
Stroke Patients: Effects of Combining Sitting Table Tennis Exercise with Neurological Physical Therapy on Brain Waves
*Corresponding Author(s):
Yongseong KimDepartment Of Physical Therapy, Nambu University, Republic Of Korea
Email:kimys2492@nambu.ac.kr
Abstract
Purpose: The purpose of this study is to analyze the brain waves and develop various exercise programs to improve the physical and mental aspects of stroke patients when neurological physical therapy and sitting table tennis exercise are applied to stroke patients.
Methods: In this study, an experiment was conducted on 15 patients diagnosed with stroke, and training was performed after changing the ping-pong table to a sitting position to apply ping-pong exercise to stroke patients. After training was conducted for 40 minutes twice a week for 4 weeks, brain waves were measured before and after. EEG was measured using Laxtha's DSI-24 equipment as a measurement tool, and data values were extracted through the Telescan program.
Results: Most of the relative beta waves showed a significant difference before and after the intervention. As for the characteristics of beta waves, this result can be seen as being highly activated during exercise or other activities.
Conclusion: Ping-pong exercise in a sitting position is a good intervention method for stroke patients, and it can help to use it as basic data in clinical practice by showing brain activity.
Keywords
Stroke; Sitting position; Ping-pong exercise; Brain wave
Introduction
Stroke is the third leading cause of death after cancer and heart disease, and its prevalence is increasing. Stroke is a disruption of the blood supply to the brain or bleeding into the brain tissue, resulting in loss of brain function, primarily motor and sensory nerves [1,2]. Approximately 80% of stroke patients have motor impairment, with more than 69% of hemiparetic patients experiencing upper limb motor impairment [3,4]. Even six months after stroke, approximately 50% of patients have chronic upper extremity deficits that limit their activities of daily living, leading to social and economic burdens, making upper extremity rehabilitation an important part of stroke care [5].
Upper extremity function and hand dexterity are crucial, given that most activities of daily living are performed with the hands [6]. Impaired upper extremity motor function impairs the ability of stroke patients to perform activities of daily living and limits their social participation [7]. Therefore, upper extremity motor function rehabilitation is important, and there are several intervention methods for upper extremity motor function rehabilitation, including exercise training robot rehabilitation, bilateral upper extremity training activities, forced guided exercise therapy, mirror therapy, and unilateral upper extremity exercises [8]. Among them, table tennis, a unilateral upper extremity exercise, can help improve upper extremity motor function by using the hands and arms to hit the ball [9].
In addition, table tennis is an activity that involves constant interaction between visual perceptual information and movement execution [10], requiring efficient visual navigation strategies for accurate, concise, and fast movement execution [11]. The region of the brain involved in the development of visual perception, attention, and memory is the parietal lobe, which houses eye movement control functions [12]. Among the various eye movements, rapid eye movements are the fastest eye movements that track moving objects and provide sensory and reflexive visual stimuli [13]. The posterior parietal lobe is located at the back end of the cerebral cortex, where we receive information through vision and is responsible for interpreting visual information [14]. Eye movements can also be considered physical therapy for the visual system, which includes the brain and eyes, and are progressive motor skills of the eye that restore or develop normal seeing skills [15]. Overall, table tennis is a great sport for eye movements because the ball movement is faster and sharper than other sports.
EEG is a technology that measures electrical activity in the brain and provides information about the brain's health by analyzing patterns of activity. Stroke is a brain injury caused by impaired circulation in the brain, and changes in EEG play an important role in the diagnosis and treatment of stroke. Therefore, EEG can be used as a very useful tool for early diagnosis, prevention, treatment, and rehabilitation of stroke [16]. We looked at studies that applied EEG to stroke patients. For example, many interventions have been applied to stroke patients, such as immersive virtual reality, behavioral observation training, task-oriented training, and visual and auditory coordination stimulation [17-20]. However, there is a lack of research on EEG during unilateral upper extremity exercise in stroke patients.
It has been suggested that stroke patients may experience decreased muscle strength and movement anxiety due to hospitalization, and existing studies have not included table tennis in neurological patients. Furthermore, there is a lack of studies looking at brain changes with table tennis [21]. Therefore, the purpose of this study is to determine what changes occur in the EEG when playing table tennis in a seated position.
Research Methods
Study population
This study was conducted on 15 patients who were diagnosed with stroke and were hospitalized at W Rehabilitation Hospital in Gwangju Metropolitan City from August to October 2021. All subjects were fully informed about the study and signed an informed consent form. The inclusion criteria for the study were: stroke onset more than 6 months ago, current paralysis, ability to maintain a sitting position for more than 40 minutes, no vision or hearing problems, and voluntary signing of the consent form.
Measurement methods and tools
- EEG measurement
In this study, EEG was measured using DSI-24 equipment (Wearable sensing Laxtha, USA). It is a research-grade wireless dry electrode EEG headset designed to quickly apply 21 sensors at 10-20 international system locations. There are 21 sensors in 10-20 locations (Figure 1). Since EEG measurements can be interfered with by external factors, we created an environment for the measurement before proceeding. For accurate measurements, two to three repeated measurements were performed, minimizing movement and keeping the eyes open for one minute. Data were extracted using the TELESCAN program (Laxtha, USA).
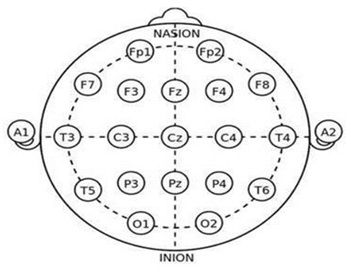 Figure 1: Internal. 10-20 electrode system.
Figure 1: Internal. 10-20 electrode system.
Intervention methods and procedures
Patients in this study received unilateral upper extremity exercises after basic inpatient treatment at a neurological hospital. In order to perform unilateral upper limb exercises, subjects who met the study selection criteria were selected who voluntarily agreed to participate in the study and then played table tennis, one of the unilateral upper limb exercises. Before playing table tennis, the researchers explained and educated the subjects about the basic knowledge of table tennis in advance. To prevent stroke patients from falling, the table tennis table was modified into a sitting position. In a sitting position, the subject held the ping-pong bat with the non-paralyzed hand and exchanged the ping-pong ball with the experimenter. One research assistant was positioned next to the subject to prevent falls. The subjects' table tennis performance was measured for 40 minutes once a day, twice a week, for a total of 4 weeks.
Data analysis
The data collected in this study were analyzed using SPSS version 22.0 for Windows. Descriptive statistics were used to calculate the mean and standard deviation to determine the general characteristics of the subjects. The parametric Paired t-test was used for electrodes that met normality using the Shapiro-Wilk test, and the non-parametric Wilcoxon signed-rank test was used for electrodes that did not meet normality, and the significance level was set at p < .05.
Results
General characteristics of the study subjects
This study was conducted on 15 stroke patients, 11 males and 4 females, with a mean age of 48.53±5.54 years, a mean height of 169.80±5.35 cm, and a mean weight of 65.27±10.03 kg. The BMI index was 22.51±2.38 km/m², the paralyzed side was 13 on the right and 2 on the left, and the etiology was 12 cerebral hemorrhage and 3 cerebral infarction (Table 1).
|
Ping-Pong exercise group (N=15) |
|
|
Gender (Male/Female) |
11/4 |
|
Type of stroke (Infarction/Hemorrhage) |
12/3 |
|
Paretic side (Rt./Lt.) |
13/2 |
|
Age (year) |
48.53±5.54 |
|
Height (cm) |
169.80±5.53 |
|
Weight (kg) |
48.53±5.35 |
|
Body mass index (km/m²) |
22.51±2.38 |
Table 1: General characteristics of the subject.
Mean±standard deviation
EEG
The inter-electrode changes in relative beta waves in this study were significantly different (p < 0.05) at electrodes Fp1, Fp2, F3, and F7 in the frontal and parietal regions (Table 2). T3, T4, T5, and T6 electrodes in the temporal lobe region showed significant differences (p < 0.05) (Table 2). Pz, O1, and O2 electrodes in the parietal and posterior parietal lobe regions (p < 0.05) (Table 2).
|
Location |
Pre |
Post |
Post-pre difference |
t/z |
p |
|
Fp1 |
0.233±0.023 |
0.264±0.044 |
-0.031±0.059 |
-2.201 |
.028* |
|
Fp2 |
0.196±0.024 |
0.238±0.043 |
-0.042±0.054 |
-3.012 |
.009** |
|
Fz |
0.270±0.037 |
0.306±0.059 |
-0.036±0.068 |
-2.031 |
.062 |
|
F3 |
0.231±0.026 |
0.276±0.034 |
-0.045±0.053 |
-2.338 |
.019* |
|
F4 |
0.251±0.025 |
0.251±0.025 |
-0.018±0.052 |
-1.377 |
.190 |
|
F7 |
0.220±0.031 |
0.254±0.037 |
-0.034±0.059 |
-2.389 |
.017* |
|
F8 |
0.188±0.048 |
0.213±0.049 |
-0.024±0.073 |
-1.304 |
.213 |
|
Cz |
0.306±0.045 |
0.341±0.054 |
-0.035±0.071 |
-1.917 |
.076 |
|
C3 |
0.248±0.029 |
0.263±0.049 |
-0.014±0.056 |
-1.007 |
.331 |
|
C4 |
0.321±0.059 |
0.309±0.054 |
0.012±0.067 |
0.689 |
.502 |
|
T3 |
0.366±0.022 |
0.275±0.048 |
0.090±0.054 |
6.419 |
.000** |
|
T4 |
0.356±0.029 |
0.270±0.056 |
0.086±0.062 |
5.349 |
.000** |
|
T5 |
0.416±0.026 |
0.296±0.048 |
0.120±0.061 |
7.546 |
.000** |
|
T6 |
0.434±0.012 |
0.279±0.043 |
0.154±0.043 |
13.801 |
.000** |
|
Pz |
0.439±0.096 |
0.350±0.056 |
0.088±0.119 |
-2.701 |
.007* |
|
P3 |
0.624±0.132 |
0.362±0.078 |
0.042±0.125 |
-1.793 |
.073 |
|
P4 |
0.386±0.027 |
0.362±0.077 |
0.024±0.086 |
1.074 |
.301 |
|
O1 |
0.450±0.029 |
0.295±0.038 |
0.154±0.056 |
10.688 |
.000** |
|
O2 |
0.480±0.020 |
0.328±0.056 |
0.152±0.060 |
9.656 |
.000** |
Table 2. Electrode-specific changes in relative beta waves.
Paired t-test (**p <.05)
Wilcoxon signed rank test (*p <.05)
Discussion
This study aimed to find out what changes occurred in the EEG of stroke patients after playing table tennis in a seated position for four weeks. The results showed a significant increase in relative beta waves at electrodes Fp1, Fp2, F3, and F7 in the frontal and parietal regions. On the other hand, relative beta waves were significantly decreased at electrodes T3, T4, T5, and T6 in the sensory areas of the temporal lobe and at electrodes Pz, O1, and O2 in the parietal and occipital lobes. The motor areas, C3 and C4 electrodes, did not show significant differences, which may be due to a variety of factors, including the fact that motor-related activity occurs in different brain regions, the intensity and duration of motor-related activity, and interindividual differences [22].
Post-stroke EEG measurements can reveal changes in interhemispheric balance, changes in activity in areas connected to the injured region, and reorganization of the body representation map, identifying reorganization of brain regions that support clinical recovery [23-25]. Although table tennis, a unilateral upper extremity exercise, was performed in a seated position in this study, it is thought to increase the anterior cingulate and parietal lobe regions in exercises with greater physical activity in the upper extremities. A 2014 study examined EEG activation in table tennis players and found increased activation in the frontal and parietal lobes during table tennis [26]. These results suggest that table tennis is conducive to activating the frontal and parietal regions of the brain because it involves a lot of upper extremity use and requires quick judgment and reaction [27].
The frontal and parietal lobes are associated with executive functions of the brain, such as motor planning, execution, and control, and various studies have shown that activity in the frontal lobe is associated with motor-related cognitive functions and motor-related tasks, such as sensory processing, motor planning, execution, control, and feedback [28]. This is consistent with previous studies that have reported that motor recovery in stroke patients is positively correlated with relative beta waves in the parietal lobe [29]. The generation and inhibition of goal-directed movements, such as hitting a ball with a ping-pong stick in a stroke patient, occurs in nested brain regions of neural networks [30,31]. Interactive inhibition occurs throughout the entire process from action planning to execution, and inhibitory control allows the human brain to develop adaptive and flexible behavioral strategies to regulate movement [32]. Thus, circuit reorganization and neuroplasticity in the injured brain occurs [25,26].
Stroke patients often experience sensory deficits [33]. Loss of somatosensory or visual fields negatively impacts functional outcomes in patients with hemiparesis [34]. It is not surprising that motor recovery can be enhanced by sensory stimulation. In patients with severe motor impairment, changes in effective connectivity can be seen after stroke at all stages of sensorimotor integration, from primary sensory areas to associated multisensory areas, and among the various neurofeedback protocols used to inhibit or enhance brain waves, sensorimotor training protocols have been suggested as an effective way to improve motor performance [35,36]. In the present study, changes in brain waves with golf putting performance were shown to increase or decrease various brain wave activities in specific areas of the cerebral cortex to develop a neural system for correct patterns or activities [37].
In this study, EEG activity decreased in the temporal lobe, parietal lobe, and posterior parietal lobe. These areas are sensory areas, and it is thought that the decrease in EEG activity is due to the tactile sensation from the table tennis bat and ball, the auditory sensation from the sounds that occur regularly in table tennis, and the visual sensation from constantly looking at the ball. However, the mechanisms for the changes in EEG activity in stroke patients are uncertain [16,38,]. Therefore, further research is needed to understand the mechanisms of sensory deficits and EEG changes in stroke patients. In the present study, stroke patients were receiving general neurological treatment at the hospital, but many of them had difficulties due to their long stay in the hospital, so the researcher conducted a study to increase their participation in exercise as a means of motivation. In the interviews of the participants, most of them had experienced sports activities at school in the past, and they seemed to be motivated to exercise by reminiscing about the past.
Limitations of this study include the fact that the EEG results were obtained by combining physical therapy and table tennis exercise in a single group of stroke patients. Although this study did not provide evidence that the changes in EEG could be attributed to table tennis exercise alone, previous studies have shown that table tennis exercise can help restore brain function in stroke patients, and this study confirmed that it can be used in combination with physical therapy. The changes in brain waves identified in this study can be interpreted as indicative of recovery of brain function in stroke patients. Consequently, this study confirms that the combination of physical therapy and table tennis exercise in stroke patients can help restore brain function, which is consistent with previous studies.
The advantage of a before-and-after comparison study of a single group is that it is a temporally separated before-and-after study within a single group, so that the differences between the before-and-after groups can be identified to determine the causal relationship between cause and effect. This is one of the most powerful ways to identify causal relationships and is an alternative method when a control group is not available [39]. For these reasons, the before-and-after design of a single-group comparison study is necessary. However, while there were changes in the outcomes of the study, the lack of a comparison group makes it impossible to say whether the changes were natural over time or due to table tennis. Therefore, future studies should include a control group. In conclusion, this study confirmed that playing table tennis in a seated position changed EEG in stroke patients. Future studies should be conducted with different methods.
References
- Chang JS, Lee SY, Lee MH, Choi YW, Lee HM, et al. (2009) The Correlations between Gait Speed and Muscle Activation or Foot Pressure in Stroke Patients. J Kor Soc Phys Ther 21: 47-52.
- Sharp SA, Brouwer BJ (1997) Isokinetic strength training of the hemiparetic knee: Effects on function and spasticity. Arch Phys Med Rehabil 78: 1231-1236.
- Langhorne P, Coupar F, Pollock A (2009) Motor recovery after stroke: a systematic review. Lancet Nrurol 8: 741-54.
- Luke C, Dodd KJ, Brock K (2004) Outxomes of the Bobath concept on upper limb recovery following stroke. Clin Rehabil 18: 888-898.
- Hatem SM, Saussez G, Della Faille M, Prist V, Zhang X, et al. Rehabilitation of motor function after stroke: A multiple systematic review focused on techniques to stimulate upper extremity recovery. Front Hum Neurosci 10: 442.
- Pendleton, HM, Schultz-krohn (2006) Pedretti’s Occupational Therapy (6th edn) San Jose: Mosby.
- Gracies JM, Marosszeky JE, Renton R, Sandanam J, Gandevia SC, et al. (2000) Short-term effects of dynamic lycra splints on upper limb in hemiplegic parients. Arch Phys Med Rehabil 81: 1547-1555.
- Choi SW (2008) Analysis of upper limb and ball kinematics during impact according to golf putting distance. Korea University. Dissertation of Master's Degree.
- Naderi A, Degens H, Rezvani MH, Shaabani F (2018) A retrospective comparison of physical health in regular recreational table tennis participants and sedentary elderly men. J Musculoskelet Neuronal Interact 18: 200-207.
- Park SH (2012) A Study on Differences in Prediction Accuracy and Visual Search Strategies According to Table Tennis Proficiency and Visual-Audible Information Presentation Level. Dissertation of Master's Degree, Seoul National University.
- Williams AM, Vickers J, Rodrigues S (2002) The effects of anxiety on visual search, movement kinematics, and performance in table tennis: A test of Eysenck and Calvo’s processing efficiency theory. J Sport Exerc Psychol 24: 438-455.
- MacAskill MR, Anderson TJ (2016) Eye movements in neurodegenerative diseases. Curr Opin Neurol 29: 61-68.
- Lim AJ (2011) Effects of eye exercise programs on postural control and visual perception in children with spastic cerebral palsy. Dissertation of Master's Degree, Inje University.
- Niedermeyer E, da Silva FL (2005) Electroencephalography: basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins.
- Koo BO, Bae SS, Kim HS, Lee DH (2002) The Effect of Eye Movement on Balance Improvement by Plegia Side of Adult Hemiplegic Patient. J Kor Soc Phys Ther 14: 1-19.
- Finnigan S, van Putten MJAM (2013) EEG in ischaemic stroke: quantitative EEG can uniquely inform (sub-) acute prognoses and clinical management. Clinical Neurophysiology 124: 10-19.
- Choi HW (2020) Effects of Immersive Virtual Reality Training on Posture Control and EEG in Stroke Patients. Dissertation of Master's Degree, Nambu University.
- Lee HJ (2016). The effect of a home exercise program using motion observation on exercise ability in patients with chronic stroke. Dissertation of Master's Degree, Yongin University.
- Shin NR (2020) Effects of task-oriented proprioception training on balance and gait in acute stroke patients. . Dissertation of Master's Degree, Hallym University.
- Hong CW (2021) The effects of a digital pegboard training occupational therapy program using visual and auditory feedback on hand function and visual perception in stroke patients. Dissertation of Master's Degree, Woosong University.
- Veerbeek JM, Koolstra M, Ket JC, van Wegen EE, Kwakkel G (2004) Effects of augmented exercise therapy on outcome of gait and gait-related activities in the first 6 months after stroke: a meta-analysis. Stroke 35: 2701-2707.
- Pfurtscheller G, Lopes da Silva FH (1999) Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110: 1842-1857.
- Dimyan MA, Cohen LG (2011) Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol 7: 76-85.
- Hara Y (2015) Brain plasticity and rehabilitation in stroke patients. J Nippon Med Sch 82: 4-13.
- Crema A, Bassolino M, Guanziroli E, Colombo M, Blanke O, et al. (2022) Neuromuscular electrical stimulation restores upper limb sensory-motor functions and body representations in chronic stroke survivors. Med 3: 58-74.
- Wang Y, Guan H, Zhang Y (2014) Differences in EEG oscillatory patterns between elite and sub-elite table tennis players. Journal of sports science & medicine 13(3):
- Tsai YH, Wu SK, Yu SS, Tsai MH (2022) Analyzing Brain Waves of Table Tennis Players with Machine Learning for Stress Classification. Applied Sciences 12:
- Watanabe M (2002) Frontal executive functions and gait in older adults. Aging Neuropsychology and Cognition 9: 214-2
- Foong R, Ang KK, Quek C, Guan C, Phua KS, et al. (2020) Assessment of the Efficacy of EEG-Based MI-BCI With Visual Feedback and EEG Correlates of Mental Fatigue for Upper-Limb Stroke Rehabilitation. IEEE Trans Biomed Eng 67: 786-795.
- Mirabella G (2014) Should I stay or should I go? Conceptual underpinnings of goal-directed actions. Front Syst Neurosci 8: 206.
- Hampshire A, Sharp DJ (2015) Contrasting network and modular perspectives on inhibitory control. Trends Cogn Sci 19: 445-452.
- Carey LM, Matyas TA, Baum C (2018) Effects of somatosensory impairment on participation after stroke. Am J Occup Ther 72: 7203205100p1-10.
- Tolonen U, Sulg IA (1981) Comparison of quantitative EEG parameters from four different analysis techniques in evaluation of relationships between EEG and CBF in brain infarction. Electroencephalogr Clin Neurophysiol 51: 177-185.
- Han L, Law-Gibson D, Reding M (2002) Key neurological impairments influence function-related group outcomes after stroke. Stroke 33: 1920-1924.
- Grefkes C, Fink GR (2011) Reorganization of cerebral networks after stroke: New insights from neuroimaging with connectivity approaches. Brain 134: 1264-1276.
- Cheng MY, Huang CJ, Chang YK, Koester D, Schack T, et al. (2015) Sensorimotor Rhythm Neurofeedback Enhances Golf Putting Performance. J Sport Exerc Psychol 37: 626-636.
- Pourbehbahani Z, Saemi E, Cheng MY, Dehghan MR (2023) Both Sensorimotor Rhythm Neurofeedback and Self-Controlled Practice Enhance Motor Learning and Performance in Novice Golfers. Behavioral Science 13: 65.
- Jordan KG (2004) Emergency EEG and continuous EEG monitoring in acute ischemic stroke. J Clin Neurophysiol 21: 341-352.
- Blume BD, Ford JK, Baldwin TT, Huang JL (2010) Transfer of Training: A Meta-Analytic Review. Journal of Management 36: 1065-1105.
Citation: Seo S, Kim Y (2024) Stroke Patients: Effects of Combining Sitting Table Tennis Exercise with Neurological Physical Therapy on Brain Waves. J Phys Med Rehabil Disabil 10: 090.
Copyright: © 2024 Seungwon Seo, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

