
Skin, Light and their Interactions, an In-Depth Review for Modern Light-Based Skin Therapies
*Corresponding Author(s):
Kamal AlhallakAlbany Cosmetic And Laser Center, Edmonton, Alberta, T6V1J6, Canada
Email:kalhallak@albanylaser.ca
Abstract
Light-based therapies, including laser, have evolved over the last decades in the dermatology and cosmetic fields. Practitioners should have a deep understanding of the mechanism of action and the effect of different laser parameters on treatments outcomes and safety profiles. Moreover, practitioners would not be able to improve the existing laser treatment protocols or develop new ones without understanding the light fundamentals and different laser-skin interactions. This review article attempts to bridge the theoretical concepts of laser and skin anatomy with the practical applications of light-based treatments.
Skin Anatomy
Human skin has three main layers; the names of these layers, from the most superficial to the deepest ones, are Epidermis, Dermis, and Subcutaneous layers [1]. Figure 1 is an illustration for different layers of normal skin, which a hair follicle, as shown in figure 1. 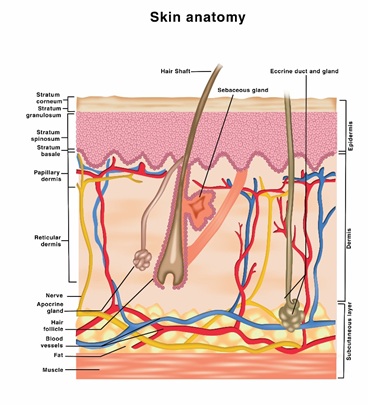
Figure 1: An illustration of different layers of normal skin, which a hair follicle.
Each one of the three skin layers contains sub-layers of living and non-living skin cells as follows:
Epidermis.
As the skin’s outermost layer, the Epidermis is responsible for some essential cosmetic quality of its appearance and texture [2]. The facial Epidermis thickness is an average of 0.1 mm and has four sub-layers: the stratum corneum, the stratum granulosum, Stratum spinosum, and Stratum Basale [3].
The renewal of the Epidermisis essential for layer functionality and cosmetic quality. In healthy skin, the Epidermis renewal cycle is about one month, which is the time required for the living keratinocytes from the Basale to desquamate and migrate to the top of the skin [4].
Stratum corneum (SC.)
It comprises about 15 layers of non-living keratinocytes (corneocytes) coated with phospholipids film layer and connected by corneodesmosomes as shown in the figure below. This layer functions as a physical barrier against pathogens and UV light. Moreover, it controls water loss via evaporation and maintains skin hydration [5].
On average, the Stratum corneum is made of 15-20 layers of corneocytes with 10-40 micron in thickness. The rest of the Epidermis layers are formed due to the multiple differentiation stages of the keratinocytes due to the migration and desquamating process. This layer has the least amount of Water among all other skin layers [6,7].
Stratum granulosum (S.G.)
This is also known as the granular layer, consisting mainly of striated squamous cells arranged in 1 to 3 rows containing lamellar granules and tonofibrils. It is important to note that besides the palms and soles, skin lacks a well-defined stratum lucidum and stratum granulosum [8]. Figure 2 illustrates the structure of the SC layer.
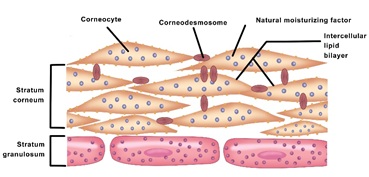 Figure 2: is an illustration of the structure of SC and SG layers.
Figure 2: is an illustration of the structure of SC and SG layers.
Stratum Spinosum (SS.)
Also known as the Spinous layer consisting mainly of a cuboidal cells arranged in multiple layers and synthesizes keratins that function to support structures. The cells are adherent by specialized cells known as desmosomes [9].
Stratum Basale (SB.)
Also known as the basal cells layer, is the deepestt layer of the Epidermis. The layer consists of tall columnar cells that are constantly undergoing cellular division and help form new Keratinocytes (keratinization) that will replace the lost once from stratum corneum; the process takes about one month [10], as shown in figure 3.
Further down the Stratum Basale, the cell layer is attached to a basement membrane that serves as a demarcationor a boundary between the Epidermis and Dermis. The layer also contains the pigment-producing cells; Melanocytes [11].
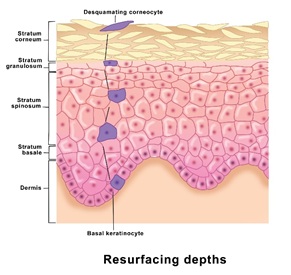 Figure 3: An illustration of differentiation and migration of the Basal Keratinocyte, starting from SB to SC layer.
Figure 3: An illustration of differentiation and migration of the Basal Keratinocyte, starting from SB to SC layer.
Melanocytes
The Epidermis is also responsible for giving us our skin color due to its high pigment melanin content. Melanocyte produces two types of Melanin: Pheomelanin and Eumelanin. Typically, one square mm of skin contains 1000 and 2000 melanocytes and can produce two Melanin types, Pheomelanin and Eumelanin [12].
The type of Melanin in the Epidermis determines the skin color, Pheomelanin is prominent in light skin, while Eumelanin in dark skin. However, there is no difference in the number of Melanocytes between light and dark skin. The production of Melanin starts from the amino acid Tyrosine via the enzyme Tyrosinase [13].
Once produced, the Melanocytes store Melanin in small sacks called Melanosomes. In light skin, melanosomes are small and contain only a few tightly packed melanin granules. In darker skin, melanosomes are larger and contain many loosely-distributed Melanin granules [14], as shown in figure 4.
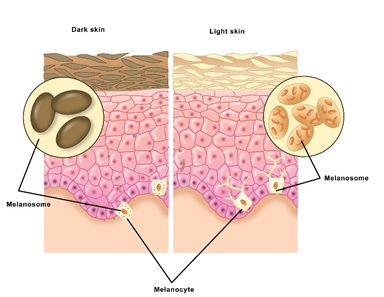 Figure 4: An illustration ofthe difference between dark and light skin regarding the Melanin type and packaging in Melanocyte.
Figure 4: An illustration ofthe difference between dark and light skin regarding the Melanin type and packaging in Melanocyte.
Each one of the Melanocyte establishes connections with an average of 40 keratinocytes via cellular extensions called dendrites as shown in figure 5. Melanin is then transferred to Keratinocytes via a keratinocyte-initiated process. This process includes Melanin Exocytosis from Melanocytes, followed by Endocytosis of Melanin by the Karyocytes. Any irregularities in Melanin production and distribution will result in dyschromia (hyper or hypopigmentation) [15].
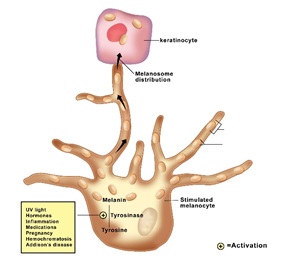 Figure 5: An illustration of a Melanocyte connection with a keratinocyte cell.
Figure 5: An illustration of a Melanocyte connection with a keratinocyte cell.
Dermis
Lies between the Epidermis and the subcutaneous layer and is about 2 mm in thickness. This middle layer of skin contains structural protein in the form of collagen in bulk and elastin in minimal quantities, with a rich intertwining blood supply.
The Dermal Extracellular Matrix (ECM) synthesis. The ECM includes structural proteins (such as collagen and elastin), glycosaminoglycans (such as hyaluronic acid), and adhesive proteins (such as fibronectin and laminins) [16].
The hydrophilic Hyaluronic acid binds Water and increases skin hydration. The ECM component’s loss is responsible for most of the skin aging signs regarding sagging and laxity. Epidermis and Dermis’s borders are not straight lines, but wavy forming the shape of intertwined fingers. The Dermal fingers (extension) are called Papilla, and Epidermal fingers (extensions) are called Rete ridges [17].
Hair follicles, nerves, lymphatic vessels, and sweat glands also resides in the Dermal layer of the skin and referred to as Appendageal structures or adnexa [18].
Subcutaneous layer
Also known as the hypodermis, which is the lowermost skin’s layer comprising mainly fat (adipose). This layer provides protection from injury, produces heat, and serves as a cushion for the body [9].
Histology of normal skin
Normal thickness of the Epidermis (top layer) comprises several layers of squamous cells with the delicate basket-weave keratin (stratum corneum) on the surface. The Dermis (bottom part) comprises sparse fibroblasts with abundant extracellular collagen bundles and embedded capillaries lined by a single layer of endothelial cells [19]. Figures 6A&6B shows the histology section using hematoxylin-eosin with two different magnifications, 10 and 40, respectively.
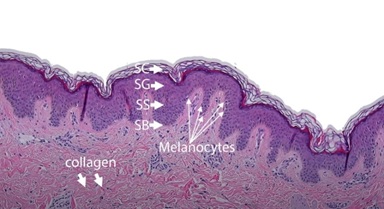 Figure 6A: Layers of the Epidermis and Dermis (hematoxylin-eosin, magnification 10). Reproduced with permission from the Royal Society of Chemistry [20].
Figure 6A: Layers of the Epidermis and Dermis (hematoxylin-eosin, magnification 10). Reproduced with permission from the Royal Society of Chemistry [20].
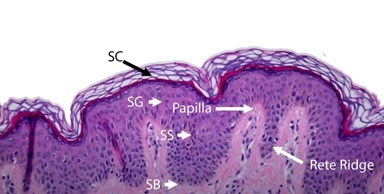 Figure 6B: Layers of the epidermis and Rete rigids (hematoxylin-eosin, magnification 40) Reproduced with permission from the Royal Society of Chemistry [20].
Figure 6B: Layers of the epidermis and Rete rigids (hematoxylin-eosin, magnification 40) Reproduced with permission from the Royal Society of Chemistry [20].
Changes due to intrinsic aging
Human skin is affected by the normal aging process, like all other organs. The aging process is mainly induced by oxidative stress and results in a thinner dermal layer and low-quality epidermal layer [21,22]. As this process is invertible; However, it is interdependent with external ageing factors such as photoaging.
Changes Due To Photoaging
Epidermis (EP.)
The extended exposure to the sun’s UV light affects Keratinocyte’s maturation and migration process and the skin’s ability to shed the old Corneocytes. Therefore, the Stratum Corneum gets thicker, and the texture gets rougher, which results in poor light reflection (dullness) [20]. Moreover, the Epidermis lose some of their functionality as a barrier allowing a higher evaporating rate (dryness) and higher irritant and pathogen penetration. Furthermore, the UV light might disrupt Melanin’s production, storing, and transferring, resulting in several hyperpigmentation manifestations (Melasma, lentigines, freckles), hypopigmentation (vertigo) both [23].
Dermis (DER)
Overall, human skin loses 1% of its ECM components per year. However, the UV light accelerates this rate. Since the Epidermis would not block the UV light efficiently, the damaging Effect might extend to ECM. The UV light induces some enzymes responsible for Elastin and Collagen degradation. Therefore, Photoaging accelerates ECM structural proteins’ loss and denaturation of Hyaluronic acid (dermal atrophy). Moreover, UV light might affect blood vessels in the Dermis layer, resulting in visible telangiectasias and erythema. Melanocyte’s hyperactivation might result in high Melanin concentration in the dermal level, such as in Melasma [24].
Figure 7 points out the main changes induced by photoaging, such as: reduced elastin and collagen, Dermal and Hypodermal atrophy, thinner Epidermis, and thicker SC layer.
Figure 8 shows histology differences between photoprotected and photoexposed skin [20].
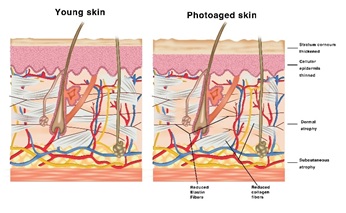 Figure 7: Depiction of the main differences between young skin and photoaged skin.
Figure 7: Depiction of the main differences between young skin and photoaged skin.
Table 1 shows the average thickness of different layers of the skin on different parts of the female face [25].
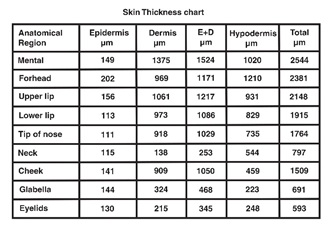 Table 1: Average thickness chart.
Table 1: Average thickness chart.
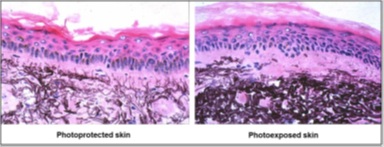 Figure 8: The difference in elastic fibre destruction in photoexposed hypertrophic skin.
Figure 8: The difference in elastic fibre destruction in photoexposed hypertrophic skin.
The photoprotected skin shows the normal architecture, in contrast to photoexposed, which shows excess mature elastic fibre deposition, which is truncated and dystrophic. Reproduced with permission from the Royal Society of Chemistry [20].
Classification Of Photoaging
Practitioners should have the tool to objectively assess their clients and photo aging state to provide evidence-based treatments. The Glogau photoaging classification is a valuable tool if the practice does not make a skin analyzer available. Figure 9 shows different stages of photoaging with detailed characteristics of each stage.
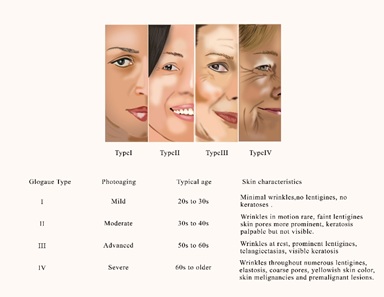 Figure 9: Different stages of photoaging and its corresponding Glogau classification.
Figure 9: Different stages of photoaging and its corresponding Glogau classification.
Another system to objectively assess the photoaging is the Photonumeric scales developed by Elliset, et al. as shown in figure 10 [26]. The photo-numeric scale assesses assign hypertrophic facial photoaging between 0 to 8, where0 = no photoaging, 2 = mild, 4 = moderate, 6 = severe, and 8 = very severe. Interdigitate values, i.e. 1, 3, 5, and 7, could also be assigned if felt appropriate [26] (Figure 10).
Light-Based Treatment
This term is widely used to describe all light-based (Lasers and Intense Pulsed Light) procedures for correct cosmetic and medical procedures. Practitioners in the aesthetic field use the term“photorejuvenation” to describe methods intended to improve the skin conditions in general or correct a particular skin concern [27].
Therefore, choosing the correct technology requires a comprehensive understanding of the skin anatomy, the treated cosmetic issue, and the principle of laser and IPL. All photo-based treatments have one of three mechanisms of action; Selective Photothermolysis [28], chemical photosensitization or Photoacoustic effect [29,30].
Light-tissue Interaction
Theoretically, a laser beam can travel in a vacuum till infinity if there is no interaction. However, once a laser beam collide with an objection (skin tissue), it would go through a combination of four different physical processes: absorption, reflection, transmission, and scattering. The object’s physical characteristics and the laser beam parameters would define the most prominent type of interaction. Figure 10 illustrates the four different possible outcomes of the light-skin interaction.
 Figure 10: Photo-numeric scale for photoaging, Reproduced with permission from the Royal Society of Chemistry [20].
Figure 10: Photo-numeric scale for photoaging, Reproduced with permission from the Royal Society of Chemistry [20].
Our goal is to maximize the light absorption by a specific skin component at a particular depth in terms of light-based therapy in the aesthetic field. We use the term “chromophore” to refer to the skin component with the highest laser absorption properties [31].
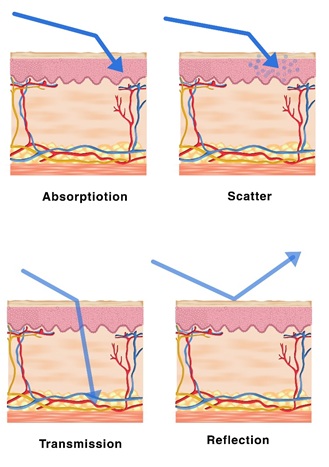 Figure :Illustrates the four different possible outcomes of the light-skin interaction.
Figure :Illustrates the four different possible outcomes of the light-skin interaction.
Skin’s Chromophores
All photo rejuvenation processes target specific skin components in the Dermis and Epidermislayers; the chromophores. These chromophores selectively absorb photons’ energy and turn it into thermal energy (heat) [32]. The main three chromophores in human skin are:
- Melanin for pigmented lesion and laser hair removal (absorbs light of wavelengths between 400 and 1100 nm, with no peaks)
- Water for wrinkle and dermal ECM induction (show a significant absorption peak at 3000 nm)
- Oxyhemoglobin for vascular lesion (shows strong absorption at 400-600 nm with peaks at 418, 542, and 577).
Melanin and Oxyhemoglobin share a vast portion of the absorption spectrum. Therefore, lasers designed to target Melanin (755 nm Alexandrite) have wavelengths with good melanin selectivity and low Oxyhemoglobin affinity [33].
The concept of photorejuvenation is to target a specific chromophore to or enhance/prohibit a specific process [34] or remove unwanted lesions [35]. In case of removing unwanted lesions, the thermal energy that builds up in the skin lesion that contains the chromophore ends up burning the chromophore without damaging any other skin cell, theoretically. This concept is “selective photothermolysis.”
Selective Photo Thermolysis
Photo energy (light) needs a medium to turn itself into thermal energy (heat); this medium is called chromophore (we will discuss the three skin chromophores in detail). A Chromophore is a molecule (or part of a molecule) that absorbs light in a certain spectrum range. The colour of a chromophore is the light minus that specific absorbed spectrum.
To absorb photon energy, a chromophore should have the ability to turn it into a different form, such as thermal energy (heat). Therefore, a chromophore temperature would increase due to heat build-up, and depending on the heating rate; there are three possible scenarios;
- The light energy has a low intensity, and the chromophore temperature heat does not rise significantly. Thus, the chromophore confines the heat and slowly dissipates the extra heat to the surrounding, and eventually cools down to normal temperature without disrupting the surroundings
- The light energy has a medium intensity, and the chromophore temperature heat rises significantly. However, the chromophore will try to cool down and return to a normal temperature as fast as possible. Thus, the chromophore will dissipate the extra heat to the surroundings at a higher rate, raising their temperature. The chromophore and its surrounding will eventually cool down with or without physiochemical changes. This is referred to as a bystander effect, as shown in figure 11. The Effect is one of the main theories that explain the laser hair removal mechanism of action
- The light energy has a high intensity, and the chromophore temperature heat rises dramatically. The chromophore will try to cool down and return to a normal temperature as fast as possible. However, this cooling rate would not be sufficient, and the temperature will reach a point that induces physicochemical changes. The chromophore will cease to exist in the same physiochemical properties, and the surroundings might (might not) experience thermal-induced changes. This is referred to as the selective photo thermolysis principle.
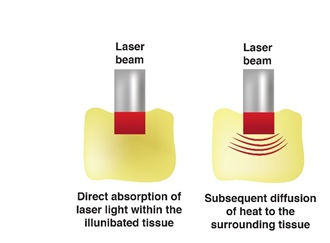 Figure 11: illustrates the heat diffusion after laser exposure commonly called the bystander effect of the laser.
Figure 11: illustrates the heat diffusion after laser exposure commonly called the bystander effect of the laser.
Most of the aesthetic field’s photo-based treatments use the selective photo thermolysis principle and its bystander effect to induce skin tissue changes. For this to happen, there are three key conditions:
- The targeted tissue should have a higher concentration of a specific chromophore than its surroundings.
- We should use a specific wavelength to target that chromophore selectively.
- We could control the temperature rise rate to induce the required photo thermolysis and/or bystander effect, not more or less.
Chemical photosensitization
Acne Vulgaris light-based treatments rely, in part, on the photosensitization of porphyrins into oxygen free-radicals with antibacterial properties [36].
Photoacoustic effect
This is one physical property that s specific to the laser in short and ultra short pulse width. In a brief explanation, a laser could induce intense vibration on a microscopic level that is mainly used for pigmentation and tattoo removal [37].
Light parameters
- Wavelengths (nm)
The wavelenght is related to the light color. Laser light is extremely monochromatic (one color) when compared to other sources of light. All of the photons (energy) that makeup the laserr beam have a fixed phase relationship (coherence) with respect to one another [38].
The selection of the wavelength emerges from the selective photothermalsis principle. Therefore, the wavelength should present the highest affinity between the laser and the lesion, hence the highest absorption. However, we should take into consideration the Effect of wavelength on skin-laser physical interactions [39].
Lower wavelength is associated with lower penetration due to high scattering. This makes lower wavelength laser not suitable for dermal level lesions. Moreover, it is associated with a higher risk of Epidermal damage. We will discuss different available wavelengths and their indication in a later section. Figure 12 shows the relative penetration of the most commonly used wavelength [40].
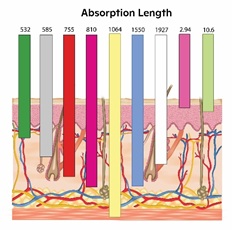 Figure 12: Show the relative penetration of the most commonly used wavelength.
Figure 12: Show the relative penetration of the most commonly used wavelength.
Scientifically, we express a chromophore’s ability to absorb light in a specific wavelength by the absorption coefficient (cm-1). A higher absorption coefficient means a higher affinity between the wavelength and the chromophore [41].
Figures 13 A and B show the three targeted chromophores in the skin and the absorption and coefficient of each wavelength [42]. We will discuss the meaning of chromophores and their relevance in aesthetic treatment in detail. As shown in Figure 13 B, Melanin and Hemoglobin do not show any photo absorption after 1064 nm, as Water becomes the main targeted chromophore.
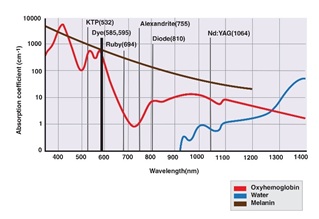 Figure 13 A: shows the absorption peak of hemoglobin, Melanin and Water for lasers between 532 and 1064 nm.
Figure 13 A: shows the absorption peak of hemoglobin, Melanin and Water for lasers between 532 and 1064 nm.
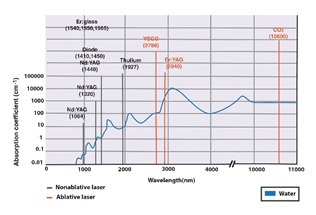 Figure 13 B: shows the absorption peak of Water for lasers between 1064 and 10600 nm.
Figure 13 B: shows the absorption peak of Water for lasers between 1064 and 10600 nm.
Tablet 2 shows the absorption coefficient and ratio of the three wavelengths commonly used in a picosecond range. A higher ratio indicates more specificity towards one chromophore [43].
 Table 2: Shows the absorption coefficient and ratio of 532 nm, 1064 nm and 755 nm.
Table 2: Shows the absorption coefficient and ratio of 532 nm, 1064 nm and 755 nm.
On the contrary, Intense Pulsed Light (IPL) is not a laser, as it is polychromatic (spectrum of wavelengths) and non-coherent. However, a practitioner can use a specific bandwidth by applying a specific filter with an upper or lower cut-off wavelength or replacing the handpiece [44]. As discussed in Lasers, each wavelength band has a different penetration depth, tissue interaction, and selective chromophores [45].
Figure 14 shows the depth of penetration of different IPL spectrums produced by different filters. The Blue light is the unfiltered IPL which includes the full spectrum between 420-1200 nm. As shown in figure 14, the full spectrum has the least penetration; A higher filter number correlates with high cut-off wavelength and deeper penetration. For example, The 690 Red filter cut all light with a wavelength less than 690 nm, thus produces light between 690 nm and 1200 nm for deeper penetration.
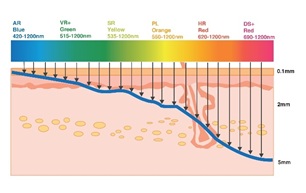 Figure 14: The depth of penetration for different IPL spectrum produced by different filters.
Figure 14: The depth of penetration for different IPL spectrum produced by different filters.
- Fluence (J/cm2)
If the laser beam is a string, then the fluence would be the string’s density/thickness. As inferred by the unit (J/cm2), it is a measure for energy (in joules) delivered to the treated area of one cm2. Low fluence may result in less than satisfactory results, while high fluence may result in burns and adverse events [46].
Therefore, practitioners should be conservative when choosing the fluence to ensure efficacy while preserving safety. A higher affinity between the laser and skin lesion allows the practitioner to increase the fluence without risking the cells and tissues around the targeted lesion. Notice that the fluency unit does not have the “time” factor in it.
- Pulse widths (fraction of a second)
The concept of pulse width adds the “time” dimension that was missing from the fluence. It is essential to understand that a laser beam does not deliver energy continuously. However, it provides the energy in waves (pulses of photo-energy) with an interval between each pulse (inter-pulse delay times) [47].
To simplify this concept, let us say that we have two different laser beams A and B (Figure 15), with the following parameters
- A: energy flow of 200 J/sec, and pulse width of 30 msec (top)
- B: energy flow 200 J/sec, and pulse width of 10 msec (bottom)
Figure A tissue was exposed to both laser for 100 msec; the skin would receive the same 20 J of photo-energyin two pulses from both lasers; each is 10 J.
However, laser B will provide the energy to the skin lesions in a more intense (higher peak) but shorter pulse width than A. In another word, the continuous delivery time of the energy is different. Laser A delivers the energy in a total of 60 msec out of the 100 msec, but laser B takes only 20 msec out of the total exposure time to deliver the energy (100 msec).
A laser Short pulse duration produces a more efficient photo thermolysis process to reach the clinically required temperature faster without thermal diffusion to unwanted tissues [48]. Practitioners should choose pulse width according tothe target’sthermal relaxation, which is the time (in a fraction of a second) required by an object to dissipate 63% of the excess thermal energy (heat) [49]. We will discuss this concept in detail later on, but an object with longer thermal relaxation time can confine the heat for longer times and spare unwanted heat diffusion. Therefore, targeting a lesion with a shorter relaxation time, such as melanosomes, is more challenging. Such targets dissipate (relax) heat to the surrounding tissue, making it hard to reach the required temperature inside the lesion itself.
Moreover, unwanted heat diffusion might result in side effects in healthy tissues such as burns and post-inflammatory hyper Pigmentation (PIH). Therefore, practitioners should use a shorter pulse when targeting certain types of pigmentation to induce photothermolysis without giving the lesion time to cool down (by dissipating the heat to unwanted tissues) [37]. The fast heat build-up raises the lesion temperature to the required level to induce photo thermolysis, minimizing heat dissipation and unwanted side effects [50]. The following figure shows a simple schematic illustration for laser A (top) and laser B (bottom). The areas coloured in red refers to intra-pulse delay, which is the time between two consequent pulses.
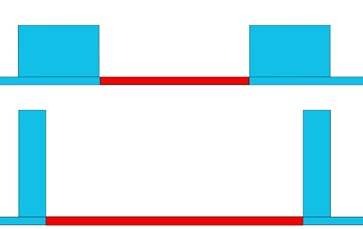 Figure 15: Shows a simple illustration of two different lasers (A and B) with the same fluence and different pulse width. The blue colour is the laser pulse, and the red is the inter-pulse delay.
Figure 15: Shows a simple illustration of two different lasers (A and B) with the same fluence and different pulse width. The blue colour is the laser pulse, and the red is the inter-pulse delay.
We wish to emphasize that this example is oversimplified, and relaxation time is not the same as the intra-pulse delay time but is an intrinsic characteristic of the target. Figure 16 shows one laser pulse with the same fluence but different pulse width, long and short; A and B, respectively. As shown in figure 15 A, the long pulse works well for larger targets and long relaxation times but less effective in smaller targets. It would not be practical to induce clinical temperature in small targets using a long pulse laser.
On the contrary, the short pulse is more effective in irradiation smaller targets with short relaxation time. The heat accumulation, induced by the short-pulsed laser, effectively exceeds the heat diffusion rate to reach clinical temperature in the targeted lesion [51].
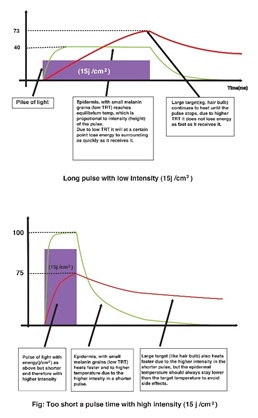 Figure 16: Shows one pulse of two different lasers (A and B) with the same fluence and different pulse width and how they affect large and small targets differently. The top (A) is a long pulse, and the bottom (B) is a long pulse.
Figure 16: Shows one pulse of two different lasers (A and B) with the same fluence and different pulse width and how they affect large and small targets differently. The top (A) is a long pulse, and the bottom (B) is a long pulse.
We could use the Er: Yag Laser’s pulse width to illustrate the relation between the pulse width and dynamic heat change (thermal diffusion). This laser is ablative and drills a hole in the exposed tissues. The following figure shows that the hole’s depth is almost the same regardless of the pulse width. However, long pulse width generates more heat to the surrounding tissue, as shown in figure 17.
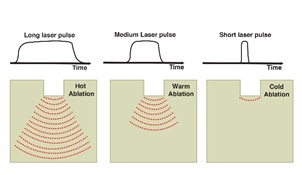 Figure 17: Shows the Effect of the pulse width on the heat diffusion after irradiation with Er: Yag laser.
Figure 17: Shows the Effect of the pulse width on the heat diffusion after irradiation with Er: Yag laser.
The difference is more profound if we compare a long and short pulse width laser. The following graph shows the difference in energy intensity (peak) of flash lamp-pumped pulsed dye laser and Q-switched Nd: YAG laser.
The short-pulsed laser provides a concentrated, high-intensity laser pulse usually illustrated as a needle shape, as shown in figure 18 [52]. It is essential to realize that all pulse widths (from Ultra-short to Ultra-long) are equally important but have different applications in aesthetic fields. Moreover, the same laser wavelength has different applications and indications according to the pulse width. Other synonyms of pulse width are pulse duration and dwell time.
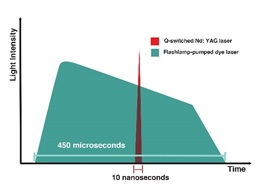 Figure 18: shows the difference between pulse width and shape between Flashlamp dye laser (in green) and nanosecond laser (red), recreated from [52].
Figure 18: shows the difference between pulse width and shape between Flashlamp dye laser (in green) and nanosecond laser (red), recreated from [52].
For Example, Nd: Yag Laser comes in Ultra-longpulsed (sec) for lipolysis [53], long-pulsed (msec) for laser hair removal [53], quasi-long microsecond (µsec) for pigmentation [54], short nanosecond (nsec) for tattoo removal [55], and Ultrashort picosecond (psec) pulse width for skin rejuvenation [56].
- Train of sequentialsub-pulses
Some systems can deliver the required total pulse energy in a train of consequent shots (sub-pulses) with a short intra-pulse delay [57]. The total pulse width is equal to the sum of the sub-pulses duration and the intra-pulse duration.
Figure 19 is an illustration of the M22 IPL interface [58].The interface shows a total pulse width of 15 msec divided into three sub-pulses with 3.8, 3.2, and 3 sub-pulse widths; we refer to the sum as active light emission time. The intra-pulse delay is 2.6 (between the first and second sub-pulse) and 2.4 msec (between the second and third sub-pulse). The total pulse width here is the sum of all sub-pulses widths and intra-pulse delay (3.8+2.6+3.2+2.4+3.0=15 ms)
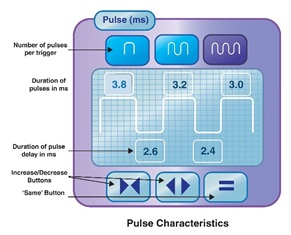 Figure 19: An illustration of the M22 IPL interface from Lumenis.
Figure 19: An illustration of the M22 IPL interface from Lumenis.
Photo energy may be delivered in two different patterns: Figure 20 A shows the traditional pattern of light-based therapy, in which the photo energy is delivered in a continuous-solid pulse, and B shows the same every delivered in three equal sequentialsub-pulses. It is important to emphasize that figure 20 B shows only two pulses, with three sub-pulses each, not six.
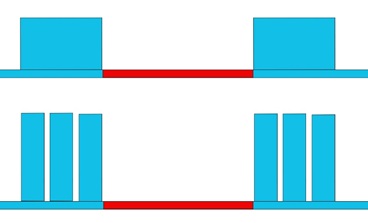
Figure 20: (A (top): Shows two solid pulses, and B (bottom) shows two pulses, each one of them is delivered in three equal sub-pulses.
This technology is mainly utilized in IPL platforms to provide a higher safety profile, especially in patients with darker skin.
An older example of this technology is the Surepulse configuration in the new Icon IPL from Cynosure for hair removal. It provides the energy in two micro pulses; the first one is 20 msec, then 100 msec intra-pulse delay, and finally a 10-msec pulse (total of 130 msec). The advantage is to provide a higher peak power with the same total energy of a longer pulse, as shown in Table 3 [59]. More advanced IPL platforms provide up to four sequential pulses to enhance safety and tolerability.
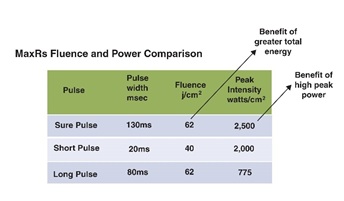 Table 3: Shows the advantages of using the Surepulse in Cynosure Icon IPL MaxR handpiece.
Table 3: Shows the advantages of using the Surepulse in Cynosure Icon IPL MaxR handpiece.
- Pulse shape
Modifying pulse width is a new advancement in laser and IPL that new practitioners usually overlook. A normal pulse’s energy distribution is bell-shaped, with a summit and two tails; A pulse with wide tails indicates energy waste [57]. The new technology converts the pulse from a bell shape to a square; some examples are the Advanced Fluorescence Technology (AFT) in Alma IPL systems and the squared-adaptive structured pulse in Fotona laser.
The goal of modifying the pulse shape is to improve equal energy distribution and enhance some properties, such as ablation, coagulation, or Photoacoustic phenomena. As we will discuss with the 10600 nm CO2 laser, the ablation/coagulation ratio differs according to the pulse shape (CW, UltraPulsed, Superpulse) [60].The chopped continuous delivery method provides high coagulation propertiescompared to the pulsed and superpulsed [61], as shown in figure 21 A.
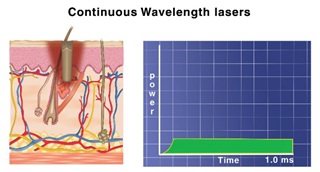 Figure 21 A: Shown the pulse shape and Effect of the CO2 laser delivered in CW mode.
Figure 21 A: Shown the pulse shape and Effect of the CO2 laser delivered in CW mode.
The Superpulse mode has high peak power and relatively long pulse width, which balances ablation and coagulation, as shown in figure 21 B.
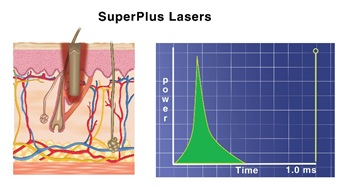 Figure 21 B: Shown the pulse shape and Effect of the CO2 laser delivered in superpulse mode
Figure 21 B: Shown the pulse shape and Effect of the CO2 laser delivered in superpulse mode
The Ultrapulse deliversan ultrashort burst of energy and shifts the Effect towards ablation with minimal coagulation effect, as shown in figure 21 C.
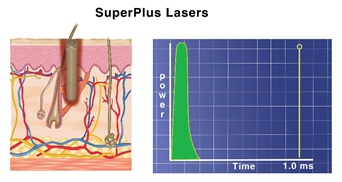 Figure 21 C: Shown the pulse shape and Effect of the CO2 laser delivered in Ultrapulse mode.
Figure 21 C: Shown the pulse shape and Effect of the CO2 laser delivered in Ultrapulse mode.
- Spot size (mm)
Spot size correlates to the lens aperture that defines the diameter of the laser beam. All laser devices have adjustable spot sizes. Moreover, any adjustment in spot size would affect the maximum laser beam fluence. It is possible to get a much high laser fluence with a smaller spot size (2mm) than with a bigger spot size (20mm). More importantly, The spot size also affects the physical interaction between the laser and the skin and penetration depth. Smaller spot size is associated with high scattering and less penetration than laser spot size [62], as shown in figure 22.
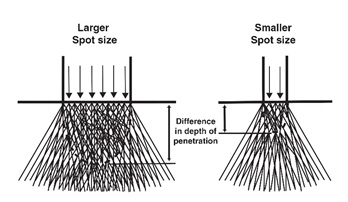 Figure 22: schematic representation of the spot-size-dependent depth of penetration, recreated from [62] with permission.
Figure 22: schematic representation of the spot-size-dependent depth of penetration, recreated from [62] with permission.
Therefore, a larger spot size had a higher safety profile due to less epidermal interaction. This Effect is significant when moving from 1 to 20 mm in size. However, the spot size has an insignificant effect on the penetration depth after 20 mm. This concept is only correct within the mm range and does not apply to the fractional laser, as we will discuss later (Figure 23).
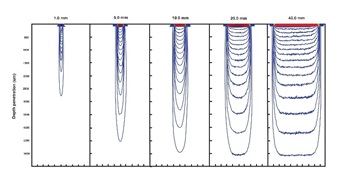 Figure 23: Calculated penetration profiles for uniform 1, 5, 10, 20 and 40 mm width beam of equal incident fluence obtained by Monte Carlo simulation using typical skin parameters for wavelengths of 525-1100 nm, recreated from [62] with permission.
Figure 23: Calculated penetration profiles for uniform 1, 5, 10, 20 and 40 mm width beam of equal incident fluence obtained by Monte Carlo simulation using typical skin parameters for wavelengths of 525-1100 nm, recreated from [62] with permission.
- Depth of penetration
The depth of laser penetration results from combining all laser parameters such as wavelength, fluence, pulse width, and spot size. Figure 24 is a summary of how these factors would affect the depth of penetration. The practitioner should consider the thickness of the skin on the treated area and the depth of the treated lesion.
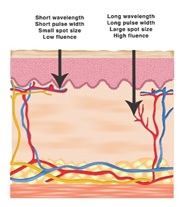 Figure 24: Summarize the Effect of different laser parameters on the depth of penetration.
Figure 24: Summarize the Effect of different laser parameters on the depth of penetration.
Table 4 shows the absorption of different laser wavelengths by different layers and components of the human skin [63].
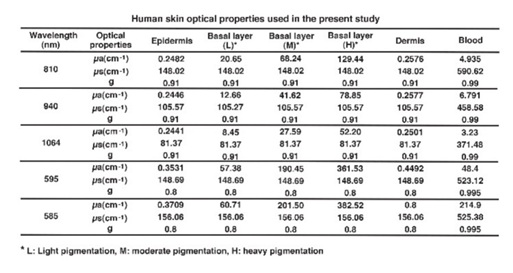 Table 4: Human skin optical properties used in the present study.
Table 4: Human skin optical properties used in the present study.
- Repetition rate (Hz) for the device
It is essential to understand that the pulse rate we discuss here is for the machine, not for the laser itself. The repetition rate for the laser device is related to the laser-firing rate. All laser machines have manual and automatic settings; the manual settings mean the laser fires only when the operating practitioner pushes the button.
Most practitioners start slow, so they fire the laser every 20 seconds. However, with experience, they increase the laser firing rate (repetition rate), which might be tiring for the thump. Once a practitioner gets comfortable with a repetition rate, they can use the auto setting to program the device to fire the laser in fixed intervals (for example, on 1 Hz), which means the machine will fire the laser once every second.
Therefore, the practitioner should have enough experience to move the hand piece swiftly before the laser fires automatically again. Otherwise, the laser might result in adverse effects such as skin burns or fat atrophy. Remember that the repetition rate is affected by the spot size, as a larger spot size would limit the laser source’s ability to fire more frequently. For example, some platforms could profile a 3 Hz rate with 5mm spot size, but only 1 Hz repetition in 20 mm.
Skin Lesion Characteristics
As we mentioned before, lase-skin interaction depends on both the laser parameters and objection characteristics.
Absorption and Contrast
Different skin lesions should be targeted relatively to the most abundant chromophore, as discussed before. The same chromophore might exist in the lesion’s cells and surrounding cells, such as Melanin, in both normal hyperpigmented skin. Moreover, two competitive chromophores may co-reside in some skin lesions, such as Melanin and Oxyhemoglobin in telangiectasia [32].
Therefore, we should consider the concentration level and the absorption of each chromophore before choosing a laser. The term “contrast” refers to the relative concentration of the chromophores and the absorption ratio. The most practical example is the laser of choice in hair removal; the 755 nm Alexandrite laser is more effective in Laser Hair Removal (LHR) due to the Melanin’s high absorption in the hair follicle.
Therefore, the 755 nm Alexandrie laser is considered the LHR laser in skin type I-II and III as the Keratinocytes do not have a high Melanin concentration. Thus, a high contrast between the lesion and the surrounding skin.
Melanin does exist in darker skin types in the hair follicle and Epidermis cells in relatively similar quantities. However, there is low contrast between the lesion and the surrounding skin. The Melanin’s high absorption of the Alexandrite makes it a less favourable choice in darker skin type III-VI and out of the choice list in skin type V due to the lack of contrast.
It is worth mentioning that while it is not recommended, the 755 nm Alexandrite laser can be used in darker skin type but required fine-tuning of the laser parameters [64].
Therefore, in such cases, we prefer to use a laser wavelength with less absorption by the chromophore, such as the 1064 nm ND-Yag. If the lesion is too pronounce (intense ecthyma in Rosacea), practitioners should adjust the parameters to decrease the treatment intensity by application one or more of the followings; decreasing the fluence, increasing the pulse width, using a longer wavelength, decreasing the overlap, or using a train of sub-pulses.
- Skin phototypes
The skin phototype assessment is one of the most critical steps that a practitioner should perform before laser or light-based treatments.
Fitzpatrick assessment
The Fitzpatrick assessment is the most used method to determine the skin phototype due to the ease of implementation. The original assessment was developed in 1972 to assess skin sensitivity to sun exposure via skin colour and tendency to burn due to sunlight exposure. Lower Fitzpatrick skin numbers indicate that skin is prone to burn than to tan.
However, practitioners have improved the original assessment in the dermatology field to include several items such as ethnicity and eye colour. The Improved Fitzpatrick assessment is still the most used tool to determine skin phototype. Most newly adapted skin type assessment forms have four different fields: Genetic background and deposition, genetic deposition, reaction to sun exposure, and tanning habits with 10-12 multiple-choice questions, as shown in tables 5 & 6.
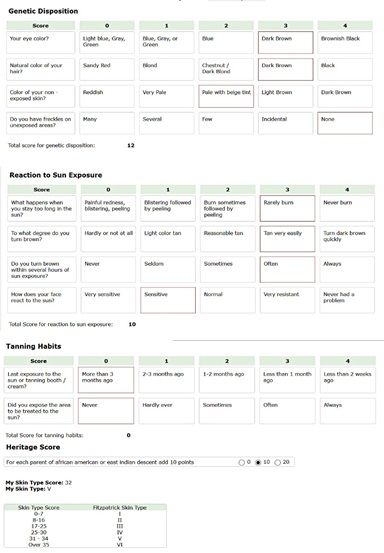 Table 5: Shows a skin type assessment with twelve multiple-choice questions that takes genetic and heritage disposition into account.
Table 5: Shows a skin type assessment with twelve multiple-choice questions that takes genetic and heritage disposition into account.
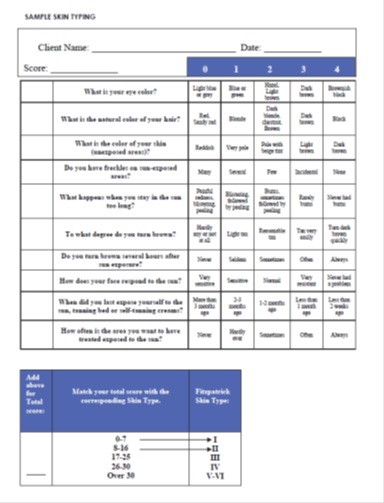 Table 6: Shows a simple skin type assessment with ten multiple-choice questions
Table 6: Shows a simple skin type assessment with ten multiple-choice questions
The practitioner should help the Client choose one of the four suggested answers and grade each question accordingly. The Client’s skin phototype is directly related to the total score of the questioner. A higher score is related to higher skin type and higher risks with photo treatments such as Epidermis burns and Post-inflammatory pigmentation.
The following table is a modern skin type assessment that includes three factors: genetic disposing of, tanning habits, sun exposure and heritage score. Several other assessments have been published over the years, such as Lancer, Goldman, FANOUS and Tayler scale [65]. Some studies argued that all these assessments are subjective and do not provide a reliable measure for the laser-skin interaction [66].
Despite the improvements, several studies have criticized the objectivity and validation of the self-reported assessment. Therefore, other methods, such as Wood’s lamp and skin colorimeter, have been developed to evaluate skin phototype better.
Skin reflectance colorimeter
The new handheld colorimeters emit light of a specific spectrum and measure the light’s wavelength and intensity reflected by the skin [67]. This data is converted into colorimetric value and suggests skin colour. One deficiency of these devices is that they measure only a small area of the skin. Therefore, the practitioner should perform multiple tests and ensure to include the darker spots if they existed.
Older devices, such as Mexameter MX 18(Courage+Khazakauses, Germany), only a narrow band reflectance spectrophotometer, Other devices such as Antera 3D (Miravex Limited, Ireland) uses seven different wavelengths. However, these devices’ data is not absolute and may change according to external variables such as previous tanning and room temperature.
Other than the Mexameter and Antera 3D, we are aware of three handheld devices to assess skin color Chromameter (Minolta, Japan) and DSM III (Cortex technology, Daminark), and Skintel (Cynosure).
Some new LHR platforms’ hand piece is integrated with an internal skin colorimeter to help practitioners choose the best treatment settings. For example, the Skintel Melanin reader is integrated with the Cynosure Icon IPL and Vectus diode laser. After taking three readings for the treatment area, the software converts the reading into Menalin Index (MI) and communicates with the treatment platform to suggest a test spot parameter [68], as shown in figure 25.
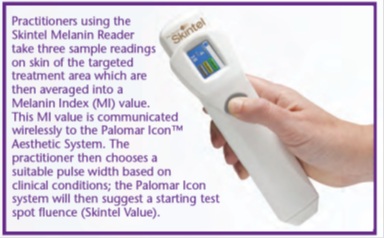 Figure 25: Shows the Skintel device, a handheld Melanin reader by Cynosure [68].
Figure 25: Shows the Skintel device, a handheld Melanin reader by Cynosure [68].
It is important to understand that these skin type does not correlate with an absolute MI value but a range [69], as shown in figure 26; For example, the Melanin Index of 20 is shared between skin type III and IV. Therefore, practitioners should always seek and observe clinical endpoints.
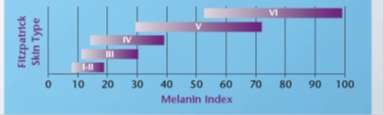
Figure 26: Shows the the MI range for each skin type [68].
- Thermal relaxation time (TRT) and size
As explained before, as the lesion’s chromophores receive laser pulses, the heat starts building up. However, the lesion’s cells that contain the chromophore are not thermally isolated from surrounding cells. Therefore, it starts dissipating the thermal heat to adjacent cells.
Thermal relaxation is an object’s ability to cool down by dissipating the thermal energy to the surroundings[49]. The TRT is a measure of the time (in a fraction of a second) required by an object to dissipate 63% of the excess thermal energy (heat). TRT is directly related to the density and size of the chromophore in the lesion.
A lesion with high laser absorption and short relaxation time would damage the surrounding cells. Therefore, a short-pulsed laser with high energy is the laser of choice as it removes the lesion before it can dissipate the heat and damage surrounding cells. Q-switch nsec and the newer Picosecond lasers are examples of high-energy short-pulsed lasers.
The secondary (by standard) effect of thermal relaxation should be controlled to exert the desired clinical outcomes without side effects. For example, Laser hair removal relies on the hair strand’s ability to relax the heat by dissipating it to the follicle’s germinative cells. However, if the heat exceeded the clinical limit, it would result in burns and other side effects.
Small targetslike Melanosomes cool down extremely fast, and they do not retain the photo-thermal energy (heat) to a specific clinical level. Therefore, such targets should be exposed to a high-energy short-pulsed laser. The TRT and the pulse width are strongly tied together but from different perspectives, as discussed later.
- Depth of the lesion
All laser targets for photo rejuvenation purposes exist in the epidermal and dermal level, a total of 2.1 mm. Therefore, we can adjust the laser settings to provide the required penetration to reach the lesion and protect the other tissues-less than optimal laser penetration (too shallow or too deep) results in unwanted adverse reactions.
Most new laser systems are equipped with a computing unit to suggest adequate parameters in relevance to the lesion depth, intensity, and skin phototype [70]. Moreover,the parameter of fractional lasers such as CO2 and Er: Yag is chosen in relevance to the required depth of ablation or coagulation. If a lesion exists in variable depth (Rosacea and Melasma), practitioners should target the deeper section and adjust parameters to correct the superficial parts.
References
- Kolárová H, Ditrichová D, Wagner J (1999) Penetration of the laser light into the skin in vitro. Lasers Surg Med 24: 231-235.
- Pozner JN, Cohen JL, Burns J, DiBernardo BE, Bass LS, et al. (2020) State of laser resurfacing 2021: A roundtable discussion. Dermatological Reviews 2: 34-46.
- Yousef H, Alhajj M, Sharma S (2020) Anatomy, Skin (Integument), Epidermis. StatPearls [Internet].
- Epstein WL, Maibach HI (1965) Cell renewal in human epidermis. Arch Dermatol 92: 462-468.
- Cua AB, Wilhelm KP, Maibach HI (1990) Frictional properties of human skin: Relation to age, sex and anatomical region, stratum corneum hydration and transepidermal water loss. Br J Dermatol 123: 473-479.
- Ya-Xian Z, Suetake T, Tagami H (1999) Number of cell layers of the stratum corneum in normal skin - relationship to the anatomical location on the body, age, sex and physical parameters. Arch Dermatol Res 291: 555-559.
- Murphrey MB, Miao JH, Zito PM (2021) Histology, Stratum Corneum. StatPearls [Internet].
- Marks R, Edwards E (1992) The measurement of photodamage. British Journal of Dermatology 127: 7-13.
- Wysocki AB (1999) Skin anatomy, physiology, and pathophysiology. Nurs Clin North Am 34: 777-797.
- Kolarsick PA, Kolarsick MA, Goodwin C (2011) Anatomy and physiology of the skin. Journal of the Dermatology Nurses’ Association 3: 203-213.
- Lin JY, Fisher DE (2007) Melanocyte biology and skin pigmentation. Nature 445: 843-850.
- Hennessy A, Oh C, Diffey B, Wakamatsu K, Ito S, et al. (2005) Eumelanin and pheomelanin concentrations in human epidermis before and after UVB irradiation. Pigment Cell Res 18: 220-223.
- D'Mello SA, Finlay GJ, Baguley BC, Askarian-Amiri ME (2016) Signaling pathways in melanogenesis. Int J Mol Sci 17: 1144.
- Lu H, Edwards C, Gaskell S, Pearse A, Marks R (1996) Melanin content and distribution in the surface corneocyte with skin phototypes. Br J Dermatol 135: 263-267.
- Dagdug VAG, Guevara HC, Arellano MI (2021) News in the Treatment of Melasma. Dermatología Cosmética, Médica y Quirúrgica 18: 307-317.
- Rippa AL, Kalabusheva EP, Vorotelyak EA (2019) Regeneration of dermis: Scarring and cells involved. Cells 8: 607.
- Topczewska JM, Ledwon JK, Vaca EE, Gosain AK (2019) Mechanical stretching stimulates growth of the basal layer and rete ridges in the epidermis. J Tissue Eng Regen Med 13: 2121-2125.
- Lavker RM, Sun T-T (1983) Epidermal stem cells. Journal of Investigative Dermatology 81: 121-127.
- Han A, Chien AL, Kang S (2014) Photoaging. Dermatol Clin 32: 291-299.
- Watson REB, Griffiths CEM (2019) Cutaneous Photoaging. Royal Society of Chemistry, UK.
- Poljšak B, Dahmane RG, Godic A (2012) Intrinsic skin aging: The role of oxidative stress. Acta Dermatovenerol Alp Pannonica Adriat 21: 33-36.
- Guinot C, Malvy DJ, Ambroisine L, Latreille J, Mauger E, et al. (2002) Relative contribution of intrinsic vs extrinsic factors to skin aging as determined by a validated skin age score. Arch Dermatol 138: 1454-1460.
- Bhawan J, Andersen W, Lee J, Labadie R, Solares G (1995) Photoaging versus intrinsic aging: A morphologic assessment of facial skin. J Cutan Pathol 22: 154-159.
- Poon F, Kang S, Chien AL (2015) Mechanisms and treatments of photoaging. Photodermatol Photoimmunol Photomed 31: 65-74.
- Chopra K, Calva D, Sosin M, Tadisina KK, Banda A, et al. (2015) A comprehensive examination of topographic thickness of skin in the human face. Aesthet Surg J 35: 1007-1013.
- Griffiths CE, Wang TS, Hamilton TA, Voorhees JJ, Ellis CN (1992) A photonumeric scale for the assessment of cutaneous photodamage. Arch Dermatol 128: 347-351.
- Elsaie ML, Lloyd HW (2008) Latest laser and light-based advances for ethnic skin rejuvenation. Indian J Dermatol. 53: 49.
- Altshuler G, Anderson RR, Manstein D, Zenzie HH, Smirnov MZ (2001) Extended theory of selective photothermolysis. Lasers Surg Med 29: 416-432.
- Maisch T, Spannberger F, Regensburger J, Felgenträger A, Bäumler W (2012) Fast and effective: intense pulse light photodynamic inactivation of bacteria. J Ind Microbiol Biotechnol 39: 1013-1021.
- Shubnyy A, Zhigarkov VS, Yusupov VI, Sviridov AP (2021) Laser bleaching of tattoos: a new approach. Quantum Electronics 51: 8.
- Waibel JS (2009) Photorejuvenation. Dermatologic Clinics 27: 445-457.
- Young AR (1997) Chromophores in human skin. Phys Med Biol 42: 789.
- Zijlstra W, Buursma A, Meeuwsen-Van der Roest WP (1991) Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin Chem 37: 1633-1638.
- Bjerring P, Christiansen K, Troilius A, Dierickx C (2004) Facial photo rejuvenation using two different intense pulsed light (IPL) wavelength bands. Lasers Surg Med 34: 120-126.
- Zoccali G, Piccolo D, Allegra P, Giuliani M (2010) Melasma treated with intense pulsed light. Aesthetic Plast Surg 34: 486-493.
- Nouri K, Villafradez-Diaz LM (2005) Light/laser therapy in the treatment of acne vulgaris. J Cosmet Dermatol 4: 318-320.
- Watanabe S (2008) Basics of laser application to dermatology. Arch Dermatol Res 300: 21-30.
- Silfvast WT (2004) Laser fundamentals. Cambridge University Press, Cambridge, UK.
- Niemz MH (2007) Laser-tissue interactions. Springer, Berlin, Germany.
- Carroll L, Humphreys TR (2006) LASER-tissue interactions. Clinics in Dermatologyx 24: 2-7.
- van Gemert MJ, Welch A (1989) Clinical use of laser-tissue interactions. IEEE Eng Med Biol Mag 8: 10-13.
- Small R (2015) A practical guide to laser procedures. Lippincott Williams & Wilkins.
- Tanghetti Md E, Jennings J (2018) A comparative study with a 755 nm picosecond Alexandrite laser with a diffractive lens array and a 532 nm/1064 nm Nd:YAG with a holographic optic. Lasers Surg Med 50: 37-44.
- Babilas P, Schreml S, Szeimies R-M, Landthaler M (2010) Intense pulsed light (IPL): A review. Lasers in Surgery and Medicine 42: 93-104.
- Husain Z, Alster TS (2016) The role of lasers and intense pulsed light technology in dermatology. Clinical, cosmetic and investigational dermatology 9: 29.
- Trivedi M, Yang F, Cho B (2017) A review of laser and light therapy in melasma. Int J Womens Dermatol 3: 11-20.
- Anderson RR (2000) Lasers in dermatology-a critical update. J Dermatol 27: 700-705.
- Fitzpatrick RE, Goldman MP, Ruiz?Esparza J (1994) Clinical advantage of the CO2 laser superpulsed mode: treatment of verruca vulgaris, seborrheic keratoses, lentigines, and actinic cheilitis. The J Dermatol Surg Oncol 20: 449-456.
- Choi B, Welch AJ (2001) Analysis of thermal relaxation during laser irradiation of tissue. Lasers in Surgery and Medicine: Laser in Surgery and Medicine 29: 351-359.
- Kono, T., Ogawaa N, Gonomeb H, Maheswari U, Yamadaa RJ (2021) A local rapid temperature rise model for analyzing the effects of irradiation on human skin in laser treatments. International Journal of Heat and Mass Transfer 171: 121078.
- Nilsen LTN ()1996 Epidermal melanin absorption in human skin. in Laser-Tissue Interaction and Tissue Optics. International Society for Optics and Photonics.
- Goo BL, Clinical effectiveness of low-fluence 585 nm Q-switched Nd: YAG laser treatment on persistent facial erythema after adult type acne treatment: A Preliminary Study.
- Lukac M, Vizintin Z, Zabkar J, Pirnat S (2009) QCW pulsed Nd: YAG 1064 nm laser lipolysis. J Laser Health Acad 4: 24-34.
- Jung JY, Hong JS, Ahn CH, Yoon JY, Kwon HH, et al. (2012) Prospective randomized controlled clinical and histopathological study of acne vulgaris treated with dual mode of quasi-long pulse and Q-switched 1064-nm Nd: YAG laser assisted with a topically applied carbon suspension. J Am Acad Dermatol 66: 626-633.
- Gorsic M, Bacak I, Ahcan UG, Topcic VH (2013) Evaluation of the efficacy of tattoo-removal treatments with Q-switch laser. J Laser and Health Academy Page no: 21-6.
- Wu DC, Goldman MP, Wat H, Chan HHL (2021) A systematic review of picosecond laser in dermatology: evidence and recommendations. Lasers Surg Med 53: 9-49.
- Town G, Ash C, Eadie E, Moseley H (2007) Measuring key parameters of intense pulsed light (IPL) devices. J Cosmet Laser Ther 9: 148-160.
- Stellar M22 - Lumenis.
- Icon Cynosure.
- Alexiades-Armenakas MR, Dover JS, Arndt KA (2008) The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol 58: 719-737.
- Lumenis M22 operator manual.
- Ash C, Dubec M, Donne K, Bashford T (2017) Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med Sci 32: 1909-1918.
- Lister T, Wright PA, Chappell PH (2012) Optical properties of human skin. J Biomed Opt 17: 090901.
- Galadari I (2003) Comparative evaluation of different hair removal lasers in skin types IV, V, and VI. Int J Dermatol 42: 68-70.
- Roberts WE (2009) Skin type classification systems old and new. Dermatol Clin 27: 529-533.
- Fasugba O, Gardner A, Smyth W (2014) The Fitzpatrick skin type scale: a reliability and validity study in women undergoing radiation therapy for breast cancer. J Wound Care 23: 358-368.
- Matias AR, Ferreira M, Costa P, Neto P (2015) Skin colour, skin redness and melanin biometric measurements: comparison study between Antera® 3D, Mexameter® and Colorimeter®. Skin Res Technol 21: 346-362.
- Yaroslavsky I, Childs J, Altshuler GB, Zenzie HH, Cohen R (2017) Objective measurement device for melanin optical density: dosimetry for laser and ipls in aesthetic treatments.
- Lloyd AA, Graves MS, Ross EV (2018) Epidermal Fluence Threshold Determination by Real-Time Melanin Measurements. Dermatol Surg 44: 1427-1436.
- Dmovsek-Olup B, Vedlin B (1997) Use of Er: YAG laser for benign skin disorders. Lasers in Surgery and Medicine: Lasers Surg Med 21: 13-19.
Citation: Alhallak K, Omran D, Tomi S, Abdulhafid A (2021) Skin, Light and their Interactions, an In-Depth Review for Modern Light-Based Skin Therapies. J Clin Dermatol Ther 7: 081.
Copyright: © 2021 Kamal Alhallak, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

