Department Of Histology And Cell Biology, Minia University, New Minia, Egypt
Abstract
Our understanding of the mechanisms responsible for decline of the human mental capabilities which accompany aging became one of the major concerns of the modern gerontology. The cerebellum is a vital organ playing a fundamental role in the postural control, equilibrium and motor coordination. Additionally, it is involved in cognitive functions such as forgetfulness, decreased ability to maintain focus and decreased problem-solving capability. Owing to its architectural and cellular simplicity, the cerebellum provides an excellent model for the study of the age-related changes at the cellular level. Moreover, the basal ganglia has received much attention over the last decades mainly because of their clinical relevance. It is now generally accepted that the basal ganglia are involved in a variety of non-motor functions, including those related to incentive and motivated behaviors. Our understanding of their cerebellum and the basal ganglia structure, organization, function and implication in diseases and age-related changes has increased equally. The most recent global analysis of the pathogenesis of aging process of the cerebellum and basal ganglia at the molecular level suggested that aging resulted in a gene expression profile indicative of an inflammatory response, oxidative stress, alteration in mitochondrial DNA, loss of homeostasis (dynamic equilibrium) in and out of the neuron and reduced neuro-tropic support in both brain regions. A strong association between the structural and functional abnormalities of the cerebellum and psychiatric disorders especially schizophrenia, depression, anorexia, anxiety, and autism was reported.
This review will discuss various aspects of these two inter-related structures, cerebellum and basal ganglia. Firstly, the structural and functional changes in them with aging. Secondarily, the factors affecting them causing age related disorders. Thirdly, the most common age-related disorders. Fourthly, the available diagnostic investigations and the prophylactic and therapeutic lines of treatments for age related disorders.
BACKGROUND
The last decade has witnessed' a significant turn in our understanding of the mechanisms responsible for decline of human mental capabilities which accompany aging. This became one of the major concerns of modern gerontology. However, it is admitted that the mechanisms of CNS aging remain far from being understood. Indeed, all aging humans will develop some degree of decline in cognitive capacity as time progresses [1].
Numerous theories of aging have been proposed [2]. The evolutionary theory of aging, oxidative damage theory and a non-adaptive programmed aging theory. Yet each of these by itself is inadequate to provide a global description of the causes of aging. For instance, the evolutionary theory, while successfully explaining how and why aging evolves, is uninformative about the specific mechanisms underlying aging. Likewise, while oxidative damage accumulation is a determinant of the rate of aging, it remains unclear (1) whether it is a primary cause of aging or a secondary event (2) why it occurs at such different rates in different species and (3) whether such differences reflect programmed or stochastic mechanisms. The theory of non-adaptive programmed aging proposes that underlying the traversal of adulthood in animals is the expression of a succession of age specific developmental genetic identities. Such a theory of programmed ageing can provide an explanatory link between the evolutionary and the oxidative damage theory. The capacity of such tripartite model to explain recent findings are explored, and some testable predictions which follow from it are set out [3].
Neurons are stable post-mitotic cells and hence apparently prone to develop age-related changes. The cerebellum is a vital organ that, besides playing a fundamental role in the postural control, equilibrium and motor coordination, is also involved in cognitive functions such as forgetfulness, decreased ability to maintain focus and decreased problem-solving capability. Owing to its architectural and cellular simplicity, the cerebellum provides an excellent model for the study of the age-related changes at the cellular level [4,5].
Additionally, the basal ganglia have received much attention during the last decades mainly because of their clinical relevance. It is now generally accepted that the basal ganglia are involved in a variety of non-motor functions, including those related to incentive and motivated behaviors. It is now clear that, in addition to afferents from the cerebral cortex, the Substantia Nigra Compacta (SNc) and thalamus, the basal ganglia receive many inputs from other sub-cortical structures, including the cerebellum, the locus coeruleus, the raphe nuclei, and the Pedunculo-Pontine Nucleus (PPN). The latter with its reciprocal connection with the stiatum, the GPi, and the SNr plays a key role in the control of posture and locomotion [6].
Our understanding of their structure, organization, function and implication in diseases and age-related changes has increased equally. Cognitive decline does not affect all individuals equally; clear associations exist between the rate and severity of cognitive decline and a variety of factors, including oxidative stress and free radical damage, chronic low-level inflammation, declining hormone levels, endothelial dysfunction, excess body weight, suboptimal nutrition, lifestyle, social network, other medical conditions, and various biomarkers. Fortunately, many of these factors are modifiable to a significant extent, and proactive lifestyle changes, cognitive training, and nutritional interventions have been shown to decrease the rate of intellectual decay and potentially reverse age-related cognitive decline [7]. Sierra & Kohanski reported that aging is the major risk factor for diseases such as macular degeneration, type 2 diabetes, atherosclerosis, cancer, pulmonary disease, Alzheimer’s Disease (AD), osteoporosis and arthritis [8].
THE CEREBELLUM ("LITTLE BRAIN")
The cerebellum is involved in the coordination of movement. The cerebellum is also partly responsible for motor learning, such as riding a bicycle. Unlike the cerebrum, this works entirely on a contralateral basis, the cerebellum works ipsilaterally. Some researchers suggested that the cerebellum is involved in the emotional domain; it acts as a mediator between the internal state and external environment for the unconscious and conscious levels of emotional process [4]. The cerebellum has been involved in a broad range of neuropsychological functions such as; Cognitive domains (i.e., attention), learning and memory, language and executive functioning [9]. Cavdar et al., provided a perspective on the role of the cerebellar cortex or nuclei upon their stimulation in eliciting or modifying a wide range of visceral responses (e.g. changes in blood pressure, heart rate, respiration, alteration in smooth muscle tone of the bladder & pupil and intestines) [10].
THE BASAL GANGLIA
The name is confusing, as generally a ganglion is a collection of cell bodies outside the central nervous system. The basal ganglia are a collection of nuclei deep to the white matter of cerebral cortex. The name includes: caudate, putamen, nucleus accumbens, globus pallidus, substantia nigra, sub-thalamic nucleus. Other groupings that may be heard are the striatum (caudate + putamen + nucleus accumbens), the corpus striatum (striatum + globus pallidus), or the lenticular nucleus (putamen + globus pallidus) [11]. She had supported a functional dissociation between the striatum and cerebellum in acquiring visual-motor skilled behaviors [12].
The relationship between the cerebellum and basal ganglia
The cerebellum and the basal ganglia are large collections of nuclei that modify movement on a minute to minute basis. Motor cortex send information to both structures and subsequently they send information right back to cortex via the thalamus (getting to the cortex got to be through the thalamus). The output of the cerebellum is excitatory, while that of the basal ganglia is inhibitory. The balance between these two systems allows for smooth, coordinated movement, and a disturbance in either system causes movement disorders [13]. Both cognitive and motor functions are controlled by brain areas such as frontal lobes, cerebellum, and basal ganglia that collectively interact to exert control over executive function. Disorders of brain integration involve disruption of executive processes, functions attributable to the frontal lobes, and articulation with motor components of the nervous system [14]. More recently, the cerebellum has also been discovered to play a role in sensory information processing as well as in cognitive functioning [15]. The brain areas such as frontal lobes, cerebellum, and basal ganglia control both of cognitive and motor function. The control messages issued by the motor cortex are themselves triggered by messages from other cortical areas. The motor cortex also communicates closely with subcortical structures such as the basal ganglia and the cerebellum, through the thalamus, which acts as a relay [16]. The previous view that the basal ganglia are centers in which massive convergence of cortical information occurred has now been replaced by a view that they process information in a highly specific manner achieving higher level behavioral control, i.e., regulation of habit learning or action selection.
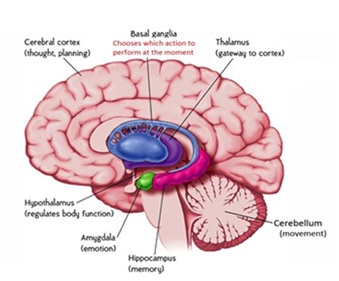
Figure 1: Diagram summarizes the functional relationship between the cortical and subcortical brain.
Structural changes of the cerebellum during aging
Diana et al., demonstrate that processes of aging have an impact on brain structures and associated behaviors differentially, with cerebellum showing earlier senescence than hippocampus [17]. As the population rapidly ages, understanding the factors that contribute to these age-related performance declines is critical for the development of appropriate targeted interventions and preventative measures. However, accumulating evidence supports a role for the cerebellum in both motor and cognitive behaviors [18]. Alho et al., added that the human brain undergoes non-uniform changes during aging [19]. The Substantia Nigra (SN), the source of major dopaminergic pathways in the brain, is particularly vulnerable to changes in the progression of several age-related neurodegenerative diseases.
PATHOLOGICAL CHANGES IN THE CEREBELLAR CELLS PROMOTING AGE RELATED DISORDERS
Pathological changes in cerebellar Purkinje Cells (PCs) with age
Purkinje cells represent the sole output neuron of the cerebellar cortex and if any changes affect their function will lead to affection on the function of the cerebellum [20]. PCs appear to be very sensitive to aging, exhibiting significant changes in both morphology and function during this condition [21]. In aged cerebellum, PCs show remarkable morphological changes. They appear as densely stained neurons with shrunken bodies and rippled dendrites with hyper-eosinophilic cytoplasm and occasional vacuolations [22]. In advanced stages there is complete loss of PCs and filling up the left empty spaces with a dense mat of astroglial fibers. Zhang et al., reported that Cerebellar Purkinje Cells (PCs), the sole output neurons in the cerebellar cortex, play an important role in the cerebellar circuit [23]. PCs appear to be rather sensitive to aging, exhibiting significant changes in both morphology and function during senescence. This article reviews such changes during the normal aging process, including a decrease in the quantity of cells, atrophy in the soma, retraction in the dendritic arborizations, degeneration in the sub cellular organelles, a decline in synapse density, disorder in the neurotransmitter system, and alterations in electrophysiological properties. Although these deteriorative changes occur during aging, compensatory mechanisms exist to counteract the impairments in the aging PCs. Additionally, the dendritic arborizations are atrophied in senile animals and this could interfere with the process of information exchange and signal transmission in the aged cerebellum. Indeed, these changes would lead to a reduction in motor control and coordination [24]. Šišková reported that the spiny branches on Purkinje neurons, the parallel and climbing fiber terminals were both intact, whereas the Purkinje dendrites degenerated progressively [25]. Synapse signaling is one of the first processes to be affected, leading to neuron morphological changes, neuron degeneration, inflammation, and ultimately behavior disorders [26]. PCs undergo significant age-related alterations including; destructed basic organelles particularly smooth endoplasmic reticulum which affect calcium homeostasis. Additionally, there marked increase in lipofuscin pigment that speed up PCs death. Aged animal models showed a significant loss of Purkinje neurons [27]. Li et al., Therefore, PCs is the first cerebellar cell affected by aging [28]. Indeed, PCs being a group of large neurons of the CNS with highly arborized dendritic trees and with lengthy projection distances need a very efficient metabolic supply and this diminish with age. This appears to be of critical importance with advancing age and, may reasonably explain the reported higher vulnerability of this type of neurons to hypoxia and ischemia.
Pathological changes in cerebellar cells in the granular cell layer in age related disorders
Andersen et al., stated that the global white matter was reduced by 26% with age; the mean volume of the PC body was decreased by 33% with no decrease in the volume of the PC nuclei [29]. A tendency towards a 16% total cerebellar volume loss was seen without a concomitant neuronal loss. No global PC or granule cell loss was detected with age, total PCs number being 28 × 106 (coefficient of variation, CV = 0.16) and total granule cell number 109 × 109 (CV = 0.17). However, a significant change was observed with age in the anterior lobe, where a selective 40% loss of both Purkinje and granule cells was found. An apparent increase in the number of granular neurons was noticed in these animals, which also presented markedly increased numbers of astrocyte cell processes demonstrated with GFAP immune-staining [30]. Excluding dense bodies which displayed a significant positive linear trend with age (Iipofuscin is the major constituent of dense bodies) the absolute volume of the cytoplasmic components did not vary significantly. There was a suggestion of mitochondrial origin of lipofuscin (beside lysosomes) in the granule cells. There was also decrease in the unclear mean volume of` the granule cells with aging (in the period between 2-24 months) rats. This could be related to important changes in the cellular synthetic activity. It was concluded that granule cells showed fair degree of morphological stability. Recently Walle et al., reported that most regions in the human cerebellum show only minor age related morphological changes except the anterior lobe showed a 40% reduction in the total number of granule and PCs [31]. The main reason of loss cortical volume in old age due to reductions in the granule cell layer. Because the integrity of the granule cell neuron is dependent on its synaptic relationship with the dendritic zone of the Purkinje neuron, loss of the latter is followed by reduction of the granule cells [30].
Pathological changes in cerebellar cells in the molecular cell layer in age related disorders
Besides the age-associated changes that occur in PCs and granule cell layers, there is also a large amount of neuronal loss in the molecular layer of the aged cerebellum. These neurons (basket and stellate cells) make inhibitory synapses with the PCs. Such a loss may weaken the inhibitory stimuli to the PCs [32].
Pathological changes in cerebellar neuroglia cells in age related disorders
Neuroglia cells also undergo age-related changes such as increase the density of Glial Fibrillary Acidic Protein (GFAP). GFAP protein levels of resident populations of astrocytes showed up regulation in aged rodent brains. This was considered a compensatory mechanism because of their role in survival and maintenance of neurons to minimize damage to the aging neurons [33]. Microglia cells showed morphological changes such as thicker processes and larger cell soma indicating activation state. Interestingly, this was associated with up regulation of pro-inflammatory cytokines IL-6, IL-18, TNFα and a down regulation of anti-inflammatory cytokine IL-4, IL-10 and TGFβ. Other pathological change affecting other neuroglia cells was observed; astrocytic processes extension into both the molecular and granular layers. Additionally, proliferation of Bergmann astrocytes was seen in folia where significant PCs take place.
Remodelling of nerve cell connectivity in the aged cerebellum
Dendrite arborization patterns of PCs are important elements for neuronal connectivity and integration [34]. Giardino et al., showed a decrease of synaptic numerical density associated with significant enlargement of synaptic size [13]. They concluded that the increase synaptic size represents a strengthening in the transmission of nerve impulse since larger junction areas can release more neurotransmitter and activate more postsynaptic receptors. Under variety of conditions including normal aging, oligodendrocytes are unable to maintain myelin sheaths [35]. There is a progressive loss of cerebellar Purkinje neurons with age as well as an increase in cerebellar behavioral deficits. Similarly, extensive stem cell loss is seen in the hypothalamus with age [36]. Matthew et al., showing that regional differences in aging are present in the brain [37]. The expression of genes related to major functions of astrocytes in relation to synaptic transmission and neuronal homeostasis is not altered in the aging brain, demonstrating that there is not a general loss of astrocyte support of neurons with age. Cerebellar nucleus neurons are crucial to the olivo-cerebellar circuit as they provide the sole output of the entire cerebellum. The relationship between mobility and cognition in aging is well established, but the relationship between mobility and the structure and function of the aging brain is relatively unknown. Shyian et al., found that the macro-microscopic dissection of persons died after 75 years old showed no significant variability of linear dimensions of cerebellar nuclei with their specific location and options. Simultaneously [38], reliable reduction of cellular density detected for Purkinje, granule and basket neurons was more pronounced in male for Purkinje cells.
Role of the mitochondria in the development of age related disorder
The process of aging is complex biological phenomena, a decline in mitochondrial function plays a key role in the aging process and increases the incidence of age-related disorders. Aging is associated with a decline in mitochondrial turnover caused by reduced mitochondrial biogenesis and/or inefficient mitochondrial degradation. A recent wave of studies has demonstrated that mitochondria were placed at the center of the ‘free radical theory of aging, because these paramount organelles are not only the main producers of energy in the cells, but also to main source of reactive oxygen species [39]. Thus, dysfunctional regulation of mitochondrial dynamics including mitochondrial metabolism and the maintenance of mitochondrial DNA might be the intrinsic causes of mitochondrial dysfunction, which contributes to oxidative stress and cell death during the aging process. The accumulation of mitochondria; DNA mutations that occur with age leads to alterations in cell-signaling pathways that can induce cell dysfunction and initiate apoptosis, irrespective of increased ROS production and oxidative stress in mitochondria [40]. These structural alterations in aging mitochondria correlate with lower ATP levels and increased generation of nitric oxide, lipid peroxidation and protein nitration. Moreover, mitochondria-smooth endoplasmic reticulum interactions are compromised due to decreased associations, which would lead to disruption in Ca2+ homeostasis and defective unfolded protein responses in aging axons [41].
FACTORS THAT INFLUENCE AGING OF THE CEREBELLUM AND BASAL GANGLIA
Genomic approaches (gene influence)
It is now commonly accepted that aging is considered as the major risk factor for many chronic diseases which usually accompanied by causative changes in the genome and epigenome. While genome sequencing and whole genome monitoring of epigenetic changes are now accurate tools to track chromosome dynamics in three dimensions (3D). This approach could dramatically enhance fundamental knowledge of the aging process [42]. Transcriptional Activator-Like Effectors (TALEs) have emerged as powerful method for genome editing. A recent study reports that this tool good to analyze telomere attrition, genome instability and epigenetic alterations that are hallmarks of aging [43].
Gender influence
Age is associated with substantial structural brain changes. While some magnetic resonance imaging studies have reported a profound age effect in men than women, others reported no sex differences [44]. They found that most brain structures were not differentially affected by aging in men and women who were healthy or had AD. The few observed differences were small and unstable across subsamples. Arani et al., reported that in the older population, there was softening of the brain tissue; however, this was not the same for all regions of the brain [45]. Some stiffness effects due to sex exist in the occipital and temporal lobes.
Oxidative stress
An increase in oxidative stress is part of normal aging process in the brain. The free radical damage and decreased energy production are common pathological pathways responsible for aging and the development of neurodegenerative disease. Consequently, with aging, some proteins increase such as enzymes that mediate energy production and oxidative stress [46,40].
Environmental factors
Oxidative stress occurs when the production of ROS exceeds the natural antioxidant systems. This imbalance can result from exposure to pro-oxidant substances ROS present as air pollutants in the atmosphere. This exposure induces inflammatory responses after a threshold is reached. Ozone is an ROS and powerful oxidizing agent capable of inducing oxidative stress state. This brain dysfunction is manifested as short- and long-term memory loss and motor deficiency in rats, all alterations that are positively related to the duration of O3 exposure. Besides causing motor deficiency and memory loss, O3can also cause neuroinflammation, neuronal damage, and alterations of the cerebral vasculature [47]. It was advised to reduce our exposure to ozone, we should avoid exercising and /or outdoor activity during afternoon and early evening hours in summer, late spring and early fall. These due to high levels of ozone were usually formed at these periods. Other environmental risk factors may also be important in the pathogenesis of neural degeneration such as air pollution, aluminum, silicon, selenium, pesticides and electromagnetic fields [48]. Aluminum in drinking water, there were evidence suggested that small amounts of aluminum could be neurotoxic and levels could accumulate selectively in certain brain tissues [49].
Loss of homeostasis
Neurons are constantly affected by extracellular changes and intracellular events so the dynamic equilibrium and the rate of energy production play important roles to give the neurons the best response to environmental stimuli and maintaining structural integrity. Aging was viewed as programmed but nonadaptive syndrome. Accordingly, at advanced ages, the developmental program which assures the fitness started to expire [50]. This resulted in a gradual loss of homeostasis and increasing the fragility. The cerebellum is a suitable model to investigate this aspect.
FACTORS AFFECTING HOMEOSTASIS IN AGE RELATED DISORDERS
Neurotransmitter (Nor Epinephrine (NE) & Acetylcholine (Ach) homeostasis
Aging is found to be associated with decline motor coordination and the ability of learning motor skills. This loss of function is correlated with a [51] dysregulation of glutathione homeostasis and alterations in glutathione-dependent enzyme activities are increasingly implicated in the induction and progression of neurodegenerative diseases. So, administration of N-acetyl cysteine or glutathione as therapeutic agents for neurodegenerative diseases help in decrease neurodegenerative changes [52].
Level of the amino acid neurotransmitters produced by intrinsic neurons
These may be excitatory (glutamate and aspartate) or inhibitory (GAB, glycine and taurine). Quantitative immuno gold electron microscopy demonstrated that neuroligins and neurexins are neuronal cell surface proteins that bind to each other and form asymmetric intercellular junctions. The activity of glutamate receptors declines in the expression of these receptors give access to programmed cell death in cerebellar granule cells [53]. Dzubay & Otis reported that in the cerebellum Metabotropic Glutamate Receptors (mGwRs) are required for distinct forms of synaptic plasticity expressed at parallel and climbing fibers synapses [54]. Aging induced impairments of the GABAergic system lead to an inhibitory/excitatory imbalance, this will lead to decreasing neuron's ability to respond with plastic changes to environmental and cellular challenges, leaving the brain more valuable for exposure to damaging effects [55]. Cerebellar taurine content was significantly lowered in aged rats. Complementary dietary supplementation regimens of taurine in old age aid healthy modulation of glutamatergic, neurotropic, and other relevant inflammatory pathways, protect against/the reversal of excite-toxicity, the rebalancing of glutamate, GABA, mitochondrial and brain function [56].
Ca2+, K+ and Na+ homeostasis
The intracellular free calcium (Ca2+) concentration plays complex signaling roles in brain. Calcium regulates neuronal plasticity underlying learning, memory and neuronal survival. Dysregulation of Ca2+leads to brain cell death and degeneration after ischemic stroke, long-term neurodegeneration in Alzheimer's disease, Parkinson's disease. Multiple lines of evidence have implicated Ca2+ dysregulation in brain aging and dementia. These changes have been associated with age-related deficits in learning and memory. Aging-related increases in the Ca2+ spikes and currents result from changes in local Ca2+, levels. The increased Ca2+ transients result in dysregulation of multiple Ca2+- dependent processes and, through different pathways, in accelerated functional decline during aging [57]. The mitochondrial dysfunction because of age dependent alterations in Ca2+ homeostasis. It has also been observed in cerebellar granule neurons in brain slices. These aging related changes not only increase the Ca2+ entry into the neurons but also decrease the capacity of neurons to buffer Ca2+ [58]. Aging is also associated with elevated intracellular calcium levels and altered calcium homeostatic mechanisms in hippocampal neurons [59]. More recently Fuchs et al., concluded that voltage-gated potassium channels (KV) play an important role in acquisition of membrane excitability in neurons [60]. There were age related changes in the distribution of KV1-1 and KV1-2 channel subunits in the rat cerebellum. These changes as result from loss of regulation of Ca2+ channel in the senescent period.
Protein and lipid homeostasis
Proteins are among the predominant products of gene expression and contribute significantly to the shape and functionality of the cell. In aging and disease, damaged proteins accumulate, leading to both loss-of-function and toxicity. The stability of the proteome is crucial to the health of the cell, and contributes significantly to the lifespan of the organism. Aging and many age-related diseases have in common the expression of misfolded and damaged proteins. The chronic expression of damaged proteins during disease may have devastating consequences on protein homeostasis (proteostasis), resulting in disruption of numerous biological processes. Understanding the various contributors to protein misfolding, and the mechanisms by which misfolding, and accompanied aggregation/toxicity, is accelerated by stress and aging. Common features of protein conformation diseases are the accumulation of protein deposits - aggregates, inclusion bodies, and plaques. These features, which are characteristic of misfolded protein species, are present in neurodegenerative diseases of aging such as Parkinson’s disease, amyotrophic lateral sclerosis and AD. The accumulation of misfolded and aggregated proteins associated with the aging related conformational diseases indicates a failure of folding homeostasis added that microglia isolated from aging mouse brains, having a profound gene expression pattern related to proinflammatory processes, phagocytosis, and lipid homeostasis [61,62]. This inflammation may contribute to the progression of neurodegeneration, and have prognostic value detecting the onset and progression of aging and neurodegeneration detecting.
Neurotropic and growth factors homeostasis
Neurotrophic factors are secreted protein that display important role in the synaptic and neuronal growth, myelination, differentiation, and survival of neurons [63]. Neurotrophic factors signaling are also severely affected in aging process which can be correlated with cognitive decline. The latter is related to alterations of neurotrophic factors level such as Brain-Derived Neurotrophic Factor (BDNF), Nerve Growth Factor (NGF) and Glial Cell-Derived Neurotrophic Factor (GDNF). There is strong relationship between aging and these factors. BDNF helps to protect neurons from damage caused by infection or injury. A preclinical study showed that a chronic BDNF deficiency leads learning deficit dependent on age in animals after seven months. GDNF has neurotrophic effects against the neuronal atrophy that causes cognitive deficits in old age, and they found that spatial learning and memory testing showed a significant gain in cognitive abilities due to GDNF exposure [64]. Budni et al., reported that there was a decrease in the levels of NGF in old age animals [65]. Considering that NGF is important for cognitive functions, and it was found to have decreased with aging. Insulin-Like Growth Factor (IGF)-1 is responsible for, neuronal survival, angiogenesis, neurogenesis, excitatory and inhibitory neurotransmission, regulation of food intake, and cognition. In addition, any aberrant decline in IGF1 values was suggested to play a role in the development of Alzheimer’s disease [66].
Homeostasis across the Microvascular endothelia in aging
In the aging, there are characteristic morphological and molecular alterations such as vessel wall thickening and reduction of nitric oxide which leading to the gradual loss of vascular homeostasis. Consequently, the risk of developing cardiovascular diseases increases with age. The age-dependent increase in free radical formation causes deterioration of the nitric oxide signaling cascade, activates prostaglandin metabolism, and promotes oxidative posttranslational protein modifications that interfere with vascular and cell signaling pathways, as a result, vascular dysfunction manifests. Compensatory mechanisms are initially activated to cope with age-induced oxidative stress, but become futile, which results in irreversible oxidative modifications of biological macromolecules [67]. Camandol & Mattson mentioned that during normal aging, there are decrease in the functionality of several energy metabolisms in brain cells including glucose transport, mitochondrial electron transport, DNA repair, and neurotrophic factor signaling [68]. Many factors likely contribute to the age-dependent brain hypometabolism. There was a negative correlation between cerebral blood flow and age. In addition, the permeability of the BBB is greater in older compared to younger individuals Brain hypoperfusion and loss of BBB integrity can result in diminished import of nutrients, and/or removal of toxins. Furthermore, a compromised BBB allows the parenchymal accumulation of blood-derived proteins (e.g., fibrinogen, immunoglobulins, albumin, thrombin, hemoglobin), and immune cells which can cause inflammation. There were reduced expression of glucose transporters in the brain with aging as well as changes in the expression of key enzymes involved in glycolysis and oxidative phosphorylation. The levels of ATP are reduced during aging, in correlation with ultrastructural alterations in mitochondria, and a reduced association of mitochondria with endoplasmic reticulum. NAD levels are critical for mitochondrial function and ATP production. An increase in the levels of NADH, with decreased total NAD and NAD+levels has been shown in human brain during normal aging. Mice with reduced GLUT1 levels display an age-dependent decrease in cerebral capillary density, reduced cerebral blood flow and glucose uptake, and increased BBB.
RESEARCH TECHNIQUES FOR DIAGNOSIS OF AGING PROCESS
Investigation of the molecular events associated with aging by lectin binding property for glycogen detection
Lectin are used to study the glycosylation state of the protein in the soluble fraction and membrane fraction of the various portions of the brain. Lectin was used to analyze the binding properties of lectins in the cerebellum and basal ganglia in 9 weeks old rats and 29 old month-old rats. It was found that the soluble fractions of white matter and basal ganglia have increased in aged rats. The binding properties of lectins in young adult rats, showed characteristic staining patterns; lectin stained strongly granular layer, a weakly molecular layer, and the medulla. However, in aged rats, different staining patterns were obtained [69].
High-Dimensional Single-Cell Mapping in CNS diseases
Using this approach in CNS research, Mrdjen et al., found that microglia, several subsets of border-associated macrophages and dendritic cells coexist in the CNS at steady state and exhibit disease-specific transformations in the immune microenvironment during aging and in models of Alzheimer's disease and multiple sclerosis [70]. Together, these data and the described framework provide a resource for the study of disease mechanisms, potential biomarkers, and therapeutic targets in CNS disease.
in situ hybridization
in situ hybridization was performed by localizations of mRNAs encoding neuronal protein subtypes, using riboprobes. The latter probes were synthesized with RNA polymerase in the presence of cDNA (Corresponding DNA) template; probe labeling was assessed by scintillation counting using PCR (Polymerase Chain Reaction) technique. Later, these riboprobes were hybridized with the coronal sections of the brain at 55°c for 24 hours. After hybridization the labeled protein subtypes mRNA signals revealed by autoradiogram [71]. For example, in situ hybridization of voltage gated K+ (K+V) potassium channel subunits genes which play an important role in acquisition of membrane excitability of neurons. Reddy et al., stated that non-coding RNAs that may play a role in psychiatric disease [72].
DNA microarray analysis
High density oligonucleotide arrays were employed to provide data on many thousands of genes to define transcriptional patterns in different brain regions. The use of this method providing new tool to measure biological age on a tissue specific bases [73]. Recently, DNA methylation markers have been used in the place of serum biomarkers to generate multivariate measures of age [74]. Hierarchical clustering approaches have also been applied to identify patterns of biomarker age changes that predict survival [75]. Based on its heritability, FI34 (frailty index) has been used in a genome-wide linkage scan to search for loci associated with healthy aging.
Fluorescent immune-histochemical (and immune-EM) techniques
These are used to investigate the cellular and sub-cellular marker localization of protein members family in brain regions [76]. In the study of immunostaining was performed on coronal slices with the free-floating method [77]. They used two animal models that reproduce vital components that predispose the brain to degeneration: aging and chronic neuro-inflammation. The interactions between neurons, astrocytes and microglia were compared in normal aged rats and adult rats infused with Lipopolysaccharide (LPS) into the 4th ventricle. Recently, Fulop, et al., introduced the term "inflammaging" which describes the progressive changes which occur in the ageing brain, characterized by a low-grade chronic up regulation of certain proinflammatory responses [78].
Magnetic Resonance Imaging (MRI)
Magnetic Resonance Imaging (MRI) uses differences in the magnetic properties of hydrogen among different tissues to generate anatomic images [79]. Ongoing advances in MRI image reconstruction now provide spatial resolution of less than 1 mm3 so that even small brain structures can be visualized and measured volumetrically, providing a distinct advantage over older cross-sectional area measurements using computed tomography. Additionally, with the advent of three-dimensional MRI acquisition techniques, it is possible to realign brain scans in whatever plane optimizes the measurement of a given structure. This approach likely improves the validity of volumetric measurements [80]. De Groot et al., used Longitudinal Diffusion MR Imaging Analysis1 to study White Matter Degeneration with aging [81]. It was concluded that longitudinal diffusion analysis indicates widespread microstructural deterioration of the normal-appearing white matter in normal aging, with relative sparing of sensor-motor fibers.
Morphometric techniques
A voxel-based morphometric analysis of age and sex-related changes in white matter volume in the normal aging brain
Females showed significantly greater total white matter volume than males (t=2.36, P=0.0096). VBM Females showed significantly greater total white matter volume than males (t=2.36, P=0.0096). VBM demonstrated statistically significant age-related differences in white matter volume between the young age-group and the middle age-group (P<0.05, FDR corrected) and between the middle age-group and the old age-group (P<0.05, FDR corrected). No interaction was found between age and sex on white matter volume (P<0.05, FDR corrected). White matter volume gradually increased before 40 years of age, peaked around 50 years of age, and rapidly declined after 60 years of age [82]. Bernard & Seidler found that older adults had smaller cerebellar volume than young adults; specifically, lobules in the anterior cerebellum were more impacted by age [83]. In sum, they demonstrate the importance of regional cerebellar volume with respect to both sensorimotor and cognitive performance, and provide additional insight into the role of the cerebellum in age-related performance declines.
Diffusion tensor imaging of white matter
Diffusion Tensor Imaging (DTI) assesses the structural integrity of white matter in the brain. Using DTI, Bennett et al., also demonstrated age-related decreases in cerebellar white matter integrity along with decreases in other brain regions [84]. Cao et al., using diffusion tensor imaging to investigate whether behavioral gains from Cognitive Training (CogTr ) would extend to White Matter (WM) microstructure, and whether training-induced changes in WM integrity would be associated with improvements in cognitive function [85]. They found that Cognitive Training (CogTr) is effective and recuperative for older adults, and can be used to fight against cognitive decline. These findings support the hypothesis that plasticity of WM can be modified by CogTr, even in late adulthood.
Stereological analysis
Using stereological analysis for assessment of the number of cell types of the cerebellum during aging, there were significantly fewer Purkinje and granule cells in the anterior cerebellum with advanced age. There was also a significant age-related association in the volume of the anterior cerebellum, and overall lower volume of cerebellar white matter [86]. More recent study suggested that no differences in the number of Purkinje or granule cells in the brains of Alzheimer's disease patients [87]. Study of Bernard & Seidler reported that across stereological studies in both human and animal models, there was evidence to indicate age-related decreases in the number of cerebellar cells, and as such, a decrease in cerebellar volume [88]. Alho et al., studied the three-dimensional and stereological characterization of the human Substantia Nigra (SN) during aging [89]. The shapes of all SNs investigated were reconstructed using fast, high-resolution computer-assisted 3D reconstruction software. They found a negative correlation between age and SN volume (p=0.04 rho=−0.53), with great variability in neuronal numbers and density across participants (from fifteen subjects aged 50–91 cognitively normal human subjects).
FUNCTIONAL ASSESSMENT OF THE AGING CEREBELLUM
Resting state functional connectivity
Resting state functional connectivity MRI (fcMRI) measures correlations in the Blood Oxygen Level Dependent (BOLD) signal between different brain regions at rest [90]. Recently, the investigations of cerebello-cortical resting state networks in young and older adults revealed that there was pattern of decreased functional connectivity in older adults [83]. The test-retest reliability of RFMRI remains largely unknown. Resting-State Functional Magnetic Resonance Imaging (RFMRI) enables researchers e.g., Zuo & Xing to monitor fluctuations in the spontaneous brain activities of thousands of regions in the human brain simultaneously [91], representing a popular tool for macro-scale functional connectomics to characterize normal brain function, mind-brain associations, and the various disorders. Bhushan et al., added that temporal Non-Local Means (tNLM) filtering can demise resting functional MRI (RFMRI) data while also retaining spatial structure that reflects ongoing dynamic brain activity [92]. Coupling Fourier transform infrared spectroscopy with focal plane array detectors at synchrotron radiation sources (SR-FTIR-FPA). Revealed that Purkinje neurons in the cerebellum are rich in cytosolic proteins and intracellular lactate that could be used in the future to study the changes in the density of the expressed binding proteins during aging process [93].
In vivo and ex vivo fluorescence microscopy
Cerebrospinal fluid-Interstitial fluid "CSF-ISF" exchange was evaluated by In vivo and ex vivo fluorescence microscopy and interstitial solute clearance was evaluated by radiotracer clearance assays in young (2-3 months), middle-aged (10-12 months), and old (18-20 months) wild-type mice. Advancing age was associated with a dramatic decline in the efficiency of exchange between the subarachnoid CSF and the brain parenchyma [94].
DISORDERS ASSOCIATE WITH AGING OF CEREBELLUM AND BASAL GANGLIA
Recent studies have reported a strong association between the structural and functional abnormalities of the cerebellum and psychiatric disorders especially schizophrenia, depression, anorexia, anxiety, attention deficit hyperactivity disorder and autism [95]. Basal ganglia dysfunction is associated with many disorders that influence movement including Parkinson's disease, Huntington disease, and uncontrolled or slow movement (dystonia) [96].
Parkinson disease
Parkinson’s disease is a neurodegenerative disorder primarily affecting the aging population. It is caused by the dysfunction of the entire basal ganglia–cortex–cerebellum system rather than by the basal ganglia in isolation. It results from a reduction of neurons that make dopamine in pars compacta of the substantia nigra. The three key symptoms of tremor, freezing, and impairments in action sequencing may be explained by considering partially overlapping neural circuits including basal ganglia, cortical and cerebellar areas [97,98]. Parkinson disease is characterized by motor dysfunctions including, tremor, difficulty in initiating and executing voluntary movements, muscular rigidity, and impaired execution of movement sequences as well as by non-motor deficits such as behavioral and cognitive impairments [99].
Cerbello olivary degeneration of holmes
Holmes tremor (also called rubral, midbrain, or cerebellar outflow tremor) is characterized by a resting tremor of a limb with marked accentuation on action, intention, and goal-oriented movement. Although the most common lesions are in the brainstem, lesions of the cerebellum and thalamus have also been reported. There was complete loss of PCs and associated astrocytes. In the inferior olivary nucleus the neuronal loss was secondary since the PC is the synaptic target of the inferior olivary nucleus neurons [100].
Schizophrenia
The cerebellum is among the most affected brain regions in schizophrenia, new research has found cerebellar volume was smaller in patients with schizophrenia compared to healthy individuals [101]. The cerebellar abnormalities in schizophrenia, such as decreased volume, decreased blood flow, and dysfunctional cortical pathways. The cerebellar volume loss in schizophrenia is possibly due to the reduction or absence of different parts of the cerebellum. A cortico-sub-cortico- cerebellar circuit has been postulated to be important in the patho-physiology of schizophrenia. This disorder was associated with decrease in the expression of synaptic proteins by excitatory neurons in the cerebellar cortex. This would disrupt neural circuits which consequently might contribute to cerebellar dysfunction, thought to occur during aging [102,95]. Studies in schizophrenic patients have brought observations supporting a cerebellar impairment: high prevalence of neurological soft signs, dys-coordination, impaired eye blink conditioning, impaired adaptation of the vestibular-ocular reflex or procedural learning tests, abnormal posture and proprioception, and lastly functional neuroimaging studies correlating poor cognitive performances with abnormal cerebellar activations [103].
Autism spectrum disorders
Known as a "spectrum" disorder because affected person can have a range of symptoms [104]. It is a neurological and developmental disorder that begins early in childhood and lasts throughout a person's life. It affects how a person acts and interacts with others, communicates, and learns. Autism spectrum disorder includes a range of motor symptoms, including repeated and stereotyped movements, impaired social interactions poor recognition of emotions, difficulty displaying physical gestures typically used in social interaction. Interestingly, it was found that cerebellar damage in infants can predict the occurrence of autism in older age. The cerebellum can influence the motor cortex [105].
Depression
It was stated that central to social signal transduction theory of depression is the hypothesis that experiences of social threat and adversity up regulate components of the immune system involved in inflammation. The key mediators of this response called pro-inflammatory cytokines can in turn elicit profound changes in behavior, which include the initiation of depressive symptoms such as sad mood, fatigue, psychomotor retardation, and socialbehavioral withdrawal. Increasingly proinflammatory phenotype may be a key phenomenon driving depression in pathogenesis and recurrence, as well as the overlap of depression with several somatic conditions including asthma, rheumatoid arthritis, chronic pain, metabolic syndrome, cardiovascular disease, obesity, and neurodegeneration [106]. Their work may also suggest new opportunities for preventing and treating depression by targeting inflammation. Previously, it is suggested that cyclic-AMP Specific phosphodiesterase in the brain (hippocampus, cerebellum and neocortex) is subject to age associated decline. The-response to rolipram (a clinically effective antidepressant drug with cAMP-selective phosphodiesterase inhibitory action) treatment is different between young and aged individuals i.e., this drug improves the general well-being state in young and prevent the emergence of depression with aging [107]. MacLullich et al., showed that the elderly humans, those individuals with rising cortisol levels with age subsequently showed loss of memory function and were susceptible to depression. Specifically, whereas structural enlargement of the basal ganglia and amygdala has been observed in bipolar disorder, in unipolar depression, these structures appear to be smaller in patients than healthy subjects [108].
Age-dependent decline in the food appetite (Anorexia)
Endogenous cannabinoids are lipid mediators that produce their effects via interaction with specific cannabinoid receptors and they have been implicated in a growing number of biological functions, including the control of appetite and food intake [109]. They found that expression of endogenous cannabinoids acting at brain receptors were reduced in the nucleus accumbens (one of basal ganglia) or another structure in the limbic forebrain and might be involved in the age-dependent decline in food intake. De Boer et al., and Yehuda & Rabinovitz reported that feeding behavior is highly complex, and is controlled by many psychological, physiological, biochemical, and immunological factors [110,111]. Anorexia of aging has a variety of consequences, including a decline in functional status, impaired muscle function, decreased bone mass, micronutrient deficiencies, reduced cognitive functions, increased hospital admission and even premature death, diseases and age-related changes.
PROPHYLACTIC AND THERAPEUTIC TREATMENTS OF AGE-RELATED DISORDER
Physical exercise
It can modify the risk factors and induce neuroprotective mechanisms which may reduce the declines in cognitive performance attributed to the normal aging process and protect against changes related to neurodegenerative diseases such as AD. It can play this role through modifying metabolic, structural, and functional dimensions of the brain and preserving cognitive performance in older adults. The results of observational studies support a dose dependent neuroprotective relationship between physical exercise and cognitive performance in older adults [112]. Study of Helen et al., suggested that yoga can be as effective as memory enhancement training in improving verbal memory performance in association with increased default mode network connectivity [113]. These findings highlight the potential clinical use of yoga for subjective cognitive complaints that should be confirmed in larger studies.
Diet
Antioxidant-rich diets improved cerebellar physiology and motor learning in aged rats [114,115]. Many natural compounds have been considered, either singularly or in combination, for supplementation therapies. These include vitamin C, vitamin E, resveratrol, curcumin, hydroxytyrosol and coenzyme Q10. These diets were identified as being high antioxidant activity and they are of a good value in treatment of aging associated diseases [116]. Curcumin strong medicinal properties are also associated with reported anti-cancer and neuroprotective effect such as in Alzheimer’s disease [117]. González-Reyes et al., identified curcumin as a neuroprotector against hemin, the oxidized form of heme, which induced damage in primary cultures of cerebellar granule neurons of rats [118]. Vitamin E is a fat-soluble vitamin and is well known as an antioxidant, its deficiency induces oxidative stress in the brain and causes memory and motor dysfunction. Vitamin E rich diet has been given much attention in recent years, regarding the prevention of age-related neuronal disorders. Rice brain, a byproduct of the rice milling process, is known to be a rich source of antioxidants including vitamin E. The effects of dietary rice brain on neuronal abnormalities such as cerebellar ataxia induced by vitamin E deficiency were investigated and revealed good improvement after its supplementation [119]. The role of Docosahexaenoic Acid (DHA) in prevention of age related disruption of brain function is an omega-3 (ω-3) Long-Chain Poly-Unsaturated Fatty Acid (LCPUFA) relevant for brain function. It has largely been explored as a potential candidate to treat AD. Clinical evidence favors a role for DHA in the improvement of cognition in very early stages of the AD. In response to stress or damage, DHA generates oxygenated derivatives called docosanoids that can activate the peroxisome proliferator-activated receptor γ (PPARγ). In conjunction this modulates inflammation, cell survival, and lipid metabolism [120]. Dietary approach (Fortasyn) including docosahexaenoic acid, uridine, choline, phospholipids, folic acid, vitamins B12, B6, C, and E, and selenium has been proposed for dietary management of AD. These diets could inhibit AD-like pathologies in aging mice. [121].
Drugs
L-carnitine administration: In aged rats it lead to restoration of the level of acetyl cholinesterase in different brain regions including the striatum and the cerebellum and reversed the age associated changes through neuroprotective effect on the aged brain by elevation antioxidants [122].
Taurine administration: A single dose immediately before and after ozone exposure was found to block per oxidation effect (caused by ozone) in the striatum of old rats. So, this will lead to improvement short and long-term memory. The useful effects of taurine as an antioxidant have been attributed to its ability to stabilize biomembranes, to scavenge ROS, and to decrease the per oxidation of unsaturated membrane lipids [123]. In addition, taurine scavenges hypochlorous acid produced by the activation of granulocytes, forming taurine-chloramine, and thus may act as an indirect antioxidant [124].
Pentifylline and nicotinic acid: As an oral treatment of elderly human volunteers aged 52-70 years for two months with a combination of pentifylline (800 mg) and nicotinic acid (200 mg) improve the cerebral blood flow of the total brain, with a more pronounced improvement in the cerebellum and frontal cortex. These volunteers experienced an improvement in memory and general well-being [125]. Nicotinic acid (Niacin) is a water-soluble B-complex vitamin that induces a profound change in the plasma levels of various lipids and lipoproteins [126]. It could strongly increase the plasma concentration of high-density lipoprotein cholesterol. So, there is increasing evidence that nicotinic acid alone or in addition to LDL cholesterol-lowering drugs can reduce the progression of atherosclerosis and reduce the risk of cardiovascular events. Lukasova et al., and Zeman et al., stated that in addition, the identification of a nicotinic acid receptor expressed in adipocytes and immune cells helped to elucidate the mechanisms underlying the anti-atherosclerotic effect of the drug through direct and indirect effects on the vascular endothelium [127,128].
Melatonin: Melatonin hormone as antioxidant, along with its protective role could play a key role in aging and senescence. It is a regulator of the sleep/wake cycle and acts as an effective antioxidant and mitochondrial function protector. A reduction in the expression of melatonin receptors has been documented in the substantia nigra of Parkinson’s disease patients. The efficacy of melatonin for preventing neuronal cell death and for ameliorating PD symptoms has been demonstrated in animal models of PD employing neurotoxins. A small number of controlled trials indicate that melatonin is useful in treating disturbed sleep in PD [129].
Ayurveda herbal mixture: “Maharishi Amrit Kalash” (MAK): Electron microscopic observations of Vohra et al., revealed various degenerative changes in the mitochondria with age [130]. Treatment of the animals with the Ayurvedic herbal mixture "Maharishi Amrit Kalash" (MAK), 500 mg/kg body wt. daily for 2 months, significantly induced the activity of antioxidant enzymes, and reversed the pathological changes to a considerable extent. MAK increased the activity of GPx significantly only in the 32-month-old animals. This shows the specificity of the action of MAK.
Diethyl Hydroxylamine (DEHA): Sharma and Singh stated that rats fed a DEHA for 30, 60 and 90 days showed a significant reduction in lipid per oxidation levels and lipofuscin contents in the cerebellum, brain stem and spinal cord [131]. Moreover, DEHA is used as a free radical scavenger and antioxidants, metabolic rate and life expectancy increase in mice fed diethyl hydroxylamine DEHA [132].
Treatment with a cellular concentrate derived from an individual's own fat: Stem Genex online group reported that treatment with a cellular concentrate derived from an individual's own fat, known as the Stromal Vascular Fraction (SVF), has on the quality of life of people with PD [133]. They concluded that SVF contains components with "regenerative" properties, including stem cells that may can ameliorate specific disease conditions.
CONCLUSION
It could be concluded that aging of the brain leads to impairments in cognitive and motor skills, and is the major risk factor for several common neurological disorders such as depression, Parkinson disease, and Schizophrenia. As we have reviewed here normal cerebellar and basal ganglia aging is associated with subtle pathological and functional alterations, in specific neuronal circuits, as opposed to large-scale neuronal loss, atrophy of cells and synapses, cytoskeleton abnormalities and reactive astrocytes and microglia. The most recent global analysis of basal ganglia and cerebellar aging at the molecular level suggested that aging resulted in a gene expression profile indicative of an inflammatory response, oxidative stress and reduced neurotrophic support in both brain regions. The cerebellar cortex is a frequently used model in neuroscience research in general and particularly for the study of age changes in the CNS neurons including deep cerebellar nuclei and neurons of the basal ganglia [134,135]. Recent studies suggest that the cerebellum is an excellent model for the study of the age-related changes at the cellular level [136,137]. Recent studies provide a clue to void the unwanted age-related disorders, first caloric restriction which retards the aging process as it selectively attenuates the age associated induction of genes encoding inflammatory and stress response. Next physical exercise, nutritional supplementation with diets high in antioxidant capacity and intake of herbal mixture can prevent and/or reverse age-related degenerative changes.
REFERENCES
- Mc Auley MT, Guimera AM, Hodgson D, Mcdonald N, Mooney KM, et al. (2017) Modelling the molecular mechanisms of aging. Biosci Rep 37: 20160177.
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U (2010) Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage 51: 501-511.
- Raz N, Gunning-Dixon F, Head D, Williamson A, Acker JD (2001) Age and sex differences in the cerebellum and the ventral pons: a prospective MR study of healthy adults. AJNR Am J Neuroradiol 22: 1161-1167.
- Clausi S, Iacobacci C, Lupo M, Olivito G, Molinari M, et al. (2017) The Role of the Cerebellum in Unconscious and Conscious Processing of Emotions: A Review. Applied Sciences 7: 521.
- Bonasera SJ, Arikkath J, Boska MD, Chaudoin TR, DeKorver NW, et al. (2016) Age-related changes in cerebellar and hypothalamic function accompany non-microglial immune gene expression, altered synapse organization, and excitatory amino acid neurotransmission deficits. Aging (Albany NY) 8: 2153-2181.
- Iansek R, Morris ME (2013) Rehabilitation in movement disorders. Cambridge University Press, New York, USA.
- Saga Y, Hoshi E, Tremblay L (2017) Roles of Multiple Globus Pallidus Territories of Monkeys and Humans in Motivation, Cognition and Action: An Anatomical, Physiological and Pathophysiological Review. Front Neuroanat 11: 30.
- Sierra F, Kohanski R (2015) Advances in Geroscience. Springer International Publishing, Berlin, Germany.
- Lidzba K, Wilke M, Staudt M, Krägeloh-Mann I, Grodd W (2008) Reorganization of the cerebro-cerebellar network of language production in patients with congenital left-hemispheric brain lesions. Brain Lang 106: 204-210.
- Cavdar S, San T, Aker R, Sehirli U, Onat F (2001) Cerebellar connections to the dorsomedial and posterior nuclei of the hypothalamus in the rat. J Anat 198: 37-45.
- Bailey R (2017) Basal Ganglia Function. Thoughtco.
- Seger CA (2008) How do the basal ganglia contribute to categorization? Their roles in generalization, response selection, and learning via feedback. Neurosci Biobehav Rev 32: 265-278.
- Giardino L, Zanni M, Fernandez M, Battaglia A, Pignataro O, et al. (2002) Plasticity of GABA(a) system during ageing: focus on vestibular compensation and possible pharmacological intervention. Brain Res 929: 76-86.
- Leisman G, Braun-Benjamin O, Melillo R (2014) Cognitive-motor interactions of the basal ganglia in development. Front Syst Neurosci 8: 16.
- Sokolov AA, Miall RC, Ivry RB (2017) The Cerebellum: Adaptive Prediction for Movement and Cognition. Trends Cogn Sci 21: 313-332.
- Leisman G, Moustafa AA, Shafir T (2016) Thinking, Walking, Talking: Integratory Motor and Cognitive Brain Function. Front Public Health 4: 94.
- Woodruff-Pak DS, Foy MR, Akopian GG, Lee KH, Zach J, et al. (2010) Differential effects and rates of normal aging in cerebellum and hippocampus. Proc Natl Acad Sci USA 26: 1624-1629.
- Bernard JA, Seidler RD (2014) Moving forward: age effects on the cerebellum underlie cognitive and motor declines. Neurosci Biobehav Rev. 42: 193-207.
- Di Lorenzo Alho AT, Suemoto CK, Polichiso L, Tampellini E, de Oliveira KC, et al. (2016) Three-dimensional and stereological characterization of the human substantia nigra during aging. Brain Struct Funct 221: 3393-3403.
- Kemp KC, Cook AJ, Redondo J, Kurian KM, Scolding NJ, et al. (2016) Purkinje cell injury, structural plasticity and fusion in patients with Friedreich’s ataxia. Acta Neuropathol Commun 4: 53.
- Zhang C, Zhu Q, Hua T (2010) Aging of cerebellar Purkinje cells. Cell Tissue Res 341: 341-347.
- Vohra BP, Sharma SP, Kansal VK (2002) Age-dependent variation in mitocliondrial and cytosolic antioxidant enzymes and lipid peroxidation in different regions of central nervous system of' guinea pigs. Indian J Biochem Biophys 38: 321-326.
- Zhang C, Zhu Q, Hua T (2010) Aging of cerebellar Purkinje cells. Cell Tissue Res 341: 341-347.
- Zhang C, Zhu Q, Hua T (2011) Effects of aging on dendritic arborizations, dendritic spines, and somatic configurations of cerebellar Purkinje cells of old cat. Pak J Zool 43: 1191-1196.
- Šišková Z (2013) How structure shapes (dys)function: A perspective to understanding brain region-specific degeneration in prion disease. Prion 7: 291-293.
- de Graaf EL, Vermeij WP, de Waard MC, Rijksen Y, van der Pluijm I, et al. (2013) Spatio-temporal Analysis of Molecular Determinants of Neuronal Degeneration in the Aging Mouse Cerebellum. Mol Cell Proteomics 12: 1350-1362.
- Woodruff-Pak DS, Foy MR, Akopian GG, Lee KH, Zach J, et al. (2010) Differential effects and rates of normal aging in cerebellum and hippocampus. Proc Natl Acad Sci USA 107: 1624-1629.
- Li C, Zhang L, Ma Q, Tang Y, He Y (2017) Stereological evidence for de/re-generation of myelin sheaths in aged brain white matter of female rats. Image Anal Stereol 36: 111-120.
- Andersen BB, Gundersen HJ, Pakkenberg B (2003) Aging of the human cerebellum: a stereological study. J Comp Neurol 466: 356-365.
- Sato J, Sasaki S, Yamada N, Tsuchitani M (2012) Hereditary cerebellar degenerative disease (cerebellar cortical abiotrophy) in rabbits. Vet Pathol 49: 621-628.
- Walløe S, Pakkenberg B, Fabricius K (2014) Stereological estimation of total cell numbers in the human cerebral and cerebellar cortex. Front Hum Neurosci 8: 508.
- Zhang C, Hua T, Zhu Z, Luo X (2006) Age-related changes of structures in cerebellar cortex of cat. J Biosci 31: 55-60.
- Reichenbach A, Derouiche A, Kirchhoff F (2010) Morphology and dynamics of perisynaptic glia. Brain Res Rev 63: 11-25.
- Kaneko M, Yamaguchi K, Eiraku M, Sato M, Takata N, et al. (2011) Remodeling of monoplanar Purkinje cell dendrites during cerebellar circuit formation. PLoS One 6: 20108.
- Sim FJ, Zhao C, Penderis J, Franklin RJ (2002) The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci 22: 2451-2459.
- Zhang Y, Kim MS, Jia B, Yan J, Zuniga-Hertz JP, et al. (2017) Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 548: 52-57.
- Boisvert MM, Erikson GA, Shokhirev MN, Allen NJ (2018) The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Reports 22: 269-285.
- Shyian DN, Galata DI, Potapov SN, Gargin VV, Shyian D, et al. (2016) Peculiarities of the cerebellum nuclei in aged persons. Georgian Med News 4: 110-115.
- Grimm A, Eckert A (2017) Brain aging and neurodegeneration: from a mitochondrial point of view. J Neurochip 143: 418-431.
- Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, et al. (2010) New insights into the role of mitochondria in aging: Mitochondrial dynamics and more. J Cell Sci 123: 2533-2542.
- Stahon KE, Bastian C, Griffith S, Kidd GJ, Brunet S, et al. (2016) Age-Related Changes in Axonal and Mitochondrial Ultrastructure and Function in White Matter. J Neurosci 36: 9990-10001.
- Dirks RA, Stunnenberg HG, Marks H (2016) Genome-wide epigenomic profiling for biomarker discovery. Clin Epigenetics 8: 122.
- Taneja R, Kennedy BK (2017) T(ell) TALE signs of aging. Cell Res 27: 453-454.
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, et al. (2009) Minute effects of sex on the aging brain: a multisample magnetic resonance imaging study of healthy aging and Alzheimer's disease. J Neurosci 29: 8774-8783.
- Arani A, Murphy MC, Glaser KJ, Manduca A, Lake DS, et al. (2015) Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. Neuroimage 111: 59-64.
- Ortuño-Sahagún D, Pallàs M, Rojas-Mayorquín AE (2014) Oxidative stress in aging: advances in proteomic approaches. Oxid Med Cell Longev 2014: 573208.
- Moulton PV, Yang W (2012) Air pollution, oxidative stress, and Alzheimer's disease. Journal of environmental and public health 472751: 9.
- Killin LO, Starr JM, Shiue IJ, Russ TC (2016) Environmental risk factors for dementia: a systematic review. BMC Geriatr 16: 175.
- Russ TC, Murianni L, Icaza G, Slachevsky A, Starr JM (2016) Geographical variation in Dementia mortality in Italy, New Zealand, and Chile: the impact of latitude, vitamin D, and air pollution. Dement Geriatr Cogn Disord 42: 31-41.
- Gems D (2000) An integrated theory of ageing in the nematode Caenorhabditis elegans. J Anat 197: 521-528.
- Bickford PC, Gould T, Briederick L, Chadman K, Pollock A, et al. (2000) Antioxidant-rich diets improve cerebellar physiology and motor learning in aged rats. Brain Res 866: 211-217.
- Johnson WM, Wilson-Delfosse AL, Mieyal JJ (2012) Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients 4: 1399-1440.
- Song JY, Ichtchenko K, Sudhof TC, Brose N (1999) Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci USA 96: 1100-1105.
- Dzubay JA, Otis TS (2002) Climbing fiber activation of metabotropic glutamate receptors on cerebellar purkinje neurons. Neuron 36: 1159-1167.
- Noda Y, Zomorrodi R, Cash RF, Barr MS, Farzan F, et al. (2017) Characterization of the influence of age on GABAA and glutamatergic mediated functions in the dorsolateral prefrontal cortex using paired-pulse TMS-EEG. Aging 9: 556-572.
- Glassford JA (2017) The Neuroinflammatory Etiopathology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Front Physiol 8: 88.
- Thibault O, Gant JC, Landfield PW (2007) Expansion of the calcium hypothesis of brain aging and Alzheimer's disease: minding the store. Aging cell 6: 307-317.
- Raza M, Deshpande LS, Blair RE, Carter DS, Sombati S, et al. (2007) Aging is associated with elevated intracellular calcium levels and altered calcium homeostatic mechanisms in hippocampal neurons. Neurosci Lett 418: 77-81.
- Zündorf G, Reiser G (2011) Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid Redox Signal 14: 1275-1288.
- Fuchs JR, Darlington SW, Green JT, Morielli AD (2017) Cerebellar learning modulates surface expression of a voltage-gated ion channel in cerebellar cortex. Neurobiol Learn Mem 142: 252-262.
- Gidalevitz T, Kikis E A, Morimoto RI (2010) A cellular perspective on conformational disease: the role of genetic background and proteostasis networks. Curr Opin Struct Biol 20: 23-32.
- Raj D, Yin Z, Breur M, Doorduin J, Holtman IR, et al. (2017) Increased White Matter Inflammation in Aging- and Alzheimer’s Disease Brain. Front Mol Neurosci 10: 206.
- Skaper SD (2012) The neurotrophin family of neurotrophic factors: An overview. Neurotrophic Factors 1-12.
- Petzold A, Psotta L, Brigadski T, Endres T, Lessmann V (2015) Chronic BDNF deficiency leads to an age-dependent impairment in spatial learning. Neurobiology of learning and memory 120: 52-60.
- Budni J, Bellettini-Santos T, Mina F, Garcez ML, Zugno AI (2015) The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis 6: 331-341.
- Werner H, LeRoith D (2014) Insulin and insulin-like growth factor receptors in the brain: physiological and pathological aspects. Eur Neuropsychopharmacol 24: 1947-1953.
- Bachschmid MM, Schildknecht S, Matsui R, Zee R, Haeussler D, et al. (2013) Vascular aging: chronic oxidative stress and impairment of redox signaling-consequences for vascular homeostasis and disease. Ann Med 45: 17-36.
- Camandola S, Mattson MP (2017) Brain metabolism in health, aging, and neurodegeneration. EMBO J 36: 1474-1492.
- Sasaki T, Akimoto Y, Sato Y, Kawakami H, Hirano H, et al. (2002) Distribution of sialoglycoconjugates in the rat cerebellum and its change with aging. J Histochem Cytochem 50: 1179-1186.
- Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, et al. (2018) High-Dimensional Single-Cell Mapping of Central Nervous System Immune Cells Reveals Distinct Myeloid Subsets in Health, Aging, and Disease. Immunity 48: 380-395.
- Colombrita C, Calabrese V, Stella AM, Mattei F, Alkon DL, et al. (2003) Regional rat brain distribution of heme oxygenase-1 and manganese superoxide dismutase mRNA: relevance of redox homeostasis in the aging processes. Exp Biol Med (Maywood) 228: 517-524.
- Reddy AS, O'Brien D, Pisat N, Weichselbaum CT, Sakers K, et al. (2017) A Comprehensive Analysis of Cell Type-Specific Nuclear RNA From Neurons and Glia of the Brain. Biol Psychiatry 81: 252-264.
- Prolla TA (2002) DNA microarray analysis of the aging brain. Chem Senses 27: 299-306.
- Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, et al. (2013) Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell 49: 359-367.
- Jazwinski SM, Kim S (2017) Metabolic and Genetic Markers of Biological Age. Front Genet 8: 64.
- Zhao Z, Chou DK, Nair SM, Tobet S, Jungalwala FB (2000) Expression of sulfoglucuronyl (HNK-1) carbohydrate and its binding protein (SBP-1) in developing rat cerebellum. Brain Res Dev Brain Res 120: 165-180.
- Cerbai F, Lana D, Nosi D, Petkova-Kirova P, Zecchi S, et al. (2012) The neuron-astrocyte-microglia triad in normal brain ageing and in a model of neuroinflammation in the rat hippocampus. PloS one 7: 45250.
- Fulop T, Dupuis G, Baehl S, Le Page A, Bourgade K, et al (2016) From inflamm-aging to immune-paralysis: a slippery slope during aging for immune-adaptation. Biogerontology 17: 147-157.
- Dager SR, Oskin NM, Richards TL, Posse S (2008) Research applications of magnetic resonance spectroscopy (MRS) to investigate psychiatric disorders. Top Magn Reson Imaging 19: 81-96.
- Strakowski SM, Adler CM, DelBello MP (2002) Volumetric MRI studies of mood disorders: do they distinguish unipolar and bipolar disorder? Bipolar Disord 4: 80-88.
- de Groot M, Cremers LG, Ikram MA, Hofman A, Krestin GP, et al. (2015). White matter degeneration with aging: longitudinal diffusion MR imaging analysis. Radiology 279: 532-541.
- Liu H, Wang L, Gang Z, Zhu Q, Song Z, et al. (2016) A voxel-based morphometric study of age- and sex-related changes in white matter volume in the normal aging brain. Neuropsychiatr Dis Treat 12: 453-465.
- Bernard JA, Seidler RD (2013) Relationships between regional cerebellar volume and sensorimotor and cognitive function in young and older adults. Cerebellum 12: 721-737.
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH Jr (2010) Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp 31: 378-390.
- Cao X, Yao Y, Li T, Cheng Y, Feng W, et al. (2016) The impact of cognitive training on cerebral white matter in community-dwelling elderly: one-year prospective longitudinal diffusion tensor imaging study. Scientific reports 6: 33212.
- Andersen BB, Gundersen HJ, Pakkenberg B (2003) Aging of the human cerebellum: a stereological study. J Comp Neurol 466: 356-365.
- Andersen K, Andersen BB, Pakkenberg B (2012) Stereological quantification of the cerebellum in patients with Alzheimer’s disease. Neurobiol Aging 33: 11-20.
- Bernard JA, Seidler RD (2014) Moving forward: age effects on the cerebellum underlie cognitive and motor declines. Neurosci Biobehav Rev 42: 193-207.
- Di Lorenzo Alho AT, Suemoto CK, Polichiso L, Tampellini E, de Oliveira KC, et al. (2016) Three-dimensional and stereological characterization of the human substantia nigra during aging. Brain Struct Funct 221: 3393-3403.
- Ferreira LK, Busatto GF (2013) Resting-state functional connectivity in normal brain aging. Neurosci Biobehav Rev 37: 384-400.
- Zuo XN, Xing XX (2014) Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: A systems neuroscience perspective. Neurosci Biobehav Rev 45: 100-118.
- Bhushan C, Chong M, Choi S, Joshi AA, Haldar JP, et al. (2016) Temporal non-local means filtering reveals real-time whole-brain cortical interactions in resting fMRI. PloS one 11: 0158504.
- Hackett MJ, Britz CJ, Paterson PG, Nichol H, Pickering IJ, et al. (2014) in situ biospectroscopic investigation of rapid ischemic and postmortem induced biochemical alterations in the rat brain. ACS chemical neuroscience 6: 226-238.
- Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, et al. (2014) Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76: 845-861.
- Phillips JR, Hewedi DH, Eissa AM, Moustafa AA (2015) The Cerebellum and Psychiatric Disorders. Front Public Health 3: 66.
- Bailey R (2017) Basal Ganglia Function. Thoughtco.
- Caligiore D, Pezzulo G, Baldassarre G, Bostan AC, Strick PL, et al. (2017) Consensus paper: towards a systems-level view of cerebellar function: the interplay between cerebellum, basal ganglia, and cortex. The Cerebellum 16: 203-229.
- Gao L-L, Wu T (2016) The study of brain functional connectivity in Parkinson’s disease. Transl Neurodegener 5: 18.
- Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, et al. (2010) Missing pieces in the Parkinson's disease puzzle. Nat Med 16: 653-661.
- Sung YF, Hsu YD, Huang WS (2009) 99mTc-TRODAT-1 SPECT study in evaluation of Holmes tremor after thalamic hemorrhage. Annals of nuclear medicine 23: 605-608.
- Moberget T, Doan NT, Alnæs D, Kaufmann T, Córdova-Palomera A, et al. (2017) Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: a multisite mega-analysis of 983 patients and 1349 healthy controls. Mol Psychiatry.
- Harrison PJ, Eastwood SL (2001) Neuropathological studies of synaptic connectivity in the hippocampal formation in schizophrenia. Hippocampus 11: 508-519.
- Picard H, Amado I, Mouchet-Mages S, Olié JP, Krebs MO (2007) The Role of the Cerebellum in Schizophrenia: an Update of Clinical, Cognitive, and Functional Evidences. Schizophr Bull 34: 155-172.
- Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson JL, et al. (2015) Harrison's Principles of Internal Medicine 19/E (Vol.1 & Vol.2) (19thedn). McGraw Hill Professional, New York, USA. Pg no: 3000.
- American Psychiatric Association (2017) Diagnostic and Statistical Manual of Mental Disorders (DSM-5). American Psychiatric Association, Washington, DC, USA.
- Slavich GM, Irwin MR (2014) From Stress to Inflammation and Major Depressive Disorder: A Social Signal Transduction Theory of Depression. Psychol Bull 140: 774-815.
- Kato H, Araki T, Chen T, Itoyama Y, Kogure K (1998) Effect of rolipram on age-related changes in cyclic AMP-selective phosphodiesterase in the rat brain: an autoradiographic study. Methods Find Exp Clin Pharmacol 20: 403-408.
- MacLullich AM, Deary IJ, Starr JM, Ferguson KJ, Wardlaw JM, et al. (2005) Plasma cortisol levels, brain volumes and cognition in healthy elderly men. Psychoneuroendocrinology 30: 505-515.
- Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G (2003). Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci USA 100: 1393-1398.
- de Boer A, Ter Horst GJ, Lorist MM (2013) Physiological and psychosocial age-related changes associated with reduced food intake in older persons. Ageing Res Rev 12: 316-328.
- Yehuda S, Rabinovitz S (2016) The Role of Essential Fatty Acids in Anorexia Nervosa and Obesity. Crit Rev Food Sci Nutr 56: 2021-2035.
- Kirk-Sanchez NJ, McGough EL (2014) Physical exercise and cognitive performance in the elderly: current perspectives. Clin Interv Aging 9: 51-62.
- Lavretsky H, Yang H, Eyre H, Leaver A, Narr K, et al. (2015) M1. Changes in the Functional Brain Connectivity and Verbal Memory Performance Following Yoga or Memory Training in Older Adults with Subjective Memory Complaints. Neuropsychopharmacology 40: 106-271.
- Vohra BP, Sharma SP, Kansal VK (2002) Age-dependent variation in mitocliondrial and cytosolic antioxidant enzymes and lipid peroxidation in different regions of CNIS of' guinea pigs. Indian J Biochem Biophys 38: 321-326.
- Shukitt-Hale B, Lau FC, Joseph JA (2008) Berry fruit supplementation and the aging brain. J Agric Food Chem 56: 636-641.
- Conti V, Izzo V, Corbi G, Russomanno G, Manzo V, et al. (2016) Antioxidant supplementation in the treatment of aging-associated diseases. Front Pharmacol 7: 24.
- Brondino N, Re S, Boldrini A, Cuccomarino A, Lanati N, et al. (2014) Curcumin as a therapeutic agent in dementia: a mini systematic review of human studies. ScientificWorldJournal 174282.
- González-Reyes S, Guzmán-Beltrán S, Medina-Campos ON, Pedraza-Chaverri J (2013) Curcumin Pretreatment Induces Nrf2 and an Antioxidant Response and Prevents Hemin-Induced Toxicity in Primary Cultures of Cerebellar Granule Neurons of Rats. Oxid Med Cell Longev 2013: 801418.
- Takahashi T, Nakaso K, Horikoshi Y, Hanaki T, Yamakawa M, et al. (2016) Rice Bran Dietary Supplementation Improves Neurological Symptoms and Loss of Purkinje Cells in Vitamin E-Deficient Mice. Yonago Acta Med 59: 188-195.
- Heras-Sandoval D, Pedraza-Chaverri J, Pérez-Rojas JM (2016) Role of docosahexaenoic acid in the modulation of glial cells in Alzheimer’s disease. J Neuroinflammation 13: 61.
- Wiesmann M, Zerbi V, Jansen D, Haast R, Lütjohann D, et al. (2016) A dietary treatment improves cerebral blood flow and brain connectivity in aging apoE4 mice. Neural Plast 2016: 6846721.
- Rani PJ, Panneerselvam C (2001) Protective efficacy of L-carnitine on acetylcholinesterase activity in aged rat brain. J Gerontol A Biol Sci Med Sci 56: 140-141.
- Rivas-Arancibia S, Dorado-Martínez C, Borgonio-Pérez G, Hiriart-Urdanivia M, Verdugo-Diaz L, et al. (2000) Effects of taurine on ozone-induced memory deficits and lipid peroxidation levels in brains of young, mature, and old rats. Environ Res 82: 7-17.
- Yildirim Z, Kilic N (2011) Effects of taurine and age on cerebellum antioxidant status and oxidative stress. International Journal of Gerontology 5: 166-170.
- Dormehl IC, Jordaan B, Oliver DW, Croft S (1999) SPECT monitoring of improved cerebral blood flow during long-term treatment of elderly patients with nootropic drugs. Clin Nucl Med 24: 29-34.
- Gille A, Bodor ET, Ahmed K, Offermanns S (2008) Nicotinic acid: pharmacological effects and mechanisms of action. Annu Rev Pharmacol Toxicol 48: 79-106.
- Lukasova M, Hanson J, Tunaru S, Offermanns S (2011) Nicotinic acid (niacin): new lipid-independent mechanisms of action and therapeutic potentials. Trends Pharmacol Sci 32: 700-707.
- Zeman M, Vecka M, Perlík F, Sta?ková B, Hromádka R, et al. (2016) Pleiotropic effects of niacin: Current possibilities for its clinical use. Acta Pharm 66: 449-469.
- Srinivasan V, Cardinali DP, Srinivasan US, Kaur C, Brown GM, et al. (2011) Therapeutic potential of melatonin and its analogs in Parkinson’s disease: Focus on sleep and neuroprotection. Ther Adv Neurol Disord 4: 297-317
- Vohra BP, Sharma SP, Kansal VK (2001) Age-dependent variations in mitochondrial and cytosolic antioxidant enzymes and lipid peroxidation in different regions of central nervous system of guinea pigs. Indian J Biochem Biophys 38: 321-326.
- Sharma D, Singh R (1999) Diethylhydroxylamime given In vivo inhibits lipid peroxidation and lipofuscin formation in the nervous tissues of rat. NISCAIR-CSIR 37: 355-358.
- Rattan SI (2003) Modulating aging and longevity. Springer Science, Netherlands.
- StemGenex (2017) Outcomes Data of Adipose Stem Cells to Treat Parkinson’s disease. US National Institutes of Health, Bethesda, Maryland, USA.
- Manto ML (2010) Cerebellar Disorders: A Practical Approach to Diagnosis and Management. Cambridge University Press, New York, USA.
- Grimm A, Eckert A (2017) Brain aging and neurodegeneration: from a mitochondrial point of view. J Neurochem 143: 418-431.
- Clausi S, Iacobacci C, Lupo M, Olivito G, Molinari M, et al. (2017) The Role of the Cerebellum in Unconscious and Conscious Processing of Emotions: A Review. Applied Sciences 7: 521.
- Bonasera SJ, Arikkath J, Boska MD, Chaudoin TR, DeKorver NW, et al. (2016) Age-related changes in cerebellar and hypothalamic function accompany non-microglial immune gene expression, altered synapse organization, and excitatory amino acid neurotransmission deficits. Aging (Albany NY) 8: 2153-2165.



