Journal of Non Invasive Vascular Investigation Category: Clinical
Type: Review Article
Spontaneous Cervical Arterial Dissection: Several Aspects of Magnetic Resonance Imaging
*Corresponding Author(s):
Dreval\' Marina VladimirovnaDepartment Of Radiology, Research Center Of Neurology, Junior Researcher, Moscow, Russian Federation
Tel:(+7) 495 490 2205,
Email:dreval@neuroradiology.ru
Received Date: Oct 11, 2016
Accepted Date: Feb 28, 2019
Published Date: Mar 14, 2019
Abstract
Spontaneous dissection of internal carotid and vertebral arteries is one of the little-known and under investigated causes of ischemic stroke, especially in young and middle-age patients. Precise and quick diagnosis of this condition has the crucial importance as the timely started treatment may decrease the risk of stroke and minimize the resulting neurological deficit. Due to variability of clinical findings, imaging of the brain tissue and major vessels of head and neck plays the leading role in the diagnosis. This article reviews the role of Magnetic Resonance Imaging (MRI) and Magnetic Resonance Angiography (MRA) in the diagnosis of cervical arteries dissection with particulate focus on the MRI and angiographic signs of dissection, their changes over time, possible pitfalls in the interpretation of the radiological findings, and differential diagnosis with other constrictive and occlusive lesions of arteries. We suggest our own protocol of MRI examination in suspected spontaneous dissection of inner carotid and vertebral arteries.
Keywords
Ischemic stroke; Magnetic resonance imaging; Spontaneous internal carotid and Vertebral artery dissection
BACKGROUND
Within the last 10 years we observe the trend towards the decrease of the age of cerebrovascular diseases victims, and stroke morbidity and mortality in individuals of economically active age group increased for over 30% [1,2]. In young and middle-aged patients major causes of stroke differ from those in elderly population, as they are represented by cardiac embolism and spontaneous dissection of the major arteries of the neck supplying blood to the brain. Spontaneous Artery Dissection (SAD) is one of the little-known and under investigated conditions; however, it accounts for about one third [3], or even half of all ischemic strokes in patients under the age of 50 [4].
OBJECTIVE
To give an overview of spontaneous dissection of inner carotid and vertebral arteries as one of the main causes of ischemic stroke in young patients, and to discuss strengths and weaknesses of MRI and MRA in diagnosis of this condition.
Definition, epidemiology and pathogenesis
The term “dissection” means intrusion of blood from the lumen of the vessel to the arterial wall through the intimal tear, and its expansion within the layers, more often between intima and media [5]. Blood accumulated within the wall (intramural haematoma; IMH) causes stenosis or occlusion of artery. Propagation of the blood to the adventitia results on the formation of the dissecting aneurism. Blood accumulated in the wall can tear intima in more distal part of artery and become connected with the main blood flow that results in formation of the double (false and true) lumen of artery [6].
SAD incidence is approximately 5 cases per 100,000/year [7]. These numbers are probably undersized since dissection as the cause of ischemic stroke often remains undetected, or it may be associated with subtle clinical symptoms or even no symptoms at all, and may become evident only on neuroimaging study, angiography or ultrasound imaging. SAD rates are similar in men and women, and in vast majority of cases (90%) it affects young people [6].
According to pathological studies, the cause of SAD may be dysplastic changes of artery wall, and, substantially less often, undifferentiated connective tissue disorders, arteritis, Ehlers-Danlos vascular syndrome, Marfan syndrome, cystic medial necrosis [7,8]. Some authors suggest that arteriopathy underlying SAD may be temporary [3,9]. The provoking factors can be minor injury of head or neck, physical strain, manipulations with neck, long-lasting uncomfortable position of head, preceding infection [6].
Dissection more often develops in the extra-cranial parts of the vessels, namely in the cervical part of Internal Carotid Artery (ICA) and in V2 and V3 segments of Vertebral Artery (VA). This may be explained by greater mobility of the arteries on these levels and their anatomic proximity to osseous structures (e.g., cervical vertebrae, styloid process) [4].
SAD incidence is approximately 5 cases per 100,000/year [7]. These numbers are probably undersized since dissection as the cause of ischemic stroke often remains undetected, or it may be associated with subtle clinical symptoms or even no symptoms at all, and may become evident only on neuroimaging study, angiography or ultrasound imaging. SAD rates are similar in men and women, and in vast majority of cases (90%) it affects young people [6].
According to pathological studies, the cause of SAD may be dysplastic changes of artery wall, and, substantially less often, undifferentiated connective tissue disorders, arteritis, Ehlers-Danlos vascular syndrome, Marfan syndrome, cystic medial necrosis [7,8]. Some authors suggest that arteriopathy underlying SAD may be temporary [3,9]. The provoking factors can be minor injury of head or neck, physical strain, manipulations with neck, long-lasting uncomfortable position of head, preceding infection [6].
Dissection more often develops in the extra-cranial parts of the vessels, namely in the cervical part of Internal Carotid Artery (ICA) and in V2 and V3 segments of Vertebral Artery (VA). This may be explained by greater mobility of the arteries on these levels and their anatomic proximity to osseous structures (e.g., cervical vertebrae, styloid process) [4].
Clinical presentation
The most often clinical signs of internal carotid artery and vertebral artery dissection include cerebral or retinal ischemia, unilateral headache, face or neck pain, and Horner syndrome. More rare signs include dysfunction of cranial nerves and throbbing noise in ears [6,7]. In almost every patient artery dissection is accompanied by pain. In ICA lesion the pain is localized in the frontotempoparietal region, on the anterior surface of neck, in the orbital area. VA dissection is characterized by occipital headache and pain on the posterior surface of neck [3,8]. Horner syndrome (ptosis, meiosis and enophthalmos) is the typical presentation of ICA dissection and may be seen in half of patients. Horner syndrome develops ipsilaterally, and in some cases it can be the only manifestation of dissection [8]. Ischemic stroke or transient ischemic attack is the most serious presentations of dissection of ICA and VA. Stroke is usually associated with favourable prognosis for life and good recovery of neurological deficit [3]. Cerebrovascular accident develops due to the stenosis or occlusion of the artery, or due to the embolism of distal branches of intracranial arteries with thrombotic masses originated from the site of the dissection [7].
Imaging diagnosis
Radiological methods play the leading role in the diagnosis of SAD [3]. Digital Subtraction Angiography (DSA) was the “golden standard” of SAD diagnostics for a long time, but recently it was shown that less invasive methods as Magnetic Resonance Angiography (MRA) and Magnetic Resonance Imaging (MRI) also have high sensitivity and specificity in the detection of artery dissection and subsequent monitoring of abnormal findings.
MRA includes Time of Flight (TOF), Phase Contrast (PC) and Contrast Enhanced (CE) techniques. Like digital subtraction angiography, all MRA methods allow revealing of typical signs of abnormal changes of artery lumen due to dissection. They include extended regular (“string sign”; Figure 1) or irregular stenosis (“wavy ribbon sign”; Figures 2, ? and b). Narrowing of internal carotid artery usually occurs in its cervical part (on the level of C1 and C2 cervical vertebrae), starts for 2-3 cm more distally than the bulb and ends before the entrance to petrous part of temporal bone. Stenosis of vertebral artery is usually localized in the V2 segment on the level of C3, C4 and C5 cervical vertebrae [10]. More than in 20% cases the VA dissection includes intracranial expansion of the lesion [4].
MRA includes Time of Flight (TOF), Phase Contrast (PC) and Contrast Enhanced (CE) techniques. Like digital subtraction angiography, all MRA methods allow revealing of typical signs of abnormal changes of artery lumen due to dissection. They include extended regular (“string sign”; Figure 1) or irregular stenosis (“wavy ribbon sign”; Figures 2, ? and b). Narrowing of internal carotid artery usually occurs in its cervical part (on the level of C1 and C2 cervical vertebrae), starts for 2-3 cm more distally than the bulb and ends before the entrance to petrous part of temporal bone. Stenosis of vertebral artery is usually localized in the V2 segment on the level of C3, C4 and C5 cervical vertebrae [10]. More than in 20% cases the VA dissection includes intracranial expansion of the lesion [4].
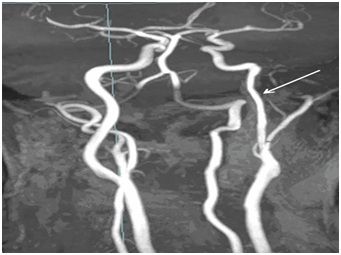
Figure 1: 3D TOF MRA, MIP. Regular stenosis (arrow) of cervical part of left internal carotid artery on the level of ?1-?3 vertebrae.
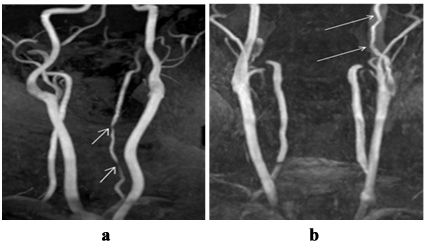
Figure 2: 3D TOF MRA, MIP: ? - irregular stenosis of V2 segment of left vertebral artery on the level of ?4-?7 vertebrae (arrows); b - irregular stenosis of cervical part of left internal carotid artery on the level of ?1-?3 vertebrae (arrows).
In the occlusion of ICA due to the dissection, angiographic signs are less specific; as the similar angiographic presentation is observed in occlusion resulted from atherothrombosis. The typical sign of ICA occlusion due to the dissection that may be seen in some patients is the pre-occlusive tapered stenosis, known as the “candle flame” or “rat tail” sign (Figure 3) [11].
In the occlusion of ICA due to the dissection, angiographic signs are less specific; as the similar angiographic presentation is observed in occlusion resulted from atherothrombosis. The typical sign of ICA occlusion due to the dissection that may be seen in some patients is the pre-occlusive tapered stenosis, known as the “candle flame” or “rat tail” sign (Figure 3) [11].
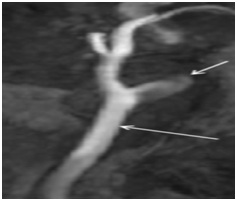
Figure 3: MRA of patient with spontaneous dissection of left internal carotid artery, MIP. Tapered pre-occlusion narrowing of internal carotid artery (“candle flamesign”, short arrow). Common carotid artery is patent (long arrow).
Other pathognomonic angiographic signs of dissection are formation of dissecting aneurysm (Figures 4, ? and b) and double artery lumen (true and false; Figures 5, ? and b).
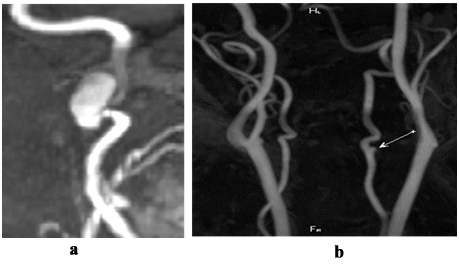
Figure 4: MRA, MIP: ? - dissection of cervical part of internal carotid artery with formation of dissecting aneurysm (arrow); b - dissection of V2 segment of vertebral artery with formation of dissecting aneurysm (arrow).
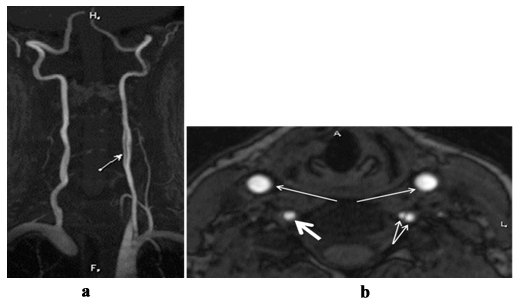
Figure 5: MRA, MIP (?) - double lumen of left vertebral artery on the level of C4-C5 vertebrae (arrow); MRA, axial plane (b) - double lumen (true and false) of left vertebral artery (thick arrows). Right vertebral artery (thick arrow) and common carotid arteries (long arrows) are normal.
Dissecting aneurysm is the local broadening of artery lumen and is often located precranialy in cervical part of ICA or in V2 segment of VA [12; 3]. In our recent study we demonstrated that the most often angiographic signs of dissection of VA and ICA is the irregular prolonged stenosis, while other typical signs are seen significantly less often; e.g., tapered pre-occlusive narrowing of artery was seen only in ICA (28%), and in VA this sign was not detected. Formation of double lumen is characteristic only for VA and it was observed only in 8% of cases [6].
The advantage of MRA compared to DSA is the opportunity to visualize the arterial wall and to reveal the signs of IMH. For this purpose ?1 fat suppression (f/s) weighted image (WI) and ?2 f/s WI in parallel (coronary) and perpendicular (axial) planes relatively to the path of main arteries of neck are used. Dissection is associated with the increase of outer diameter of artery, decrease of diameter and eccentric positioning of artery lumen. IMH in the axial plane is seen as an area of semi lunar form with sharp contours that surrounds the arterial lumen like a cuff. Intensity of MR-signal from IMH is determined by paramagnetic properties of products of haemoglobin decay, and its changes over time have some common features with the decay of intracerebral haematoma (Figure 6) [10].
Dissecting aneurysm is the local broadening of artery lumen and is often located precranialy in cervical part of ICA or in V2 segment of VA [12; 3]. In our recent study we demonstrated that the most often angiographic signs of dissection of VA and ICA is the irregular prolonged stenosis, while other typical signs are seen significantly less often; e.g., tapered pre-occlusive narrowing of artery was seen only in ICA (28%), and in VA this sign was not detected. Formation of double lumen is characteristic only for VA and it was observed only in 8% of cases [6].
The advantage of MRA compared to DSA is the opportunity to visualize the arterial wall and to reveal the signs of IMH. For this purpose ?1 fat suppression (f/s) weighted image (WI) and ?2 f/s WI in parallel (coronary) and perpendicular (axial) planes relatively to the path of main arteries of neck are used. Dissection is associated with the increase of outer diameter of artery, decrease of diameter and eccentric positioning of artery lumen. IMH in the axial plane is seen as an area of semi lunar form with sharp contours that surrounds the arterial lumen like a cuff. Intensity of MR-signal from IMH is determined by paramagnetic properties of products of haemoglobin decay, and its changes over time have some common features with the decay of intracerebral haematoma (Figure 6) [10].
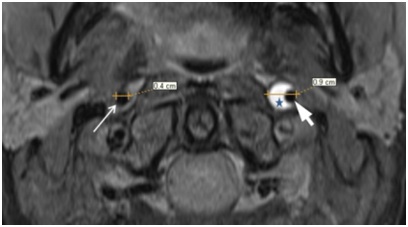
Figure 6: MRI, ?1 f/s, axial plane: outer diameter of left internal carotid artery is broadened up to 0,9 cm, lumen of artery is narrowed and located out of centre (thick arrow), surrounded by area of semilunar form (IMG) with sharp contours of hyperintensive MR-signal (asterisk). Right internal carotid artery is not changed, its diameter is 0,4cm (thin arrow).
In the acute stage of the dissection (on days from 1 to 3) IMH has is intensive signal on?1WI (Figure 7, ?) and hypo-intensive signal on ?2WI (Figure8, ?), that makes it difficult to distinguish it from surrounding tissues. Then MR-signal from IMH becomes slightly hyper intensive on ?1WI (Figure 7, b) and high intensive on ?2 (Figure 8, b). By the end of the first week signal from IMH becomes hyper intensive on ?2WI as well as on ?1WI sequences (Figure 7, c).
In the acute stage of the dissection (on days from 1 to 3) IMH has is intensive signal on?1WI (Figure 7, ?) and hypo-intensive signal on ?2WI (Figure8, ?), that makes it difficult to distinguish it from surrounding tissues. Then MR-signal from IMH becomes slightly hyper intensive on ?1WI (Figure 7, b) and high intensive on ?2 (Figure 8, b). By the end of the first week signal from IMH becomes hyper intensive on ?2WI as well as on ?1WI sequences (Figure 7, c).
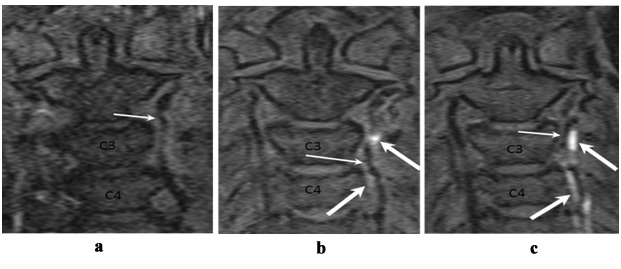
Figure 7: MRI, ?1 f/s WI, coronary plane: ? - Day 1 after clinical manifestation. No abnormal changes of MR signal intensity in the left vertebral artery wall are seen, arterial lumen is irregularly narrowed (arrow); b - Day 3 after clinical manifestation. MR signal intensity in the left vertebral artery wall on the level of C3-C4 vertebrae is increased lightly incoherently (thin arrows), arterial lumen is irregularly narrowed (thin arrow); c - Day 7 after clinical manifestation. Incoherent hyperintensive MR signal in the left vertebral artery wall on the level of ?3-?5 vertebrae (thick arrows), arterial lumen is irregularly narrowed (thin arrow).
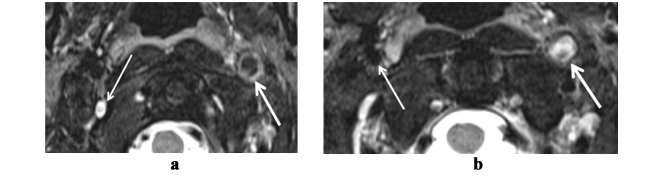
Figure 8: MRI: ? - ?2 WI, axial plane. Dissection of left internal carotid artery (thick arrow), day 2 after clinical manifestation. Hypointensive MR-signal in intramural haematoma, lumen of the artery is not visualized, outer diameter of artery is increased. Right internal carotid artery is normal (thin arrow); b - ?2 f/s WI, axial plane. Dissection of left internal carotid artery, day 4 after clinical manifestation. Hyperintensive MR-signal fromintramural haematoma, lumen of the artery is not visualized (thick arrow). Right internal carotid artery is normal (thin arrow).
Gradual further decrease of signal intensity in IMH leads to inability to visualize it in 2-3 months after hematoma formation [12]. The issue of differentiating diagnosis of intra-arterial thrombosis and dissection that caused occlusion of artery lumen often arise [13]. Usually intra-arterial thrombosis is caused by white thrombus. To our data, it produces less intensive signal on?1WI (Figure 9, b) compared to IMH (Figure 9, ?), since it contains less haemoglobin and products of haemoglobin decay than IMH [13]. Another distinguishing feature of IMH vs. intra-arterial thrombosis is increase of outer arterial diameter in case of dissection.
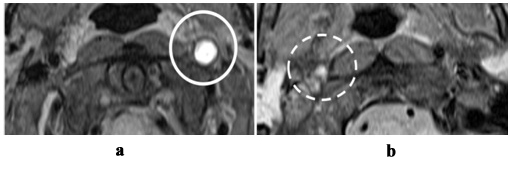
Figure 9: MRI, ?1 f/s WI, axial plane: ? - occlusion of left internal carotid artery at the result of dissection. Hyperintensive MR-signal in intramural haematoma, outherarterial diameter is increased, lumen is not visalized; b - occlusion of right internal carotid artery at the result of intra-arterial thrombosis. Intra-arterial thrombosis is characterized by slightly hyperintensive MR-signal; outherarterial diameter is not increased, lumen is not visualized.
Hyper-intensive signal from fat tissue surrounding artery can be falsely identified as IMH. Therefore, imaging sequences with fat suppression are of high importance [10]. Hyper-intensive MR-signal in vertebral venous plexus in transverse openings of cervical vertebrae due to slow blood flow may mimic sub acute IMH in the vertebral artery thus producing false-positive result. One should carefully assess the arterial lumen (it is normal if hyper-intensive signal comes from the venous plexus) and to check if there is a linear area of decreased MR signal intensity between the lumen of artery and IMH corresponding to intima on TOF MRA [14]. Atheromatous plaque may imitate local dissection of artery appearing as hyper-intensive rim on ?2WI. However, this rim usually produces isointensive MR signal on ?1WI by contrast with hyper-intensive sub acute IMH [15]. False-negative results are often in first days after the lesion, when IMH does not produce hyper-intensive signal and blends into surrounding tissues (Figure 7, ?).Therefore, in hyper acute stage of suspected dissection careful estimation of MRA data for the signs of abnormality within arterial lumen is essential [3].
In addition to aforementioned ?1WI and ?2WI, TOF MRA may be also used for IMH imaging. This MRA technique provides an opportunity to visualize IMH due to lack of signal suppression in surrounding tissues with short T1 time (e.g., methaemoglobin within IMH) (Figure 10) [5].
Hyper-intensive signal from fat tissue surrounding artery can be falsely identified as IMH. Therefore, imaging sequences with fat suppression are of high importance [10]. Hyper-intensive MR-signal in vertebral venous plexus in transverse openings of cervical vertebrae due to slow blood flow may mimic sub acute IMH in the vertebral artery thus producing false-positive result. One should carefully assess the arterial lumen (it is normal if hyper-intensive signal comes from the venous plexus) and to check if there is a linear area of decreased MR signal intensity between the lumen of artery and IMH corresponding to intima on TOF MRA [14]. Atheromatous plaque may imitate local dissection of artery appearing as hyper-intensive rim on ?2WI. However, this rim usually produces isointensive MR signal on ?1WI by contrast with hyper-intensive sub acute IMH [15]. False-negative results are often in first days after the lesion, when IMH does not produce hyper-intensive signal and blends into surrounding tissues (Figure 7, ?).Therefore, in hyper acute stage of suspected dissection careful estimation of MRA data for the signs of abnormality within arterial lumen is essential [3].
In addition to aforementioned ?1WI and ?2WI, TOF MRA may be also used for IMH imaging. This MRA technique provides an opportunity to visualize IMH due to lack of signal suppression in surrounding tissues with short T1 time (e.g., methaemoglobin within IMH) (Figure 10) [5].
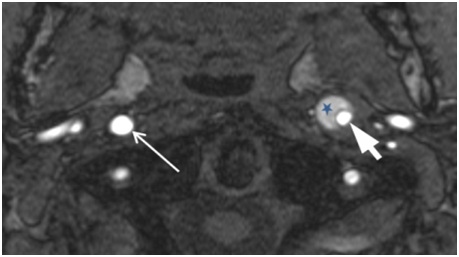
Figure 10: Magnetic resonance angiography, axial plane. In the wall of left internal carotid artery there is an area with sharp borders of semilunar form (intramural haematoma) with hyperintesive MR signal (star), arterial lumen is narrowed (thick arrow), located eccentrically, outer diameter is increased. Right internal carotid artery is normal (thin arrow).
Besides assessment of raw TOFMRA data, reconstructed Maximum Intensity Projection (MIP) image analysis is also extremely important. Usually MR signal from blood flow has higher intensity than signal from IMH. Source (raw) data allows visualization of the whole circle (diameter) of artery, and MIP reconstruction allows visualization of length and precise localization of dissection [10]. There are several most common mistakes of TOF MRA image interpretation in suspected dissection. In some cases IMH is not revealed, as hyper-intensive signal from products of haemoglobin decay (methaemoglobin) mimics blood flow on MIP images (Figures 11, ? and b). Moreover, artefact of sensitivity from skull base bones on MIP images sometimes appears as irregularnarrowing of proximal part of petrous segment of internal carotid artery and by mistake, it may be considered as dissection, especially if this narrowing is asymmetrical [10].
Besides assessment of raw TOFMRA data, reconstructed Maximum Intensity Projection (MIP) image analysis is also extremely important. Usually MR signal from blood flow has higher intensity than signal from IMH. Source (raw) data allows visualization of the whole circle (diameter) of artery, and MIP reconstruction allows visualization of length and precise localization of dissection [10]. There are several most common mistakes of TOF MRA image interpretation in suspected dissection. In some cases IMH is not revealed, as hyper-intensive signal from products of haemoglobin decay (methaemoglobin) mimics blood flow on MIP images (Figures 11, ? and b). Moreover, artefact of sensitivity from skull base bones on MIP images sometimes appears as irregularnarrowing of proximal part of petrous segment of internal carotid artery and by mistake, it may be considered as dissection, especially if this narrowing is asymmetrical [10].
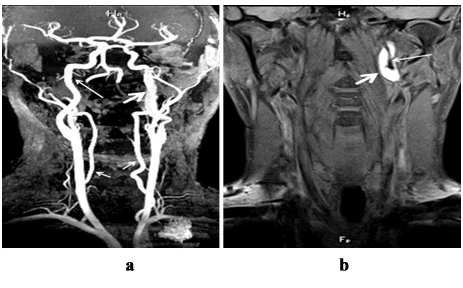
Figure 11: Dissection of cervical part of left internal carotid artery, 3 after clinical presentation: ? - magnetic resonance angiogram, MIP reconstruction. Hyperintensive signal from IMH in the wall of left internal carotid artery mimicsnormal blood flow (thick arrow), on this level the diameter of the artery is increased. Right internal carotid artery (long arrow) and both vertebral arteries (short arrows) are normal; b -?1 f/sWI, coronary plane. In the wall of left internal carotid artery the area of hyperintensive magnetic resonance signal is seen, outer diameter of artery is increased (thick arrow), internal lumen is narrowed (long arrow).
It should be mentioned that another technique of non-contrast MRA, Phase Contrast (PC) ?R?, as well as Contrast-Enhanced (??) MRA depicts only lumen of the artery and does not allow direct IMH detection. PC MRA is used in diagnosis of dissection more rarely than TOFMRA or CE MRA. The drawback of ?? MRA in comparison with DSA is lower spatial and temporal resolution; however, minimal invasiveness, absence of ionizing radiation, availability of the technique in outpatient setting are amongits undeniable advantages. The disadvantage of TOFMRA is low sensitivity to slow blood flow and poor ability to visualize curved portions of blood vessels due to lack of laminar circulation of blood and formation of turbulence. The advantage of ?? MRA and TOFMRA in comparison to DSA is readily available extension of the study with ?2 f/s and ?1 f/s WIs with focus on stenotic portion of the artery to prove or to exclude dissection. Combination of MRI and MRA provides not only effective diagnostics of dissection, but it also can be used as non-invasive approach to monitor IMH regress over time or development of complications [16].
As the development of dissection is dynamic process, changes of IMH are associated with changing of angiographic picture. Thus, stenosis of internal carotid artery and vertebral artery resolve completely or partially in 2-3 months that is the important sign of dissection (Figures 12, ? and d).
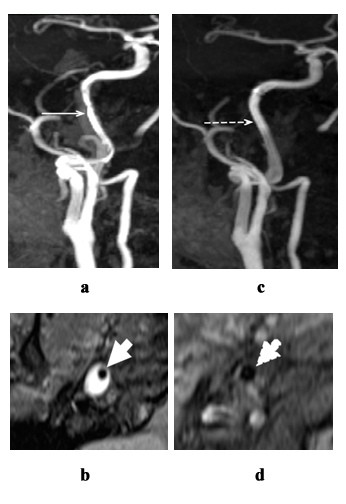
Figure 12: Changes of magnetic resonance imaging and magnetic resonance angiography in spontaneous dissection of right internal carotid artery over time; 3 weeks after clinical presentation; ?, b - irregular stenosis of right internal carotid artery; in 3 months: c, d - blood flow recovery in right internal carotid artery; ?, c - magnetic resonance angiogram, MIP reconstruction; b, d - ?1 f/s weighted image, axial plane. Hyperintensive signal from IMH in the wall of right internal carotid artery (b - star). Irregular narrowing of lumen of right internal carotid artery (?, b - solid arrows). No abnormal changes of signal from the wall of right internal carotid artery are not revealed, blood flow resolved (c, d - broken arrows).
By contrast, occlusions due to dissection resolve only in half of cases [4], and the level of residual stenos is in all cases is usually does not exceed 20% (Figures 13, ? and d) [17].
By contrast, occlusions due to dissection resolve only in half of cases [4], and the level of residual stenos is in all cases is usually does not exceed 20% (Figures 13, ? and d) [17].
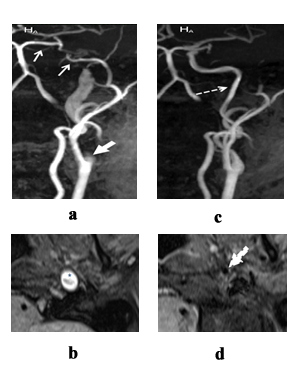
Figure 13: Changes of magnetic resonance imaging and magnetic resonance angiography in spontaneous dissection of left internal carotid artery over time; 2 weekls afer clinical presentation; ?, b - occlusion of left internal carotid artery; in 3 months: c, d - blood flow recovery in left internal carotid artery; ?, c - magnetic resonance angiogram, MIP reconstruction; b, d - magnetic resonance in ?1 f/s weighted image, axial plane. Hyperintensive signal from IMH in the wall of left internal carotid artery (b - star). Pre-occlusive conic narrowing of left internal carotid artery is the “candle flame” sign. (? - short thick arrow). Blood flow in left internal coronary artery is not seen (? - short thin arrows). No abnormal changes of signal from the wall of left internal carotid artery is revealed, blood flow is resolved (c, d - dotted arrows).
In 1/3 of cases dissecting aneurysms regress completely, about 40% decrease in size. However, the double lumen of artery that developed in the acute period of dissection remains unchanged [4].
In 1/3 of cases dissecting aneurysms regress completely, about 40% decrease in size. However, the double lumen of artery that developed in the acute period of dissection remains unchanged [4].
CONCLUSION
In patients with acute stroke, especially in young and middle age individuals without atherosclerosis, cerebral artery dissection should be considered, in particular in case when typical clinical characteristics are present (unilateral headache/pain in the neck, history of common provoking factors). For primary diagnostics it is necessary to choose the methods that allow visualization of artery wall (to detect IMH), arterial lumen (to detect angiographic signs of dissection) and brain tissue. For this purpose, MRI and MRA combination is used. A number of authors suggest the following protocol: ?1 and ?2 WIs of major arteries of head and neck with fat suppression in conjunction with ?? MRA (or 3D TOFMRA if gadolinium enhancement is contraindicated) to detect abnormalities of arteries, and MRI of brain tissue to detect cerebral lesion [1; 12].
In the Research Center of Neurology we use the following protocol for patients with suspected cerebral artery dissection:
- MRI of brain tissue(?2, ?1, ?2 FLAIR, SWI, DWI with ADC mapping);
- 3D TOFMRA of extra and intracranial arteries;
- ?1 f/s and ?2 f/s WIs of major arteries of head and neck in coronary and axial planes.
In the Research Center of Neurology we use the following protocol for patients with suspected cerebral artery dissection:
- MRI of brain tissue(?2, ?1, ?2 FLAIR, SWI, DWI with ADC mapping);
- 3D TOFMRA of extra and intracranial arteries;
- ?1 f/s and ?2 f/s WIs of major arteries of head and neck in coronary and axial planes.
REFERENCES
- Tanashyan MM, Lagoda OV, Antonova KV (2011) Cerebrovascular diseases and metabolic syndrome. M. OAO. 24 c.
- Stakhovskaya LV, Kotov SV (2013) Stroke: Handbook for physicians. In: Meditsinskoeinformatsionnoeagentstvo, Pg no: 13.
- Engelter ST, Traenka C, Von Hessling A, Lyrer PA (2015) Diagnosis and treatment of cervical artery dissection. Neurol Clin 33: 421-441.
- Kalashnikova LA, Dobrynina LA (2013) Cerebral Artery Dissection: ischemic stroke and other clinical manifestations. M Vako: 72-123.
- Ben Hassen W, Machet A, Edjlali-Goujon M, Legrand L, Ladoux A, et al. (2014) Imaging of cervical artery dissection. Diagn Interv Imaging 95: 1151-1161.
- Kalashnikova LA, Dobrynina LA, Dreval' MV, Nazarova MA (2014) [Clinical characteristics of internal carotid and vertebral arteries dissection]. Zh Nevrol Psikhiatr Im S S Korsakova 114: 4-8.
- Schievink WI, Debette S (2011) Etiology of cervical artery dissections: the writing is in the wall. Neurology 76: 1452-1453.
- Kalashnikova LA, Sakharova AV, Dobrynina LA, Mir-Kasimov MF, Cha?kovskaia RP, et al. (2010) [Mitochondrial arteriopathy as a cause of spontaneous dissection of cerebral arteries]. Zh Nevrol Psikhiatr Im S S Korsakova 110: 3-11.
- Rahme RJ, Aoun SG, McClendon J Jr, El Ahmadieh TY, Bendok BR (2013) Spontaneous cervical and cerebral arterial dissections: diagnosis and management. Neuroimaging Clin N Am 23: 661-671.
- Medel R, Starke RM, Valle-Giler EP, Martin-Schild S, El Khoury R, et al. (2014) Diagnosis and treatment of arterial dissections. Curr Neurol Neurosci Rep 14: 419.
- Debette S, Grond-Ginsbach C, Bodenant M, Kloss M, Engelter S, et al. (2011) Differential features of carotid and vertebral artery dissections: the CADISP study. Neurology 77: 1174-1181.
- Habs M, Pfefferkorn T, Cyran CC, Grimm J, Rominger A, et al. (2011) Age determination of vessel wall hematoma in spontaneous cervical artery dissection: a multi-sequence 3T cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 13: 76.
- Dreval M, Krotenkova M, Kalashnikova L, Dobryinina E, Konovalov R (2013) Differential diagnosis of cervical artery dissection and intra-arterial thrombosis using MRI and MRA. Insights Imaging 4: 230.
- Naggara O, Louillet F, Touzé E, Roy D, Leclerc X, et al. (2010) Added value of high-resolution MR imaging in the diagnosis of vertebral artery dissection. AJNR Am J Neuroradiol 31: 1707-1712.
- Kurosaki Y, Yoshida K, Fukumitsu R, Sadamasa N, Handa A, et al. (2015) Carotid artery plaque assessment using quantitative expansive remodeling evaluation and MRI plaque signal intensity. J Neurosurg 11: 1-7.
- Coppenrath EM, Lummel N, Linn J, Lenz O, Habs M, et al. (2013) Time-of-flight angiography: a viable alternative to contrast-enhanced MR angiography and fat-suppressed T1w images for the diagnosis of cervical artery dissection? Eur Radiol 23: 2784-2792.
- Dreval M , Krotenkova M, Kalashnikova L, Dobryinina L, Konovalov R (2015) Patency dynamics of spontaneous cervical arteries dissection. Neuroradiology 57: 45.
Citation: Vladimirovna DM, Andreevna KL, Anatol'evna DL, Nikolaevna SA, Viktorovna DE, et al. (2019) Spontaneous Cervical Arterial Dissection: Several Aspects of Magnetic Resonance Imaging. J Non Invasive Vasc Invest 4: 014.
Copyright: © 2019 Dreval\' Marina Vladimirovna, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!