
Structure of the Ways of Lymphatic Drainage in the Liver under Conditions of Remote Tumor Growth
*Corresponding Author(s):
Zhumadina SMKazakh State Agrotechnical University, Astana, Kazakhstan
Tel:+7 87979815971,
Email:ms.zhumadina@mail.ru
Abstract
Keywords
TOPICALITY
It is known that the initial parts of the lymphatic system of the liver are Disse's space bounded by hepatocyte microvillion one side, and by endothelial cells lining the liver sinusoidson the other hand [3]. It is assumed that the lymph in the liver arises from plasma components that are filtered through the fenestra of sinusoidal endothelial cells into the Disse's space, then into the interstitial Mall’s space and lymph capillaries and the portal tract vessels [3].
Goal of research
RESEARCH METHOD
Experimental studies were carried out on CBA male mice weighing 18-20 g at the age of 3 months. Animals were kept on a standard diet with free access to water and food. Work with animals was carried out in accordance with the "Rules of work with the use of experimental animals".
Two groups of animals had been used in the experiment. The first group included intact mice; the second group included animals with the development of the tumor process. Hepatocarcinoma-29 (G-29) cells were used to induce the tumor process. Hepatocarcinoma-29 was obtained and verified by employees of the Institute of Cytology and Genetics of the Siberian Branch of the Russian Academy of Sciences and was kindly provided for our research [4]. CBA mice were vaccinated with G-29 cells into the abdominal cavity. After 10 days, an ascitic fluid was collected, and suspended in 10-fold volume of saline and injected with 0.1 ml to intact animals into the muscle of the right thigh. Material sampling for research was performed after 3, 7, 13 and 30 days of the experiment. Animals were removed from the experiment under ether anesthesia by the method of cranio-cervical dislocation.
Liver samples were fixed in 10% neutral formalin solution, processed by standard histological method and embedded in paraffin. Paraffin sections were stained with hematoxylin and eosin and using antibodies. All stages of the Immunohistochemical (IHC) reaction (dewaxing, unmasking, incubation with primary antibodies, etc.) were performed according to the protocol of the antibody manufacturer. Monoclonal antibodies to LYVE-1 (DCSImmunoLine) and Podoplanin (MONOSAN) were used.
For submicroscopic examination, liver samples were fixed in 4% paraformaldehyde solution prepared on Hanks medium, fixed for 1 hour in 1% OsO4 solution (Osmium Tetroxide) (Sigma, USA) in phosphate buffer (pH = 7.4), dehydrated in ethanol of increasing concentration and concluded in epon (Serva, Germany). Semifine sections with a thickness of 1 μm were obtained on a Leica EM UC7 ultra microtome (Germany/Switzerland), stained with toluidine blue, studied under a LEICADME light microscope (Germany), and photographed using the Avigilon computer program. Micrographs were morphometric using the ImageJ computer program. The volume density of the parenchyma and stroma of the liver, the numerical density of hepatocytes and their nuclei, binuclear hepatocytes using a closed test system of 140 points were estimated.
Ultrathin sections with a thickness of 70-100 nm were contrasted with a saturated aqueous solution of uranyl acetate and lead citrate and examined in a JEM 1010 electron microscope (Japan). The sizes of Disse’s spaces were determined using the ImageJ computer program. Statistical data processing was performed using the Statistica 6.0 program. The mean values and standard deviation were calculated, the significance of differences was calculated using the Mann-Whitney U-test and was taken with p<0.05.
RESEARCH RESULTS
Yu I Borodin formulated the concept of the lymphatic region as a morphofunctional unit, which includes the transport and detoxification component. It covers the regional lymphatic apparatus of the organ (part of the body) and the pool of its lymphoslect. In the lymphatic region, there are 3 lymphatic drainage links: interstitial nonvascular mass transfer paths from the pericellular space to the roots of the lymphatic bed, lymphatic vessels (capillaries, postcapillaries, vessels) and regional lymph nodes. The bed of the non-vascular microcirculation of the liver is rather complicated and consists of at least 2 cascades: the Disse’s space and the periportal Mall’s space [5].
When studying the structure of the liver in the dynamics of tumor growth of G-29 in the muscle tissue of the thigh of experimental animals, there was a tendency to decrease the bulk density of hepatocytes. By the 30th day of the experiment, the volume density of hepatocytes in animals with tumor growth was 22% (p<0.05) lower than in control animals (Figure 1a).
At that, for this research period, the value of the numerical density of hepatocytes was decreased by 30% (p<0.05) (Figure 1b) and the volume density of sinusoidal spaces was increased by 88% (p<0.05) (Figure 1c).
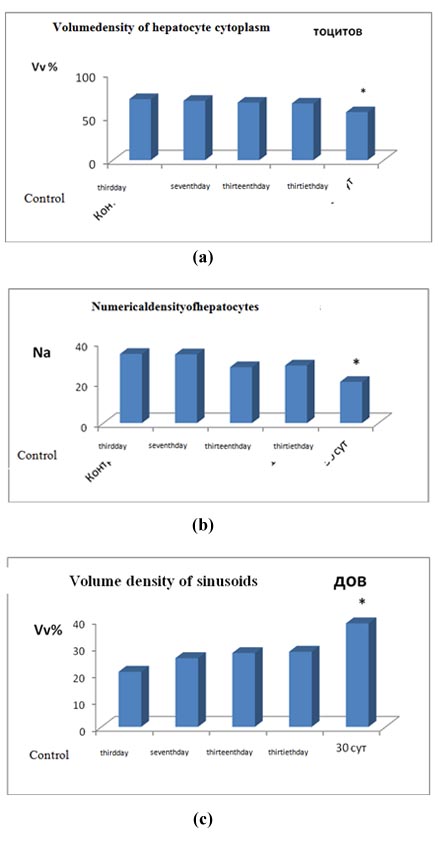
Figure 1: Structural organization of the liver under conditions of distant tumor growth. (a) Volume density of hepatocyte cytoplasm (Vv); (b) Numerical density of hepatocytes (Na); (c) Volume density of sinusoids (Vv).
3rd day, 7th day, 13th day, 30th day is a time of development of hepatocarcinoma in the thigh area of experimental animals; * - significance of differences with control p<0.05.
Immunohistochemical staining on the Podoplanin lymphatic marker, in which sinusoidal endothelial cells of the liver are stained on the basis of literature data, clearly demonstrated the expansion of sinusoids of the liver during tumor growth, most pronounced on the 30th day of the experiment (Figure 2a) [4].
Podoplanin+ lymphatic capillaries with moderately dilated lumens were detected in the portal tract (Figure 2b). An increase in the size of the liver's pre limfatik as - Disse’s spaces and swelling of sinusoidal endothelial cells was noted (Figure 2c). Observed structural changes in the initial parts of the lymphatic drainage of hepatocytes under conditions of remote tumor growth indicated an increase in lymphatic processes in the liver, probably due to the presence of toxic metabolites in the blood associated with the development of the tumor.
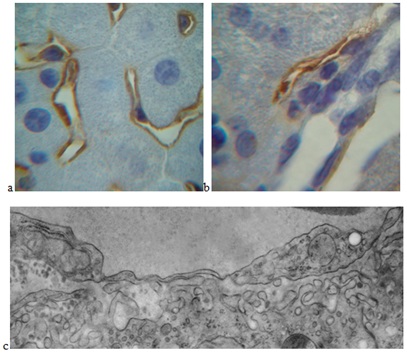
Figure 2: : Structural organization of the pathways of the lymphatic drainage of the liver under conditions of the development of hepatocarcinoma in the thigh region of an experimental animal.
(a) Enlarged gaps of the sinusoids of the liver after 30 days of the experiment. Immunohistochemical staining on the Podoplanin endothelial marker of lymphatic vessels. An increase of 10x100.
(b) Lymphatic capillary in the portal tract after 3 days of the experiment. Immunohistochemical staining on the Podoplanin endothelial marker of lymphatic vessels. An increase of 10x100.
(c) Expanded Disse’s space and swelling of the cytoplasm of an endothelial sinusoidal cell after 13 days of the experiment. An increase of x20000.
At morphometric analysis of the initial parts of the lymphatic drainage of hepatocytes in the dynamics of tumor growth, a significant change in the size of Disse’s spaces had been revealed. After 3 days of the experiment, an increase in the sizes of Disse’s spaces by 13% (P<0.0001) was noted, and after 7 days the value of this parameter did not differ from the corresponding value in the control. On the thirtieth day of the experiment, the size of Disse’s spaces increased by 21% (P<0.0001). On the 30th day of the experiment, the value of this parameter returned to its original value (Table 1).
|
Control |
3rd day |
7th day |
13th day |
30th day |
|
0.290±0.035 |
0.330±0.082*** |
0.300±0.098 |
0.367±0.049*** |
0.280±0.087 |
Table 1: Sizes of Disse’s spaces in the liver of animals under conditions of distant tumor growth.
***P
It is known that the size of Disse’s spaces under normal conditions is from 0.2 to 1 μm. The metabolism between hepatocytes and plasma of blood coming from sinusoids occurs in the Disse’s space. The cell membrane of hepatocytes in contact with the Disse’s space forms microvilli, increasing the cell surface area 6 times. In the Disse’s space, Ito cells are located, which are the most important participants in fibrogenesis.
Lymph is formed in the process of lymphatic drainage of the extracellular fluid through the thin-walled capillaries of sinusoids. Due to the permeability of capillaries, the metabolism is carried out and tissue fluid, proteins, fats (Michurin and others) are delivered into the blood [6].
Under the conditions of the development of hepatocarcinoma, toxic products are formed in the body, which are formed as a result of tumor growth, which enter the liver through the bloodstream. At the same time, the liver, as the central organ of detoxification and metabolism, is most susceptible to the toxic effects of malignant growth products. Under these conditions, an effective lymphatic drainage of the liver is extremely important for the normal function of hepatocytes.
Due to a change in the lymphatic drainage of the liver under conditions of distant tumor growth, destructive changes were noted [7]. Volume density of lysosomes increased, volume density of lipid inclusions decreased: by 90 and 70% on the 13th and 30th days respectively (p<0.05; Table 2). The glycogen volume density decreased by 67% (p<0.05) on the 3rd day of tumor development. On the 3rd day of the experiment, the mitochondrial volumetric density decreased by 22% (p<0.05), and on the 30th it was 68% of the control (p<0.05). On the 30th day of the experiment, the volume density of cisterns of the endoplasmic reticulum, the numerical density of attached and free polysomal ribosomes significantly decreased (Table 2).
|
Parameter |
Control |
Experimental Group |
|||
|
3rd day |
7thday |
13th day |
30th day |
||
| Mitochondria, Vv | 37.52±1.17 | 29.22±1.01 | 34.22±1.24 | 34.78±0.58 | 25.17±0.87 |
|
Granular EPR, Vv |
33.63±2.45 |
38.74±1.30 |
31.2±1.32 |
30.15±1.80 |
20.18±0.77* |
|
Ribosomefixed, NA |
85.75±3.63 |
73.23±2.46 |
78.16±3.56 |
77.40±3.62 |
29.36±1.34* |
|
Ribosomesfreepolysomal, NA |
40.12±0.99 |
22.24±0.64* |
13.22±1.51* |
10.46±0.83* |
11.54±0.36* |
|
Primary lysosomes,Vv |
2.00±0.11 |
2.67±0.47 |
1.72±0.05* |
3.15±0.20 |
2.73±0.15 |
|
Secondary lysosomes,Vv |
1.06±0.11 |
3.88±0.64 |
1.61±0.10* |
1.78±0.11 |
2.37±0.14* |
|
Lipidvacuole,Vv |
8.00±0.71 |
6.21±0.65 |
6.11±0.31 |
1.78±0.11* |
2.37±0.40* |
|
Glycogen,Vv |
14.21±0.59 |
4.71±1.02* |
16.72±1.19 |
13.5±0.51 |
17.75±0.91* |
Table 2:The results of a morphometric study of hepatocytes under the conditions of distant tumor growth (?±m).
It is known that the lymphatic system maintains tissue homeostasis by regulating the outflow of tissue fluid and returning it to venous blood. It plays an important role in the absorption and transportation of dietary fat. In addition, the vessels of the lymphatic system serve as the main conductors of antigens and antigen-presenting cells from the periphery to the lymph nodes, and that is crucial for immune surveillance and acquired immunity [7].
In our research, the dynamics of changes in lymphatic drainage of hepatocytes in the process of distant tumor growth was noted. On the third day of the research, a significant increase in the size of the initial parts of the lymphatic drainage of the liver - Disse’s spaces was associated with the actively developing tumor growth in the muscle tissue of the thigh of experimental animals [3]. The second more significant increase in the size of Disse’s spaces was due to the maximum development of the tumor process [10]. The decrease in the size of the Disse’s spaces on the 30th day of the experiment, apparently, was due to the depletion of the drainage system of the liver under conditions of increasing toxic load due to the further development of the tumor process.
Note: Vv is the volume density of the structures (% of the volume of the cytoplasm), NA is the numerical density of the structures (the number in the test area), EPR is the endoplasmic reticulum. * p
Lymph in the liver arises from plasma components that are filtered through the fenestra of sinusoidal endothelial cells into Disse’s space, then into the interstitial Mall’s space and lymphatic capillaries and the portal tract vessels [4]. In our research, after 30 days of the experiment, significant enlargements of the lumens of the lymphatic capillaries and vessels of the portal tracts in the liver were observed under conditions of distant tumor growth, indicating an increase in lymphatic outflow from the organ (Figure 3).
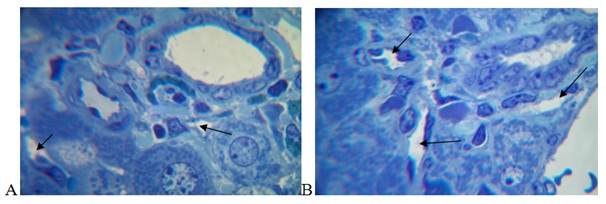
CONCLUSION
REFERENCES
- Germano D, Daniele B (2014) Systemic therapy of hepatocellular carcinoma: Current status and future perspectives. World J Gastroenterol 12: 3087-3099.
- Lupinacci RM, Paye F, Coelho FF, Kruger JA, Herman P (2014) Lymphatic drainage of the liver and its implications in the management of colorectal cancer liver metastases. Updates Surg 66: 239-245.
- Bgatova NP, Makarova OP, Pozhidayeva AA, Borodin YI, Rachkovskaya LN, et al. (2015) Effects of Lithium Nano-Scaled Particles on Local and Systemic Structural and Functional Organism Transformations Under Tumour Growth. Achievements in the Life Sciences 8: 101-111.
- Kalinin VI, Zhukova NA, Nikolin VP (2009) Hepatocarcenoma-9 - metastatic transplantable tumor of mice that causes cachexia. Extrememental Biology Bulletin 148: 664-669.
- Yu IB, Michurina SV, Yu I, Belkin AD (2012) Lymphatic region and hepatic hematolymphatic barrier are normal. In the book: Lymphology. (Ed) Konenkova Yu I Borodin MS, Lyubarsky Novosibirsk: Manuscript 215-220.
- Michurina SV, Borodin IuI, Kolesnikov SI, Ischenko IIu, Konenkov VI (2015) Liver and Its Lymph Region at Benzo[a]pyrene Effects in an Experiment. Vestn Ross Akad Med Nauk 242-248.
- Tanaka M, Iwakiri Y (2016) The Hepatic Lymphatic Vascular System: Structure, Function, Markers, and Lymphangiogenesis. Cell Mol Gastroenterol Hepatol 2: 733-749.
- Bgatova NP, Borodin YI, Makarova VV, Pozhidaeva AA, Rachkovskaya LN, et al. (2014) Effects of Nanosized Lithium Carbonate Particles on Intact Muscle Tissue and Tumor Growth. Bul Expert biol 157: 102-108.
Citation: Bahbaeva SA, Bgatova NP, Taskaeva YS, Zhumadina SM (2019) Structure of the Ways of Lymphatic Drainage in the Liver under Conditions of Remote Tumor Growth. Adv Ind Biotechnol 2: 007.
Copyright: © 2019 Zhumadina SM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

