
Exclusion criteria included dermatological disorders or poor health that could interfere with the accurate evaluation of skin health; previous sensitivities to any of the ingredients of the study products or known allergies to topical products or their ingredients; subjects with clinically significant unstable medical disorders; subjects who had used retinols or other topical with actives in the neck or décolleté in the previous 30 days, or recent excessive exposure to sunlight, tanning beds, or self-tanner within 30 days study entry; subjects with excessive laxity, including deep lines and wrinkles along neck/décolleté; use of topical medications on the neck/décolleté for any skin condition (including acne); any resurfacing procedure (ablative or non-ablative laser or chemical peel in the past 3 months that extended beyond the jawline); use of oral isotretinoin in the last 6 months; pregnancy; planning to become pregnant; or nursing during the course.
The participants underwent 4 study visits including one screening/baseline visit and 3 follow-up visits. At the initial screening visit, participants consented prior to any study procedure. Medical history, concomitant medications, and study eligibility were assessed.Investigator assessments and photographs were taken at weeks 4, 8, and 12.The subjects were given a cleanser, sunscreen, and RNC, and asked to return to the office for follow-up visits at 4, 8, and 12 weeks. The Gentle Cleanser and RNC were used in the morning and evening, respectively, and sunscreen was applied in the morning. At each follow-up visit, standardized photos were taken of the neck and décolleté, and the questionnaires were completed. The investigator’s assessment was completed at week 12. This study was reviewed and monitored by the Veritas IRB.
Assements
- Investigator assessments were performed at baseline and 12 weeks after the examination of signs of fine/coarse lines, crepiness, texture, laxity, dryness, redness, pigmentation, and overall global appearance. These were graded according to a 9 point numeric scale divided into absent, mild, moderate, and severe categories (Supplementary Table 1).
- The subject assessments were performed at 4, 8, and 12 weeks. Questions were directed to subjects relating to specific skin characteristics, such as condition, appearance, and tone, and overall subject satisfaction was gauged (Supplementary Table 2).
- Photographic analysis from each visit supplemented assessments above
Data Analysis
All data points collected during the follow-up were compared to their baseline data points. The statistical analyses were performed by an independent statistician.
- Statistical methods
- Investigator’s assessments: Mean, standard deviation, and paired t-tests were used to summarize and compare changes in investigator assessments from baseline to week 12.
- Participants’ assessments: Paired t-tests were used to compare the percentages of favorable ratings from weeks 4 to 8 and 12.
Results
- Investigator’s assessments
Table 1 and Figures 1A-B summarize the investigator’s assessments at baseline, week 12, and changes from baseline to week 12. There were statistically significant reductions in severity from baseline to week 12 in both neck and décolleté, in all parameters including coarse, crepiness, dryness, wrinkles, laxity, redness, texture, uneven skin, and global/overall appearance. The magnitude of severity reduction range from -1.0 to -2.0 on a scale of to 1-5 (p-values <.01).
|
Location |
Parameter |
Baseline Mean (SD) |
Week12 Mean (SD) |
Change Mean (SD) |
P-value |
|
Neck |
Coarse |
4.3 (1.6) |
2.9 (1.3) |
-1.5 (1.6) |
0.0007 |
|
Crepiness |
4.5 (1.4) |
2.8 (1.1) |
-1.8 (1.2) |
<.0001 |
|
|
Dryness |
4 (1.6) |
1.3 (0.6) |
-2.7 (1.8) |
<.0001 |
|
|
FineLinesWrinkling |
4.8 (1.1) |
3 (0.9) |
-1.8 (0.9) |
<.0001 |
|
|
Laxity |
4.3 (1.6) |
2.7 (0.8) |
-1.6 (1.5) |
0.0001 |
|
|
Redness |
3.5 (1.5) |
1.9 (1.4) |
-1.6 (2.2) |
0.0047 |
|
|
Texture |
4.5 (1.3) |
2.7 (0.8) |
-1.8 (1.3) |
<.0001 |
|
|
Uneven |
4 (1.6) |
2.3 (0.9) |
-1.8 (1.7) |
0.0003 |
|
|
GlobalOverall |
4.4 (1.3) |
2.7 (0.7) |
-1.8 (1) |
<.0001 |
|
|
Decollete |
Coarse |
3.6 (1.3) |
2.8 (1.2) |
-0.8 (1.4) |
0.0244 |
|
Crepiness |
3.4 (1.1) |
2.2 (0.8) |
-1.2 (1) |
<.0001 |
|
|
Dryness |
3.9 (1) |
1.7 (0.9) |
-2.3 (1.3) |
<.0001 |
|
|
FineLinesWrinkling |
3.7 (1) |
2.6 (1) |
-1.1 (1) |
0.0001 |
|
|
Laxity |
3.4 (0.9) |
1.8 (0.8) |
-1.7 (1.2) |
<.0001 |
|
|
Redness |
3.3 (1.2) |
2.2 (1.4) |
-1.1 (1.4) |
0.0039 |
|
|
Texture |
3.9 (1) |
2.4 (0.8) |
-1.5 (1.1) |
<.0001 |
|
|
Uneven |
4.5 (1.4) |
3.3 (1.3) |
-1.3 (1.2) |
0.0002 |
|
|
GlobalOverall |
3.9 (0.9) |
2.8 (0.6) |
-1.1 (0.9) |
<.0001 |
Table 1: Investigators assessments.
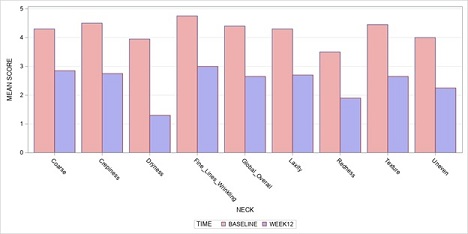 Figure 1A: Investigators assessments of neck - baseline vs 12 weeks
Figure 1A: Investigators assessments of neck - baseline vs 12 weeks
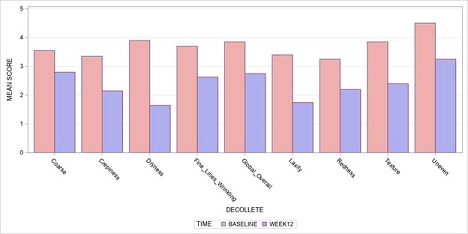 Figure 1B: Investigators assessment of décolleté-baseline vs 12 weeks.
Figure 1B: Investigators assessment of décolleté-baseline vs 12 weeks.
- Participants’ assessment
Table 2 shows the percentage of participants’ favorable ratings in weeks 4, 8, and 12. The rating about ‘felt nice’ and ‘softer/smoother skin’ was the highest (>95%) and consistent from week 4 to weeks 8 and 12. At the 12 week point, 100% of participants expressed overall satisfaction.
|
Parameter |
% Favorable Rating
|
||
|
Week4 |
Week 8 |
Week 12 |
|
|
Overall |
70.0 |
90.0 |
95.0 |
|
Appearance |
60.0 |
90.0 |
90.0 |
|
Chest lines wrinkles |
45.0 |
60.0 |
65.0 |
|
Confident |
65.0 |
60.0 |
75.0 |
|
Continue to use |
90.0 |
95.0 |
90.0 |
|
Crepiness on chest |
65.0 |
65.0 |
70.0 |
|
Crepiness on neck |
65.0 |
65.0 |
80.0 |
|
Felt nice |
95.0 |
100.0 |
100.0 |
|
Neck lines/wrinkles |
55.0 |
65.0 |
75.0 |
|
Radiance/clarity |
45.0 |
60.0 |
60.0 |
|
Recommend product |
85.0 |
95.0 |
90.0 |
|
Redness/discoloration |
35.0 |
50.0 |
45.0 |
|
Skin tone |
55.0 |
80.0 |
75.0 |
|
Softer/smoother |
85.0 |
95.0 |
95.0 |
|
Tighter/lifted |
45.0 |
55.0 |
65.0 |
|
Youthful |
55.0 |
80.0 |
85.0 |
|
Overall satisfaction |
95.0 |
85.0 |
100.0 |
Table 2: Participants’ Assessment.
Discussion
The neck and décolletéare anatomic areas, each with its own aging nuances. The neck area is affected by gravitational forces, crepiness, and thinned aging skin, causing laxity and loss of skin tone. However, early changes in skin texture, hydration, redness, and laxity precede the loose skin phase. The décolleté is recognized as an area that suffers from extreme dehydration, more so than most other anatomic areas [4].
In this open-label, single-center study using a Restorative Neck Complex with TriHex Technology® on the neck and chest, we demonstrated significant improvements in dryness, texture, crepiness, laxity, and overall appearance (Figures 2&3). Participants with greater than mild-to-moderate neck laxity were excluded from the study to focus on detailed skin changes. Each of these detailed skin changes showed a significant improvement, as assessed by the investigator.
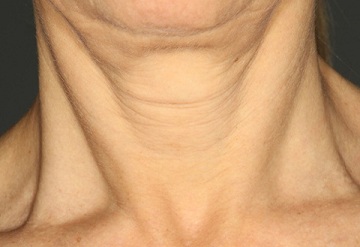 Figure 2a: Baseline neck appearance pre-treatment.
Figure 2a: Baseline neck appearance pre-treatment.
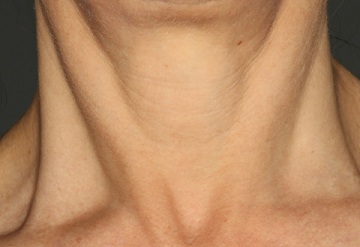 Figure 2b: Neck appearance after 12 week treatment.
Figure 2b: Neck appearance after 12 week treatment.
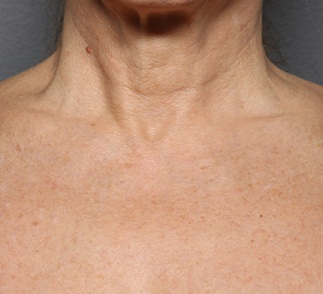 Figure 3a: Baseline décolleté appearance pre-treatment.
Figure 3a: Baseline décolleté appearance pre-treatment.
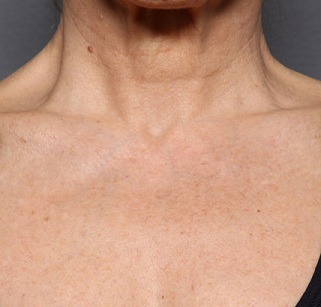 Figure 3b: Décolleté appearance after 12 weeks.
Figure 3b: Décolleté appearance after 12 weeks.
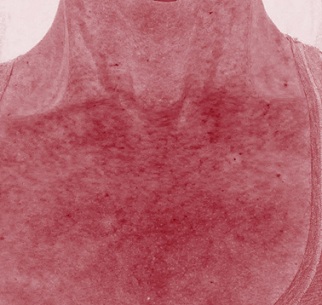 Figure 3c: Décolleté appearance in red channel (vascular analysis cross polarized Canfield technology) pre-treatment.
Figure 3c: Décolleté appearance in red channel (vascular analysis cross polarized Canfield technology) pre-treatment.
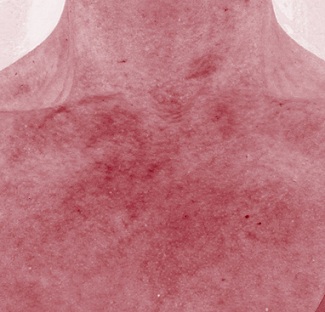 Figure 3d: Red channel appearance 12 weeks post topical treatment.
Figure 3d: Red channel appearance 12 weeks post topical treatment.
The overall changes observed were consistent with those of previous studies related to TriHex technology. In particular, modification of the ECM and restructuring of the dermal foundation allow for more efficient crosstalk and signaling between cells and proteins. This manifests as more efficient healing when used as a preconditioning and postprocedure agent [5,7,8], or as a maintenance program with emphasis on general skin health [9,10]. In this product (RNC) profile, the primary focus for topical therapy of the neck and decollate is to improve skin texture, elasticity, pigmentation, barrier function, contour, and overall health, with a great emphasis on elastin stimulation.
Thus, in addition to TriHex,extra elastin-stimulating activities include - acetyl tetrapeptide-2, which increases FBLN5 and LOXL1 levels and proteins actively involved in elastin synthesis, resulting in a reduction in skin flaccidity [11]. As previously noted the TriHex (Palmitoyltripeptide 1 and Palmitoylhexapeptide 12) remodels the extracellular matrix by clearing the fragmented collagen and elastin and then stimulating neocollagenesis and elastogenesis [12,13]. Anethum Graveolens/Dill extract induces LOXL synthesis which dramatically decreases from the age of 18 [14]. Increased levels of LOXL in the skin aid in the assembly of elastin fibers, resulting in improved mechanical properties of the skin [14].
To improve pigmentation, phytoene/phytofluene derived from saltwater algae protects against the effects of Ultraviolet (UV) radiation. They have antioxidant and anti-inflammatory properties, which reduce pro-inflammatory cytokine levels, inhibit MMP-1 production, combat pigmentation, and exhibit UV absorbency properties [15]. Niacinamide contributes to barrier health by balancing ceramide and skin cholesterol levels and reducing pigmentation by interfering with melanosome transfer from melanocytes to keratinocytes [16-18]. Finally, hydroceramide is added as a skin barrier defense by providing ceramides and lipids at a slightly acidic pH to the epidermis, optimizingskin antimicrobial defense, and epidermal enzyme activity [4].
Thus, by combining these active agents into a formulation targeting the specialized needs of the neck and decollete areas, the product was developed and tested for its impact on relevant skin aging characteristics. The limitations of this study included its open-label nature, small sample size, and invalidated scale.That is, the combination of statistically significant reductions in severity from baseline to week 12 in both the neck and décolleté, in all parameters including coarse, crepiness, dryness, wrinkles, laxity, redness, texture, uneven skin, and global appearance, combined with 100% participant satisfaction, demonstrates the efficacy of the product in these unique anatomic areas.
Conclusion
Used daily as a regimenfor the treatment of photoaged skin of the neck and décolleté over 12 weeks, RNC, a novel topical product formulated using TriHexTechnology®and elastin-stimulating active ingredients, proved very effective in improving general skin health. Statistically significant reductions occurred in both the neck and décolleté, crepiness, dryness, wrinkles, laxity, redness, texture, uneven skin, and global appearance combined with 100% participant satisfaction.
Acknowledgement
Statistical analysis provided by Tuyen Hoang, PhD, Biostatistics, Institute for Clinical and Translational Science, University of California, Irvine, CA Alastin Skincare, Inc., provided funding for the study.
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
SH: Conception, design, analysis, and interpretation of data involved in drafting the manuscript or critically revising it.
NC: Acquisition of data, revising manuscript critically
ADW: Conception, design, involved in drafting manuscript
Conflict of Interest Declaration
Alastin Skincare Inc (Carlsbad CA), a Galderma company, funded this study. Alan Widgerow is Chief Scientific Officer of Galderma. The other authors have no conflict to declare
References
- Shadfar S, Perkins SW (2014) Anatomy and physiology of the aging neck. Facial Plast Surg Clin North Am 22: 161-170.
- Kim E, Cho G, Won NG, Cho J (2013) Age-related changes in skin bio-mechanical properties: the neck skin compared with the cheek and forearm skin in Korean females. Skin Res Technol 19: 236-241.
- Calame A, Widgerow A (2017) Histological Changes Associated with Extracellular Matrix-Remodeling Topical Therapy. Dermatology Case Reports 2:1000126.
- Luebberding S, Krueger N, Kerscher M (2013) Age-related changes in skin barrier function - quantitative evaluation of 150 female subjects. Int J Cosmet Sci 35: 183-190.
- Gold MH, Sensing W, Biron JA (2019) A topical regimen improves skin healing and aesthetic outcomes when combined with a radiofrequency microneedling procedure. J Cosmet Dermatol.
- Widgerow AD, Napekoski K (2020) New approaches to skin photodamage histology-Differentiating 'good' versus 'bad' Elastin. J Cosmet Dermatol.
- Vanaman Wilson MJ, Bolton J, Fabi SG (2017) A randomized, single-blinded trial of a tripeptide/hexapeptide healing regimen following laser resurfacing of the face. J Cosmet Dermatol 16: 217-222.
- Widgerow AD, Cohen SR, Fagien S (2019) Preoperative Skin Conditioning: Extracellular Matrix Clearance and Skin Bed Preparation, A New Paradigm. Aesthetic Surgery Journal 39: 103-111.
- Widgerow AD, Jiang LI, Calame A (2018) A single-center clinical trial to evaluate the efficacy of a tripeptide/hexapeptide antiaging regimen. J Cosmet Dermatol 18: 176-182.
- Widgerow AD, Ziegler ME, Garruto JA, Bell M (2022) Effects of a Topical Anti-aging Formulation on Skin Aging Biomarkers. J Clin Aesthet Dermatol 15: 53-60.
- Lubrizol (2013) Product monograph: UplevityTM. Lipotec.
- Widgerow AD, Fabi SG, Palestine RF, Rivkin A, Ortiz A, et al. (2016) Extracellular Matrix Modulation: Optimizing Skin Care and Rejuvenation Procedures. J Drugs Dermatol 15: 63-71.
- Widgerow A (2016) Topica Skin Restoration Technology – Advances in Age Management Strategies. Modern Aesthetics Page no: 1-8.
- Cenizo V, Andre´ V, Reymermier C, Sommer P, Damour O, et al. (2006) LOXL as a target to increase the elastin content in adult skin: a dill extract induces the LOXL gene expression. Exp Dermatol 15: 574-581.
- Vilchez C, Forjan E, Cuaresma M, Bedmar F, Garbayo I, et al. (2011) Marine carotenoids: biological functions and commercial applications. Mar Drugs 9: 319-333.
- Hakozaki T, Minwalla L, Zhuang J, Chhoa M, Matsubara A, et al. (2002) The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. British Journal of Dermatology 147: 20-31.
- Navarrete-Solis J, Castanedo-Cazares JP, Torres-Alvarez B, Oros-Ovalle C, Fuentes-Ahumada C, et al. (2011) A Double-Blind, Randomized Clinical Trial of Niacinamide 4% versus Hydroquinone 4% in the Treatment of Melasma. Dermatol Res Pract 2011: 379173.
- Wohlrab J, Kreft D (2014) Niacinamide - mechanisms of action and its topical use in dermatology. Skin Pharmacol Physiol 27: 311-315.
Supplementary Tables
|
Clinical Sign |
Absent |
Mild |
Moderate |
Severe |
|
Fine Lines/Wrinkling |
0 |
1 2 3 |
4 5 6 |
7 8 9 |
|
Coarse Lines/Wrinkling |
0 |
1 2 3 |
4 5 6 |
7 8 9 |
|
Crepiness (crinkley) |
0 |
1 2 3 |
4 5 6 |
7 8 9 |
|
Texture |
0 |
1 2 3 |
4 5 6 |
7 8 9 |
|
Laxity (loose skin) |
0 |
1 2 3 |
4 5 6 |
7 8 9 |
|
Dryness |
0 |
1 2 3 |
4 5 6 |
7 8 9 |
|
Redness |
0 |
1 2 3 |
4 5 6 |
7 8 9 |
|
Uneven pigment/brown spots |
0 |
1 2 3 |
4 5 6 |
7 8 9 |
|
Global: Overall appearance of amount of photo damage |
0 |
1 2 3 |
4 5 6 |
7 8 9 |
Supplementary Table 1: Investigator Assessment at Baseline and Week 12.
|
Please check one box per question. |
|
||||||
|
Agree Strongly |
Agree |
Neither Agree nor Disagree |
Disagree |
Disagree Strongly |
|||
|
|
Improved the overall condition of my skin |
|
|
|
|
|
|
|
|
Improved the overall appearance of my skin |
|
|
|
|
|
|
|
|
Made my skin appear more youthful |
|
|
|
|
|
|
|
|
Improved the look of crepiness on my neck |
|
|
|
|
|
|
|
|
Improved the look of crepiness on my chest |
|
|
|
|
|
|
|
|
Improved the appearance of my neck fine lines and wrinkles |
|
|
|
|
|
|
|
|
Improved the appearance of my chest fine lines and wrinkles |
|
|
|
|
|
|
|
|
Reduced redness and discoloration |
|
|
|
|
|
|
|
|
My skin feels softer and smoother |
|
|
|
|
|
|
|
|
Improved my overall skin tone |
|
|
|
|
|
|
|
|
Made my skin appear tighter and lifted |
|
|
|
|
|
|
|
|
Increased the radiance and clarity of my skin |
|
|
|
|
|
|
|
|
Made me feel more confident in the way my skin looks |
|
|
|
|
|
|
|
|
Product felt nice on my skin |
|
|
|
|
|
|
|
|
I would continue using this treatment regimen |
|
|
|
|
|
|
|
|
I would recommend this treatment regimen to others |
|
|
|
|
|
|
Supplementary Table 2a: Subject assessments.
|
Circle answer below that you feel best describes your Overall Satisfaction
0 = Completely Dissatisfied 1 = Moderately Dissatisfied 2 = Mildly Dissatisfied 3 = Neither Dissatisfied nor Satisfied 4 = Mildly Satisfied 5 = Moderately Satisfied 6 = Completely Satisfied
|
Supplementary Table 2b: Subject Satisfaction.
Citation: Humphrey S, Croll N, Widgerow A (2023) Single Center Evaluation of Restorative Neck Complex with TriHex Technology® in Aging Skin of the Neck and Décolleté. J Clin Dermatol Ther 9: 0127.
Copyright: © 2023 Shannon Humphrey, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

