
Study on the Mechanism of Hypertension Caused by Sorafenib in Liver Cancer-Bearing Rats Based on VEGF Signal Pathway
*Corresponding Author(s):
Kaicheng WangDepartment Of General Medicine, People’s Hospital Of Huishan, Wuxi, China
Tel:+86 18006181090,
Email:391113516@qq.com
Tingwang Jiang
Department Of Key Laboratory, The Second People’s Hospital Of Changshu, The Affiliated Changshu Hospital Of Xuzhou Medical University, Changshu, China
Tel:+86 15995921425,
Email:jtwgyp@163.com
Abstract
To explore the mechanism of hypertension caused by sorafenib in liver cancer-bearing rats based on Vascular Endothelial-Growth Factor (VEGF) signal pathway. After the three groups (blank, liver cancer and Spontaneously Hypertensive Rat (SHR) groups, n = 30) were administered orally with sorafenib (2 mg/kg), the non-invasive tail arterial pressure measurement was employed to determine the changes in blood pressure (BP). Enzyme-linked immunosorbent assay measured the changes of VEGF and Nitric Oxide (NO) in serum. The expressions of Kinase insert Domain Receptor tyrosine kinase (KDR-CD), endothelial Nitric Oxide Synthase (eNOS), Bax and Bcl-2 were detected by Western blot. TUNEL assay detected the Apoptosis Index (AI) of cardiomyocyte. CD31 immunohistochemical staining was observed the changes of Myocardial Capillary Density (MCD). The BP and VEGF levels of the blank and SHR groups were not significantly (P > 0.05) different from those in the before treatment cohort, while the BP of the liver cancer group was significantly (P < 0.05) higher. VEGF level was significantly (P < 0.05) lower than before. Compared with the blank group, the levels of NO, KDR-CD, eNOS, Bcl-2/Bax and MCD in the liver cancer group were significantly (P < 0.05) lower than those in the after administration, while the myocardial cell AI increased significantly (P < 0.05). After antagonist intervention, the BPs and myocardial AI of the liver cancer and SHR groups were significantly lower than before the intervention. Besides, levels of KDR-CD, eNOS, Bcl-2/Bax protein, MCD, serum VEGF and NO were significantly higher than before the intervention (P<0.05). Induction mechanism of hypertension induced by sorafenib may be owing to the inhibition of the VEGF signaling pathway, and reduction of endothelial cells proliferation leading to blockade of NO synthesis. This causes vasoconstriction, while promoting myocardial cell apoptosis and decreasing capillary density, thereby inducing the occurrence of hypertension.
Keywords
Hypertension; Liver Cancer; Nitric Oxide; Sorafenib; Vascular Endothelial Growth Factor (VEGF)
Introduction
Sorafenib is a multi-kinase inhibitor drug commonly used in the clinical treatment of various tumors [1]. As a vascular targeting drug, its mechanism of action is to inhibit the abnormal formation of neovascularization in tumor tissues by blocking vascular endothelial factor receptors and the blood supply to tumors, thereby inhibiting the growth and proliferation of tumor cells [2,3]. Currently, sorafenib has been widely used in the treatment of liver tumors. However, the drug has been reported to exhibit variety of adverse reactions, which are common after 3-4 weeks of administration, among which hypertension has been found to be a serious complication with a high incidence of 16.0% - 42.6% [4]. The difficultness in diagnosing and treating sorafenib-induced hypertension may directly lead to a reduction in the dose of sorafenib, amid the patient permanently discontinuing the usage of the drug in severe cases [5]. Existing studies have described the Vascular Endothelial-Growth Factor (VEGF) pathway as the main target for vascular-targeting drugs and it was closely related to sorafenib-induced hypertension, but the physiological regulatory mechanism is still unclear. As a highly specific vascular endothelial mitogenic factor, VEGF can promote endothelial cell division and proliferation, induce angiogenesis and increase capillary permeability [6]. Besides, abnormal expression of VEGF has been observed in patients with hypertension induced by sorafenib in previous work [7]. Also, in spontaneously hypertensive rats (SHR), the activation of VEGF signaling pathway could promote nitric oxide (NO) synthesis that caused peripheral vascular dilatation to prevent hypertension occurrence [8]. Despite these studies, available evidence suggests that investigation on the mechanism of VEGF signaling pathway and sorafenib-induced hypertension is still lacking. Herein, the alterations in relevant physical and chemical indexes in normal nude, SH nude and tumor-bearing mice after administration of a certain sorafenib dose were studied to explore the mechanism underlying hypertension induced by sorafenib, which may aid the provision of coping strategies for clinical treatment.
Materials And Methods
Animals and cells
Sixty male Wistar rats and 30 homologous SHR male rats (all aged 12 weeks, 200 ± 10 g) were purchased from Beijing Weitong Lihua Laboratory Animal Co., Ltd., with laboratory animal production license number of SCXK (Beijing) 2017-0001. The rat feeding environment was as follows: room temperature (22 ± 2°C), humidity (60 -70%), 12 h / 12 h interval lighting, standard feed and free drinking water. The H22 hepatocellular carcinoma cell line was bought from the Cancer Research Institute of Chinese Academy of Medical Sciences. All animals used fully comply with local and national ethics, as well as licensing requirements of Laboratory Animal Management Assessment and Accreditation (AAALAC) International.
Drugs and reagents
Sorafenib (registration certificate number, H20130137) was supplied by the Bayer Medical & Health Co., Ltd., Germany. Next, the VEGF receptor antagonist (SU5416) was purchased from American APExBIO Company. The VEGF Enzyme-Linked Immunosorbent Assay (ELISA) kit was bought from Wuhan Huamei Co., Ltd. Also, NO kits were provided by Nanjing Jiancheng Bioengineering Research Institute. The RIPA protein lysate was produced by Shanghai Biyuntian Company, while the ECL color rendering solution was obtained from Thermo Company, USA. Abcam Corporation, USA provided the rabbit anti-rat KDR-CD, rabbit anti-eNOS, rabbit anti-Bax, rabbit anti-Bcl-2, rabbit anti-GAPDH, and horseradish peroxide-labeled goat anti-rabbit IgG. Next, 4% paraformaldehyde was purchased from Beijing solarbio science & technology Co., Ltd. The TUNEL apoptosis and BCA protein concentration detection kits were produced by Shanghai Jingke Chemical Technology Co., Ltd. Then, CD31 monoclonal antibody was supplied by the American Sigma company.
Instruments
BP-98A intelligent non-invasive sphygmomanometer was provided by Beijing Ruonong Biotechnology Co., Ltd. Small animal thermal insulation heating blanket (45 × 45 cm) was obtained from Andy teaching experimental instrument. The ultra-clean workbench was purchased from Suzhou Purification Engineering Company. The 5417R type desktop high-speed freezing centrifuge was produced by Eppendorf Company in Germany. Infinite 2000 multifunctional enzyme marker was bought from TECAN. DYCZ-24DN vertical electrophoresis tank and electric transfer instrument were provided by Beijing Liuyi Instrument Factory. Next, CUT6062 automatic paraffin slicer was supplied by German Slee Company. Gene Genius gel imaging analysis system was purchased from Bio-Rad, USA, while Micto Vitalab semi-automatic biochemical analyzer was obtained from Rittal, Netherlands.
Establishment of liver cancer tumor-bearing rat model
The resuscitation of the H22 tumor cells was carried out prior to the adjustment of the cell concentration to 5 × 106/mL. Afterwards, the inoculation of the cells into the abdominal cavity of rats under sterile conditions was performed, and after 5 days of subculture, the tumor ascites was produced. Subsequently, the rats were sacrificed and disinfected via cervical dislocation. Under aseptic conditions, the fluids in the abdomen
of the rat were extracted with a disposable syringe and diluted into 1.0 × 107/mL cell suspension using normal saline. A subcutaneous inoculation of an aliquot (0.1 mL) of cell suspension at the right armpit of rats was employed for the tumor-bearing model establishment.
Grouping and administration
The 60 male Wistar rats were divided into the blank and liver cancer groups according to the random table, with 30 rats in each group. The liver cancer group was modeled in accordance with the method described earlier (section of Establishment of liver cancer tumor-bearing rat model), while the blank group was injected with an equal volume of saline at the right armpit. In addition, 30 male homologous SHR rats (SHR group) were chosen for comparison and observation. Notably, no significant difference (P > 0.05) was statistically observed in the body weight of the three groups. Meanwhile, three groups of rats were given orally at the same dose of sorafenib (2 mg/kg, daily) [9]. After continuous administration for 1 week, the 3 groups of the rats were given VEGF receptor antagonist (SU5416) for intervention experiment, which lasted for 1 week.
Blood pressure measurement
In accordance with the DSWY-1 rat tail artery Blood Pressure (BP) measurement (Shanghai huayan instrument equipment Co. Ltd) operating instructions, the rat tail should be heated with a thermal insulation heating blanket to 38°C to soften the rat tail and turn reddish before the measurement. Afterwards, the same operator used a non-invasive sphygmomanometer to measure the caudal systolic blood pressure (SBP) and diastolic blood pressure (DBP) of each group after the pulse wave pattern was stabilized for 5 s, with each measurement taken 5 times. The mean value was taken as the final BP result for the sample. In this experiment, 10 rats [10] were randomly selected from each group at three time points to measure the SBP. The three time points were before the administration of sorafenib, after the end of the administration (before the intervention) and after the end of the receptor antagonist intervention.
Determination of serum VEGF and NO levels
The anesthetists of the rats were performed via intraperitoneal injection with 2% pentobarbital sodium (30 mg / kg), and afterwards the whole blood was collected via abdominal aortic blood collection. Subsequently, serum was obtained after centrifugation at 3000 rpm/min for 10 min at 4°C. Then, ELISA kit was employed to detect the serum VEGF level. On the other hand, serum NO level was detected with Micto Vitalab semi-automatic biochemical analyzer and nitrate reductase chemical colorimetric technique [11]. In this work, 10 rats were randomly selected from each group to detect the serum VEGF and NO levels at three time points stated in section of Blood pressure measurement.
Western blot detection of myocardial KDR-CD, endothelial nitric oxide synthase (eNOS), Bax and Bcl-2 protein expressions
At the end of sorafenib administration (before intervention), 10 rats were randomly selected from each group to detect the expressions of KDR-CD, eNOS, Bax and Bcl-2 proteins in the myocardium. At the end of the receptor antagonist intervention, the same procedure was performed. The specific experimental operation process was as follows: the rat myocardial tissue was taken, and an appropriate amount of RIPA lysate was added. After 30 min, it was centrifuged at 12000 rpm/min and 4°C for 10 min to collect the supernatant. Next, the protein concentration was detected using the BCA kit. The protein sample was mixed with the loading buffer, and denatured in a 100°C water bath for 5 min, before added to the prepared SDS-PAGE gel (5% concentrated gel, 10% separation gel) in the sample hole with each being 25 μL. When the gel was concentrated, the adjusted voltage was 60 V, while the voltage was 120 V when the gel was separated. After that, the gel was removed and transferred to the membrane at 4°C for 1.5 h. Next, the PVDF membrane was sealed with 5% skim milk powder for 2 h and the primary antibody was added (overnight) at 4°C. After washing the membrane with TBST, goat anti-rabbit IgG labeled with horseradish peroxide was added and incubated at 37°C for 2 h. Then, adding the ECL color developing solution, the images were collected by the automatic gel imaging system, and the protein levels were analyzed with GAPDH as an internal reference.
TUNEL staining to detect myocardial cell apoptosis
The tissues were rinsed with 4% paraformaldehyde twice and washed with PBS for 3 times prior to hydrolysis with protease K solution at 30°C for 15 min. After that, it was washed 4 times with PBS before PBS containing 2% hydrogen peroxide was added and reacted for 10 min. After the reaction, PBS was used for washing before the addition of the TUNNEL reaction mixture and the sealing film to react in a dark wet box for 60 min at 37°C. After rinsing with PBS, 50 μL of conberter-POD and parafilm were added to react in a dark wet box for 1 h at 37°C. After the reaction, PBS was used to rinse, and DAB was added to react for 15 min at 20°C. Afterwards, staining with hematoxylin was performed before dehydration with gradient alcohol, transparent with xylene, sealing with neutral resin, and observation under optical microscope. Next, the apoptotic positive cells were brown-yellow and were counted under the optical microscope. The number of apoptotic positive cardiomyocytes in 5 high-power visual fields accounted for the total number of cells, which was the positive Apoptosis Index (AI). At the end of sorafenib administration (before intervention), 10 rats were selected at random from each group to detect the myocardial cell apoptosis. At the end of the process of receptor antagonist intervention, the same procedure was performed.
Immunohistochemical method to determine the changes of myocardial capillary density
After the anesthesia, the rats were sacrificed quickly. The hearts were removed and myocardial tissue was cut out, before the Capillary Density (CD) was measured with CD31 immunohistochemical staining. After the myocardial tissue was fixed with 4% formaldehyde, conventional dehydration, and paraffin embedding, three consecutive slices (5 μm) were stained with CD31 immunohistochemistry. Under a 40 × high-power field of vision, the abundant micro vascular area with myocardial stroma diameter of 5-8 μm and only one layer of endothelial cells was found and analyzed via computer imaging. The number of myocardial capillaries in 5 different visual fields was measured in each slice, while the mean value of CD was used. At the end of sorafenib administration (before intervention), 10 rats were selected randomly from each group to detect the changes of CD. At the end of the receptor antagonist intervention, the same procedure was performed.
Statistical analysis
SPSS 20.0 software was applied for data processing, while Graph Pad 5.01 was used for graph drawing. The comparison between multiple groups was assessed through a single factor analysis, while the pairwise comparison was analyzed with independent sample t test. A P < 0.05 was considered as statistically significant.
Results
Changes of BP and serum VEGF levels in rats before and after sorafenib administration
Table 1 showed the BP of each rat in before and after administration groups. It could be observed (Table 1) that the difference between SBP and DBP of the blank and liver cancer groups was statistically not significant (P > 0.05) before administration. Likewise, after the administration of sorafenib, the SBP and DBP of rats in the blank and SHR groups showed no significant (P > 0.05) changes in comparison with before administration cohort. Meanwhile, the SBP and DBP of rats in the liver cancer group were significantly (P < 0.05) higher than those in the before administration.
|
|
|
|
SBP (mm Hg) |
|
|
|
DBP (mm Hg) |
|
|
|
Groups |
|
|
|
|
|
|
|
|
|
|
|
Before |
After |
t |
P |
Before |
After |
t |
P |
|
|
|
|
administration |
administration |
value |
administration |
administration |
value |
||
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
Blank |
137.45 ± 10.56 |
135.74 ± 11.63 |
0.344 |
0.367 |
99.76 ± 8.33 |
100.86 ± 9.13 |
0.281 |
0.391 |
|
|
Liver cancer |
138.89 ± 11.14 |
168.88 ± 14.42* |
5.205 |
0 |
102.84 ± 9.42 |
137.74 ± 11.11* |
7.578 |
0 |
|
|
SHR |
181.32 ± 14.51* |
183.88 ± 13.66* |
0.406 |
0.345 |
142.86 ± 15.28* |
146.53 ± 14.64* |
0.548 |
0.295 |
|
|
*P < 0.05, Compared with blank group. |
|
|
|
|
|
|
|||
Table 1: Comparison of blood pressure of rats in each group before and after treatment (n=10)
In addition, the comparison of serum VEGF levels of each rat in before and after administration groups was shown in figure 1. It was observed that the VEGF levels of rats in the blank and liver cancer groups were insignificantly (P > 0.05) different from those in the before administration cohort (Figure 1). Similarly, after administration of sorafenib, the serum VEGF levels of rats in the blank and SHR groups did not substantially (P > 0.05) changed compared with the before administration cohort, but that of those in liver cancer group was significantly (P < 0.05) lowered comparable to the before administration.
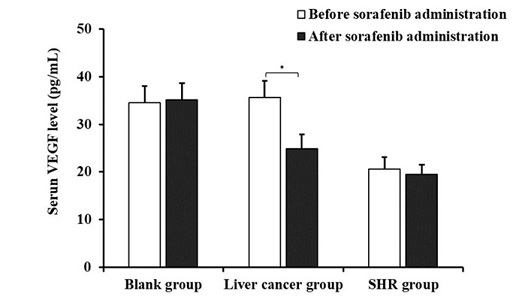 Figure 1: Comparison of serum VEGF levels of each rat in before and after administration groups (*P < 0.05, compared with before administration)
Figure 1: Comparison of serum VEGF levels of each rat in before and after administration groups (*P < 0.05, compared with before administration)
Changes in BP and serum VEGF levels in rats before and after antagonist intervention
The comparison of the BP of mice in before and after intervention cohorts was determined and the result is shown in table 2. After intervention with the VEGF receptor antagonists, the SBP and DBP of rats in the blank group did not alter significantly (P > 0.05) comparable to those in the before the intervention cohort (Table 2). Meanwhile, the SBP and DBP of rats in the liver cancer and SHR groups were significantly (P < 0.05) lower than those in the before the intervention cohort. Furthermore, as could be seen from figure 2, after the antagonist intervention, the serum VEGF level of rats in the blank group showed no significant (P > 0.05) change compared with before the intervention cohort. Nonetheless, the serum VEGF levels of the rats in the liver cancer and the SHR groups were substantially (P < 0.05) higher than those in the before the intervention.
|
|
|
|
SBP (mm Hg) |
|
|
|
DBP (mm Hg) |
|
|
|
Groups |
|
|
|
|
|
|
|
|
|
|
|
Before |
After |
t |
P |
Before |
After |
t |
P |
|
|
|
|
intervention |
intervention |
value |
intervention |
intervention |
value |
||
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
Blank |
135.74 ± 11.63 |
133.48 ± 11.48 |
0.437 |
0.333 |
100.86 ± 9.13 |
99.92 ± 10.21 |
0.217 |
0.415 |
|
|
Liver cancer |
168.88 ± 14.42* |
142.78 ± 13.69* |
4.151 |
0 |
137.74 ± 11.11* |
109.29 ± 10.86* |
5.791 |
0 |
|
|
SHR |
183.88 ± 13.66* |
163.75 ± 15.33* |
3.1 |
0.003 |
146.53 ± 14.64* |
119.63 ± 12.95* |
4.252 |
0.295 |
|
Table 2: Comparison of blood pressure of rats in each group before and after intervention (n=10).
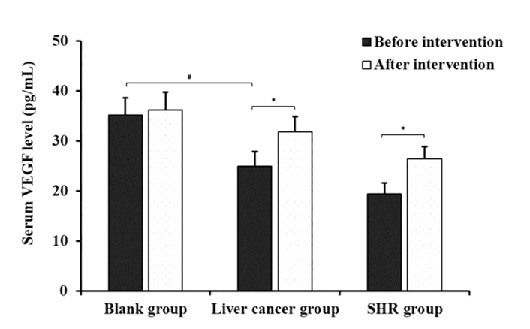 Figure 2: Comparison of serum VEGF levels of each rat in before and after intervention cohorts (*P < 0.05, compared with before intervention; #P < 0.05, compared with blank group).
Figure 2: Comparison of serum VEGF levels of each rat in before and after intervention cohorts (*P < 0.05, compared with before intervention; #P < 0.05, compared with blank group).
Changes in serum NO levels of rats before and after antagonist intervention
Figure 3 displayed the result for the comparison of serum NO levels of each rat in the before and after intervention cohorts. Before antagonist intervention, the serum NO level of liver cancer group was significantly (P < 0.05) lower than those in the blank group. However, after the intervention of antagonists, the serum NO level of rats in the blank group had not significant (P > 0.05) changed compared with those in the before the intervention cohort. Nevertheless, NO levels in the serum of rats in the liver cancer and SHR groups were significantly (P < 0.05) higher than those in the before the intervention cohort.
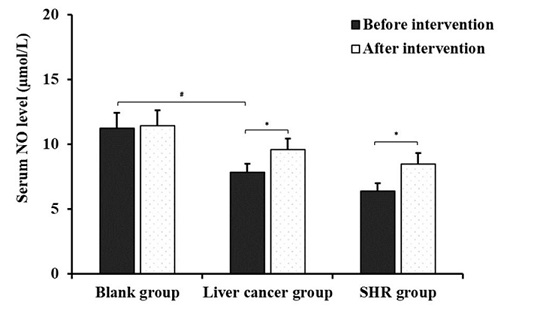 Figure 3: Comparison of serum NO levels of each rat in before and after intervention cohorts (*P < 0.05 compared with before intervention; #P < 0.05, compared with blank group).
Figure 3: Comparison of serum NO levels of each rat in before and after intervention cohorts (*P < 0.05 compared with before intervention; #P < 0.05, compared with blank group).
Changes in the levels of expression of KDR-CD, eNOS, Bcl-2 and Bax in rat myocardium before and after antagonist intervention
The comparison of expression levels of KDR-CD, eNOS, Bcl-2 and Bax in myocardium of rats before and after intervention is indicated in Figure 4. It can be seen from figure 4 (D) and (E) that the levels of myocardial KDR-CD, eNOS, Bcl-2 and Bax in the liver cancer group before intervention were significantly (P < 0.05) lower than those in the blank group. As shown in figure 4 (A) and (D), the levels of myocardial KDR-CD, eNOS, Bcl-2 and Bax in the blank group after intervention indicated no significant (P > 0.05) changes compared with those in the before intervention. As was shown in figure 4 (E) and (F) that the levels of KDR-CD, eNOS, Bcl-2 and Bax in the liver cancer and SHR groups after intervention were significantly (P < 0.05) higher than those in the before intervention.
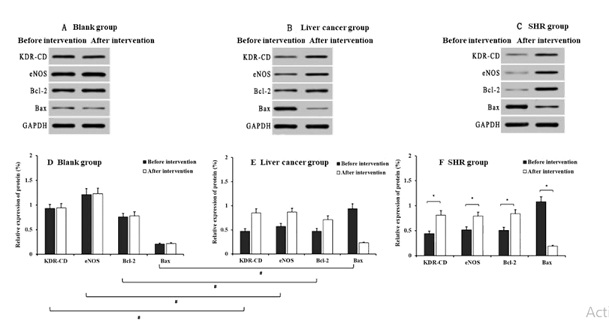 Figure 4: Comparison of KDR-CD, eNOS, Bcl-2 and Bax levels of each rat in before and after intervention groups (A: Western blot detection electrophoresis of blank group; B: Western blot detection electrophoresis of liver cancer group; C: Western blot detection electrophoresis of SHR group; D, E, F: relative expression of protein in blank group; E: relative expression of protein in liver cancer group; F: relative expression of protein in SHR group; *P < 0.05, compared with before intervention; #P < 0.05, compared with blank group).
Figure 4: Comparison of KDR-CD, eNOS, Bcl-2 and Bax levels of each rat in before and after intervention groups (A: Western blot detection electrophoresis of blank group; B: Western blot detection electrophoresis of liver cancer group; C: Western blot detection electrophoresis of SHR group; D, E, F: relative expression of protein in blank group; E: relative expression of protein in liver cancer group; F: relative expression of protein in SHR group; *P < 0.05, compared with before intervention; #P < 0.05, compared with blank group).
Changes in rat cardiomyocyte apoptosis index (AI) before and after intervention
Figure 5 shows the effect of antagonist intervention on cardiomyocyte apoptosis in rats. The normal cardiomyocyte nucleus was blue, while the apoptotic positive cardiomyocyte nucleus was brown yellow after the TUNEL staining. It can be observed from figure 5 that after sorafenib administration (before intervention), the AI of liver cancer group was significantly (P < 0.05) higher than that of the blank cohort. After intervention, there was no significant change in AI of the blank group compared with that of the before intervention (P > 0.05). However, the AI in liver cancer and SHR groups were significantly (P < 0.05) lower than that of the before intervention cohort.
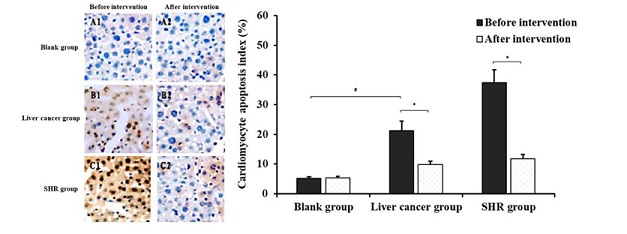 Figure 5: Effect of antagonist intervention on cardiomyocyte apoptosis in each group (*P < 0.05, compared with before intervention; #P < 0.05, compared with blank group).
Figure 5: Effect of antagonist intervention on cardiomyocyte apoptosis in each group (*P < 0.05, compared with before intervention; #P < 0.05, compared with blank group).
Alterations in rat capillary density before and after antagonist intervention
It was demonstrated that the comparison results of myocardial capillary density of rats in each group before and after intervention (Figure 6). After sorafenib administration (before intervention), the myocardial capillary density (MCD) of the liver cancer group was significantly (P < 0.05) lower than that of the blank cohort (Figure 6). After the intervention, the myocardial capillary density in the blank group did not change substantially (P > 0.05) in comparison with before intervention cohort. However, the myocardial capillary density in liver cancer and SHR groups were significantly (P < 0.05) higher than the before intervention cohort, and the differences were statistically significant.
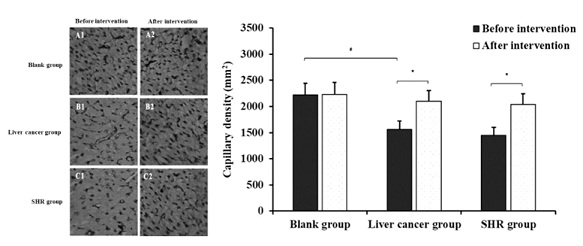 Figure 6: Comparison of myocardial capillary density of each rat in before and after intervention groups (*P < 0.05, compared with before intervention; #P < 0.05, compared with blank group).
Figure 6: Comparison of myocardial capillary density of each rat in before and after intervention groups (*P < 0.05, compared with before intervention; #P < 0.05, compared with blank group).
Discussion
Sorafenib is a vascular-targeted drug approved by the Food and Drug Authority (FDA) for as therapeutic option for advanced renal cell and hepatocellular carcinomas that cannot be surgically removed in the past decade [12]. As an oral multi-target tyrosine kinase inhibitor, various mechanisms of action have been reported. These include the inhibition of tumor angiogenesis via suppression VEGF receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), and blocking of the RAS/RAF/MEK/ERK signaling pathway as well as inhibition of the growth and proliferation of tumor cells, in order to purposely control the growth and metastasis of tumor, thereby exerting an anti-tumor effect [13]. These signaling factors and pathways exist not only in tumor cells but also in the normal cells, thus sorafenib may also affect the function of the normal cells and produce some adverse reactions common to anti-angiogenic drugs. Hypertension was reported as the most common adverse reaction of sorafenib in clinical trials and applications. It mostly appears as mild or moderate, and generally occurs 3-4 weeks after treatment, which usually culminate in a reduction or discontinuation of sorafenib treatment. Besides, sorafenib can even cause serious and irreversible adverse cardiovascular reactions, such as bleeding, Congestive Heart Failure (CHF), cerebral infarction, myocardial infarction, etc. [14,15].
The pathogenesis of hypertension caused by sorafenib is relatively complex. From the perspective of molecular pathology, its occurrence and development were correlated with the abnormal expression of the genes or proteins targeted by sorafenib [16]. The VEGF pathway is established as the main target of vascular targeting drugs with the VEGF being produced by vascular smooth muscle and endothelial cells. As an important cytokine for vascular endothelial survival, VEGF can maintain the integrity of vascular endothelial function [17]. Some studies have found that when VEGF expression was suppressed, it led to excessive mitosis of endothelial cells in local microcirculation, which in turn caused impaired endothelial function and promoted the occurrence of hypertension [18]. Pena et al., [19] observed that when patients with advanced renal cell carcinoma were treated with sorafenib, VEGF protein was abnormally expressed in the body, which interfered with the vasoconstriction process via the inhibition of NO synthase phosphorylation and protein kinase C activity. Herein, it was observed that after sorafenib administration, the SBP and DBP of rats in the liver cancer group increased significantly, while the serum VEGF level substantially reduced, suggesting that the BP abnormality induced by sorafenib may be related to the VEGF pathway. Therefore, it was necessary to deeply explore the molecular mechanism of VEGF regulation in relation to sorafenib-induced hypertension.
Existing studies have found that the pro-angiogenesis function of VEGF was mainly mediated by binding to VEGF receptors distributed in the vascular endothelial cell membrane [20]. Physiologically, KDR-CD is a receptor containing a kinase insertion region, while VEGF can activate KDR-CD and promote the proliferation of vascular endothelial cells [21]. Under normal physiological conditions, NO in the blood vessels is mainly produced by the oxidation of L-arginine catalyzed by endothelial Nitric Oxide Synthase (eNOS), which is the main vasorelaxing active substance, in the body and plays a key role in the vasodilation function [22]. Gardini et al., [23] posited that endothelial cell injury could reduce eNOS gene activity by 40% and inhibit eNOS protein expression, thereby reducing NO synthesis, which culminates in vasoconstriction and increment in BP. Collectively, these results suggest that after sorafenib administration, the serum NO level of rats in the liver cancer group significantly reduced compared with the blank group, while the KDR-CD and eNOS protein levels also declined substantially. This implies that VEGF may inhibit KDR-CD expression, reduce the proliferation of endothelial cells and block eNOS expression, thereby inhibiting the production of NO and causing vasoconstriction. Thus, an experiment was designed on the account of the administration of sorafenib and the intervention with a receptor antagonist. The results showed that the levels of serum VEGF, NO, KDR-CD and eNOS of rats were significantly reduced after the intervention in the liver cancer and the SHR groups, further affirming that this pathway was involved in the induction of hypertension by sorafenib.
Bax is a pro-apoptotic gene, which can induce apoptosis by binding to mitochondria, thereby damaging the integrity of mitochondrial membrane and promoting the release of pro-apoptotic proteins [24]. On the other hand, Bcl-2, as the main anti-apoptotic gene, can prevent Bax from binding to mitochondria and alter the permeability of mitochondrial membranes, resulting in the inhibition of cell apoptosis [25]. In addition, other study has shown that VEGF, as an important factor in maintaining vascular homeostasis, played a regulatory role in apoptosis [26]. The expressions of pro-apoptotic protein Bax and anti-apoptotic protein Bcl-2 were also detected in this study. It was observed that after sorafenib administration, the expression levels of Bcl-2 and Bax of rats in the liver cancer group significantly lowered than those in the blank group, while the AI of cardiomyocytes was significantly increased, suggesting that blockade of VEGF pathway may mediate the apoptosis of cardiomyocytes through Bcl-2 and Bax. Some studies have reported that sparse capillaries were closely related to the occurrence of hypertension in SHR rats [27]. Iliev A et al., [28] found that, compared with rats in the normal group, the apoptosis rate of endothelial cells in SHR rats was significantly increased, while the capillary density was significantly reduced, indicating that the sparse capillary caused by apoptosis might act as an important factor in the occurrence and development of hypertension. This study further observed the MCD in rats after administration of sorafenib and found that the MCD of rats in the liver cancer group was significantly lower than those in the blank group, implying that apoptosis might cause the CD to decrease, thereby inducing hypertension. Based on the administration of sorafenib and the intervention with receptor antagonist, it was found that the levels of Bcl-2 and Bax as well as the MCD were significantly increased while the AI decreased substantially in the liver cancer and SHR groups after the intervention, which further verified that the thinning of the capillary caused by apoptosis may be the underlying mechanism for sorafenib-induced hypertension.
In summary, sorafenib could induce hypertension during the treatment of liver cancer with possible underlying mechanisms including inhibition of the VEGF signaling pathway, and reduction of the proliferation of endothelial cells, which concomitantly lead to suppression of NO synthesis and occurrence of vasoconstriction. These events simultaneously promote cardiomyocyte apoptosis and cause a decrease in CD, thereby inducing the occurrence of hypertension.
Acknowledgement
This work was supported by the Jiangsu University Clinical Medicine Technology Development Fund Project (JLY20180026).
Author's Contribution
KCW, XBQ, TL conceived and designed the experiments. KCW, XBQ, TL, KSG, JL performed the experiments. XBQ, TL, KSG, JL analyzed the data. KCW, XBQ, TL, KSG, JL contributed reagents/materials/analysis tools. KCW, XBQ, TL wrote the paper. We are grateful for this. All authors read an approved the final manuscript.
Conflict of Interest Disclosure
The authors declare no declarations of interest.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Gyawali B, Shimokata T, Ando M, Honda K, Ando Y (2016) Risk of Serious Adverse Events and Fatal Adverse Events with Sorafenib in Patients with Solid Cancer: A meta-analysis of phase 3 Randomized Controlled Trials. Annals of Oncology Official Journal of the European Society for Medical Oncology 28: 246-253.
- Xu J, Cao N, Dai Y, Feng J, Liu L (2018) Inhibitory Effect of Sorafenib on Non-small Cell Lung Cancer H460 and A549 Cell Lines. Anti-tumor Pharmacy.
- Zhu Y-j, Zheng B, Wang H-y, Chen L (2017) New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacologica Sinica 38: 614-622.
- Gardini AC, Scarpi E, Marisi G, Foschi FG, Donati G, et al. (2016) Early onset of hypertension and serum electrolyte changes as potential predictive factors of activity in advanced HCC patients treated with sorafenib: results from a retrospective analysis of the HCC-AVR group. oncotarget 7: 15243-15251.
- Jiaxi Y, Xiaoyi H, Yanjun Z, Hang W, Jianming G (2018) Comparison of efficacy between sorafenib and sunitinib as first-line therapy for metastatic renal cell carcinoma and analyze prognostic factors for survival. Chinese Journal of Clinical Medicine. 40: 384-389.
- Wang J, Li C, Cao W (2019) Expression of vascular endothelial growth factor and its vital catalytic role in promotion of invasion and metastasis of hepatocellular carcinoma cells. Chinese Journal of Microbiology and Immunology 39: 73-78.
- Ping LZ, Yoshiaki H (2018) Effects of toceranib compared with sorafenib on monocrotaline-induced pulmonary arterial hypertension and cardiopulmonary remodeling in rats. Vascular Pharmacology 110: 31-41.
- Lijun W (2018) Effect of aerobic exercise on microvascular rarefaction in skeletal muscle of spontaneously hypertensive rats. Chin J Arterioscler 26: 691-697.
- Jiang Q-YCY, Li R-S, Sun H-W, Li X-J, Chai Y, et al. (2018) A Novel Quantitative Analysis Method of in Situ Tumor Image of Liver in Nude Mice Based on Image J Software. Letters in biotechnology 29: 541-546.
- Alsaied OA, Sangwan V, Banerjee S, Krosch TC, Chugh R, et al. (2014) Sorafenib and triptolide as combination therapy for hepatocellular carcinoma. Surgery 156: 270-279.
- Dianzhi S, Laboratory TC (2017) Clinical significance of oxidative stress related indexes and A-beta levels in the serum of patients Alzheimer's disease. China Medical Herald..
- Shah BK, Ghimire KB (2015) Survival trends among patients with advanced renal cell carcinoma in the United States. Urol Int 94: 133-136.
- Du Q-Q, Hang L-L, Liu C-X, Tang M, Yan C, et al. (2019) Anti-tumor activity and mechanisms of isorhamnetin in combination with sorafenib for renal cancer. Acta Pharmaceutica Sinica 54: 1424-30.
- Møller NB, Budolfsen C, Grimm D, Krüger M, Magnusson NE (2019) Drug-Induced Hypertension Caused by Multikinase Inhibitors (Sorafenib, Sunitinib, Lenvatinib and Axitinib) in Renal Cell Carcinoma Treatment. International Journal of Molecular Sciences 20: 4712.
- Kimura G, Kataoka M, Inami T, Fukuda K, Yoshino H, et al. (2017) Sorafenib as a potential strategy for refractory pulmonary arterial hypertension. Pulmonary Pharmacology & Therapeutics 44: 46-49.
- Ma R, Chen J, Liang Y, Lin S, Zhu L, et al. (2017) Sorafenib: A potential therapeutic drug for hepatic fibrosis and its outcomes. Biomedicine & Pharmacotherapy 88: 459-468.
- Xiaxiao LB (2019) Progress on Biological Function of Vascular Endothelial Growth Factor and Treatment of Traditional Chinese Medicine. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease 27: 12-15.
- Mingyue L, Wendi Y, Tesi L, You L, Sato Y, et al. (2019) VEGF promotes biliary epithelial cell viability and cystic dilation in rats with polycystic kidney. Chinese Journal of Pathophysiology 35: 1106-1111.
- Pena-Hernandez C, Rahman R, Thavaraputta S, Garg N (2019) VEGF modulation and renal effects: Case report and review of the literature. The Southwest Respiratory and Critical Care Chronicles 7: 58-63.
- Wei CM, Zhang JJ, Bao-Yuan LI, Wang CJ, Wen-Wen LV (2017) Progress in research of natural products targeting VEGF/VEGFR for cancer therapy. Chinese Traditional & Herbal Drugs.
- Marotta V, Sciammarella C, Capasso M, Testori A, Pivonello C, et al. (2017) Preliminary data of VEGF-A and VEGFR-2 polymorphisms as predictive factors of radiological response and clinical outcome in iodine-refractory differentiated thyroid cancer treated with sorafenib. Endocrine 57: 539-543.
- Chuan R, Shaomin C, Lingyun Z, Shunlin X, Lijun G (2019) Relationship between angiopoietin-2 and vascular endothelial factor and vasodilation function in hypertensive patients. National Medical Journal of China 99: 934-938.
- Gardini AC, Marisi G, Faloppi L, Scarpi E, Foschi FG, et al. (2016) ENOS polymorphisms and clinical outcome in advanced HCC patients receiving sorafenib: Final results of the ePHAS Study. Digestive & Liver Disease 48: 23.
- Hu W, Han W, Zhang S, Zhuo P (2015) Research Progress of Mitochondrial Protein Smac Relevance to the Bcl-2 Protein Family and Promoting Apoptosis Mechanism. China Resources Comprehensive Utilization.
- Opferman JT, Kothari A (2018) Anti-apoptotic BCL-2 family members in Cell Death & Differentiation 25: 37-45.
- Mangoni AA, Kichenadasse G, Thanigaimani S (2011) The Emerging Role of Vascular Endothelial Growth Factor (VEGF) in Vascular Homeostasis: Lessons from Recent Trials with Anti-VEGF Drugs. Current Vascular Pharmacology 9: 358-380.
- Plotnikov MB, Aliev OI, Sidekhmenova AV, Shamanaev AY, Anishchenko AM, et al. (2017) Dihydroquercetin Improves Microvascularization and Microcirculation in the Brain Cortex of SHR Rats during the Development of Arterial Hypertension. Bulletin of Experimental Biology & Medicine 163: 57-60.
- Iliev AA, Kotov GN, Landzhov BV, Jelev LS, Kirkov VK, et al. (2017) A Comparative Analysis of CapillaryDensity in the Myocardium of Normotensiveand Spontaneously Hypertensive Rats. Acta morphologica et anthropologica 24: 19-25.
Citation: Xin B, Tang L, Kuai S, Liang J, Wang K, et al. (2021) Study on the Mechanism of Hypertension Caused by Sorafenib in Liver Cancer-Bearing Rats Based on VEGF Signal Pathway. J Altern Complement Integr Med 7: 191.
Copyright: © 2021 Baoqiong Xin, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

