
Journal of Medicine Study & Research Category: Medical
Type: Case Report
Successful Treatment of Acute Paralysis of Lower Limbs with High Dose Steroids and Rehabilitation in Achondroplasic Dwarf: A Case Report
*Corresponding Author(s):
Shin-Tsu ChangDepartment Of Physical Medicine And Rehabilitation, Tri-Service General Hospital, School Of Medicine, National Defense Medical Center, Chung Shan Medical University, No.161, Sec. 6, Minquan East Road, Neihu District, Taipei,Taichung, Taiwan, Province Of China
Tel:+886 423741350,
Fax:+886 945605523
Email:ccdivlaser1959@gmail.com
Received Date: Mar 08, 2019
Accepted Date: Mar 18, 2019
Published Date: Apr 01, 2019
Abstract
We present a case report of a young adult with achondroplasia, who developed sudden-onset paralysis of both lower limbs due to lumbar spinal stenosis. Surgical intervention was refused, and then he underwent combination therapy with methylprednisolone and comprehensive rehabilitation, and his symptoms of paralysis ameliorated progressively. There was no previously published report discussing the medical therapy for limb paralysis in those patients. We discuss the general characteristics and symptoms of achondroplasia and emphasize that methylprednisolone administration with rehabilitation was successful for spinal stenosis with acute muscle paralysis in such kind of patients if operation is unavailable.
Keywords
Achondroplasia; Congenital spinal stenosis; Low back pain; Methylprednisolone; Paralysis
INTRODUCTION
Achondroplasia, one of the well-known forms of dwarfism, characterizes a disproportionate stature with rhizomelic short limbs and trident hands, genu recurvatum, lateral torsion, saddle nose, and macrocephaly [1-3]. Due to abnormal development of generalized skeleton, those patients often experience low back pain and neurological deficits related to spinal stenosis such as radiation pain or numbness over the lower limbs [4]. Stenosis occurred in the spinal canal has been known as abnormalities of endochondral ossification and earlier fusion in the developing vertebrae [5,6]. The symptoms of spinal stenosis associated with achondroplasia often worsen in adulthood. This delayed development of neurological problems could be resulted from herniation of the nucleus pulposus, degenerative spurring of the vertebral facets, or hypertrophy of the ligamentum flavum [7]. The most common involvement is L2-3 level [7]. Acute limb paralysis is often correlated with sudden physical exertion or an accident, which lead to subsequent surgery inevitably [3]. We report a young adult with achondroplasia, who developed sudden-onset paralysis of both lower limbs. After refusal of surgical intervention, he underwent high dose steroid and rehabilitation. Four months later, neurological deficits obviously faded away. Steroid regimen combined with comprehensive rehabilitation has never been suggested as an effective treatment for acute paralysis in achondroplasia. We offer our experience and make a broader discussion about achondroplasia, including its related problems and treatment options.
CASE REPORT
The patient was a 22 year old male who was diagnosed as achondroplasia at his infancy. None of his family members were affected. The patient was in good health except for occasional complaints of low back pain. In March 2003, he reported severe low back pain without clear history of trauma or heavily lifting. Three weeks later, symptoms had progressed to marked lower limb weakness with difficulty in ambulation. Neurosurgeon examined him and found decreased sensation to pinprick and light touch below the L2 level bilaterally. The American Spinal Injury Association (ASIA) motor scores were right/left 7/8 in lower limbs; urinary retention was also found. Paresthesia developed at both lower legs. Deep tendon reflexes decreased. Magnetic Resonance Imaging (MRI) showed severe spinal stenosis from T12 to L5. Surgical treatment with decompressive laminectomy was suggested, but refused by him and his parents. He had to be treated with intravenous methylprednisolone as protocol in the Second National Acute Spinal Cord Injury Study ( NASCIS II ) trial ( i.e., a bolus of 30 mg/kg in 15 minutes followed by 5.4 mg/kg/hour for 23 hours) [8]. Two days later, he was moved to our department due to persisted signs and symptoms even minimal improvement.
Upon observation, the patient showed short stature with typical achondroplastic characteristics, including trident hands, genu recurvatum and macrocephaly. Standing ability was remarkably reduced. Hypoesthesia below L2 dermatome presented in the both sides. There was hypoesthesia around the perianal region, but anal sphincter tone was normal. Babinski’s sign and Hoffman’s sign were negative. Urodynamic studies revealed detrusor hypereflexia, decreased bladder capacity and dys-synergia. X-ray films showed deformity of the L1 and L2 vertebral bodies (Figure 1). MRI revealed spinal stenosis from vertebrae T12 to L5 (Figures 2 and 3). The conclusion drawn from the electrophysiological studies was bilateral lumbosacral radiculopathy. However, the minimal involvement of thoracolumbar spinal cord lesion contributed to lower limb paralysis could not be excluded.
Upon observation, the patient showed short stature with typical achondroplastic characteristics, including trident hands, genu recurvatum and macrocephaly. Standing ability was remarkably reduced. Hypoesthesia below L2 dermatome presented in the both sides. There was hypoesthesia around the perianal region, but anal sphincter tone was normal. Babinski’s sign and Hoffman’s sign were negative. Urodynamic studies revealed detrusor hypereflexia, decreased bladder capacity and dys-synergia. X-ray films showed deformity of the L1 and L2 vertebral bodies (Figure 1). MRI revealed spinal stenosis from vertebrae T12 to L5 (Figures 2 and 3). The conclusion drawn from the electrophysiological studies was bilateral lumbosacral radiculopathy. However, the minimal involvement of thoracolumbar spinal cord lesion contributed to lower limb paralysis could not be excluded.
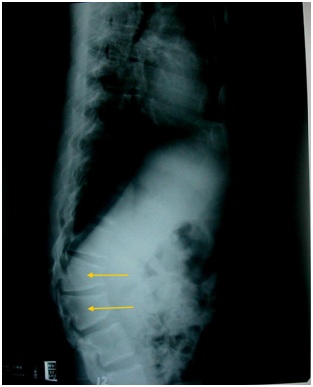
Figure 1: Lateral view of a plain film of lumbo-sacral spine revealing L1, L2 vertebral body deformity.
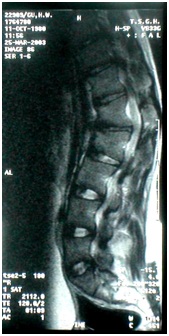
Figure 2: Sagittal T2-weighted MRI scan showing diameter of vertebral canal is roughly 12 mm.
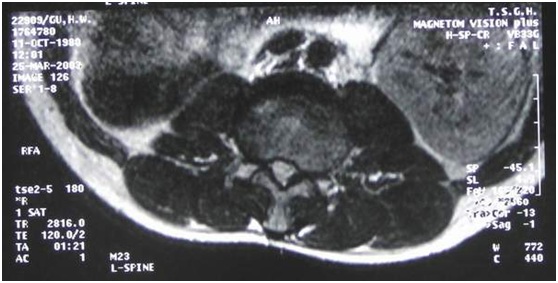
Figure 3: Axial T2-weighted MRI scan showing diameter of vertebral canal at L2, L3 level as roughly 12 mm (comparable with diameters of T12, L1; L1, L2; L3, L4; L4, L5; L5, S1).
An anteroposterior diameter of less than 10 mm is considered as “absolute stenosis” and a diameter of 10 to 12 mm as “relative stenosis”.
Because an anteroposterior diameter of less than 10mm is considered as “absolute stenosis” and a diameter of 10 to 12 mm as “relative stenosis” the patient was diagnosed with spinal stenosis from level T12 to L5.
Because his parents refused surgical decompression of the spinal column, steroid therapy was maintained. Intravenous Rinderon was given (4 mg q8h from March 24 to March 27, and then 4 mg q12h from March 27 to March 30), and then shifted to oral prednisolone (10 mg tid from March 30 to April 11). Over this period, the motor score increased to right/left 16/19 and sensation to pinprick and light touch also improved gradually and he started to receive comprehensive rehabilitation in addition to steroid administration. The rehabilitation program included stretching exercise, strengthening exercise, electrical stimulation, and postural education. He was discharged on April 11, 2003 and maintained his rehabilitation programs at outpatient. Four months later, in July 2003, he could walk unaided with a walker for 50 minutes; even bilateral drop foot remained. The motor score increased to right/left 18/20. He was able to void normally; however, constipation and mild paresthesia of lower limbs still persisted.
DISCUSSION
Achondroplasia has an estimated incidence of 1 per 26000 people, and it is the most frequent and well-known form of dwarfism [1,6]. It is caused by mutation in the FGFR3 (Fibroblast Growth Factor Receptor 3) gene; only a few cases demonstrate autosomal dominant inheritance, with the rest representing new mutations [7,9]. The function of FGFR3 is to regulate endochondral ossification by slowing the growth rate. Therefore, when mutation results in over-expression, the growth of long bones becomes limited and the height of affected adults is usually around four feet (120 cm) [10].
Characteristics of achondroplasia consisted of clinical features of appearance and neurologic complications, for instance, hydrocephalus, cervical compression and neurological deficits after spinal stenosis [7]. Spinal stenosis is related to aberrant endochondral ossification, in which there is premature fusion of the chondrification center, thickened lamina, and shortened pedicle, hypertrophied zygapophyseal joint and even lower height of the vertebral body [5]. These vertebral abnormalities create a discrepancy in the diameter of the osseous canal and neural structures that results in increased intraspinal pressure, typically at the level of L2 to L3 [6]. Patients with achondroplasia and symptomatic spinal stenosis often experience back pain, which may progress to lower extremity pain and debilitating consequences. Anatomic anomalies of the vertebral column place the patient at risk for spinal stenosis as early as the first decade and especially during adulthood [4]. Schkrohowsky et al. studies the likelihood of developing symptomatic stenosis by measuring canal diameters radiographically, and found that those “at risk” for stenosis had a significantly larger average percentage decrease in the transverse interpedicular distance from T12 to L5 (-8% vs. -19%) and a significantly greater thoracolumbar kyphosis angle (24.2-degree angle vs. 14.1-degree angle), when compared with controls [11]. A study investigated the natural history of pain associated with spinal stenosis in individuals with achondroplasia, and the results showed that patients reported significant progression of pain toward involvement of the lower extremities and significant increases in lower extremity pain severity overall. Compared with patients with back pain only, those with back pain and proximal or distal leg pain had higher self-rated pain severity; higher functional disability; and more bowel and bladder dysfunction symptoms, sleep disturbances, extremity numbness, and psychological distress [5]. A group of surgeons, major in limb lengthening operation, screened for spinal stenosis at risk in children with achondroplasia, and concluded that female sex, delayed milestones and a tight cervicomedullary junction as high risks [12].
Skeletal abnormalities are known to cause neurological morbidity and lead to a shortened lifespan [7]. The symptoms of spinal stenosis include low back pain, paresthesia, neurogenic claudication, and even sudden-onset paraplegia [3,7]. Paresthesia is described as tingling, pin-pricking, numbness, burning, or a sleepy or dead sensation in the legs; paresthesia was present in our patient. The symptoms derived from spinal stenosis are usually bilateral and asymmetric. The straight-leg-raising test is usually negative [13]. EMG study is usually abnormal, but not a specific finding [13]. The average age at onset of symptoms is 38 years. Fewer than 10% of patients have such neurological symptoms at 10 years old, compared with 20% by the end of the teens and 80% by the 50s [1]. This delayed development of neurologic problems can be caused by size of the spinal canal, herniation of the nucleus pulposus, degenerative spurring of the vertebral facets, vertebral mal-alignment, vertebral instability, increasing lumbar lordosis, and hypertrophy of the ligamentum flavum [14]. Occasionally, neurologic complications may be abrupt in onset following undue physical exertion, like our case. Hamamci et al. reported spinal cord lesion occurred in 3 cases with achondroplastic dwarfism. All the 3 cases underwent surgical intervention, and obtained better recovery [14]. Wieting & Krach also described a case of a 12-year-old achondroplastic patient who incurred an apparently nontraumatic cervical cord infarction with resultant quadriplegia. The disable case was also treated by high dose steroid together with intensive multidisciplinary rehabilitation, and obtained better outcome, like our case. Coincidently, the 12-year-old boy did not have apparent cause, like ours, too [15]. Di Fabio et al. reported a case of achondroplasia suffered from acute paraparesis after lumbar bending as well [16].
Eligible treatments for patients with lumbar spinal stenosis vary. Conservative treatment is suitable for patients with mild to moderate symptoms [17]. Operation is generally indicated in cases of acute cauda equina syndrome or in condition with rapidly worsening neurologic deficits [18]. Our case had acute-onset paralysis, which is appropriate for surgical intervention. After refusal for operation, he started to receive methylprednisolone as the NASCIS II trial [8]. When some evidence of improvement appeared, rehabilitation programs were added. Four months after discharge from the hospital, the functional recovery revealed good.
Methylprednisolone, a synthetic glucocorticoid as gold standard in the management of acute spinal cord injury, is effective due to its inhibition of lipid peroxidation and hydrolysis preventing breakdown of the cell membrane [8,19]. It also increases blood perfusion of the spinal cord when given after an acute injury [8]. It is universally knowledge that administration of methylprednisolone has been confirmed for treatment of spinal cord injury, but not consensus in spinal cord lesion yet [20,21]. The above mechanisms might provide reasonable explanation for functional improvement observed in our case, no matter what radiculopathy/myelopathy or not.
The rehabilitation program for our patient mainly included stretching exercise, strengthening exercise, electrical muscular stimulation, and postural training. The goal of stretching exercise for hamstring and lumbar paraspinal muscles and strengthening exercises for the abdominal muscles were to decrease extension forces on the lumbar spine [17]. Extension of the spine can lead to posterior protrusion of an intervertebral disc and bulging of the ligamentum flavum, which can cause more narrowing of the spinal canal [17]. Therefore we stretched the patient’s hamstring and lumbar paraspinal muscles, the agonist muscles to lumbar extension, and strengthened the abdominal muscles, the antagonist muscles to lumbar extension. We also strengthened the patient’s gluteal and quadriceps muscles to enhance ambulation. Electrical muscular stimulation was given because it might prevent muscle atrophy. Finally we instructed the patient to keep proper posture and avoid lumbar extension to prevent further deterioration or recurrence of his neurological complications.
Characteristics of achondroplasia consisted of clinical features of appearance and neurologic complications, for instance, hydrocephalus, cervical compression and neurological deficits after spinal stenosis [7]. Spinal stenosis is related to aberrant endochondral ossification, in which there is premature fusion of the chondrification center, thickened lamina, and shortened pedicle, hypertrophied zygapophyseal joint and even lower height of the vertebral body [5]. These vertebral abnormalities create a discrepancy in the diameter of the osseous canal and neural structures that results in increased intraspinal pressure, typically at the level of L2 to L3 [6]. Patients with achondroplasia and symptomatic spinal stenosis often experience back pain, which may progress to lower extremity pain and debilitating consequences. Anatomic anomalies of the vertebral column place the patient at risk for spinal stenosis as early as the first decade and especially during adulthood [4]. Schkrohowsky et al. studies the likelihood of developing symptomatic stenosis by measuring canal diameters radiographically, and found that those “at risk” for stenosis had a significantly larger average percentage decrease in the transverse interpedicular distance from T12 to L5 (-8% vs. -19%) and a significantly greater thoracolumbar kyphosis angle (24.2-degree angle vs. 14.1-degree angle), when compared with controls [11]. A study investigated the natural history of pain associated with spinal stenosis in individuals with achondroplasia, and the results showed that patients reported significant progression of pain toward involvement of the lower extremities and significant increases in lower extremity pain severity overall. Compared with patients with back pain only, those with back pain and proximal or distal leg pain had higher self-rated pain severity; higher functional disability; and more bowel and bladder dysfunction symptoms, sleep disturbances, extremity numbness, and psychological distress [5]. A group of surgeons, major in limb lengthening operation, screened for spinal stenosis at risk in children with achondroplasia, and concluded that female sex, delayed milestones and a tight cervicomedullary junction as high risks [12].
Skeletal abnormalities are known to cause neurological morbidity and lead to a shortened lifespan [7]. The symptoms of spinal stenosis include low back pain, paresthesia, neurogenic claudication, and even sudden-onset paraplegia [3,7]. Paresthesia is described as tingling, pin-pricking, numbness, burning, or a sleepy or dead sensation in the legs; paresthesia was present in our patient. The symptoms derived from spinal stenosis are usually bilateral and asymmetric. The straight-leg-raising test is usually negative [13]. EMG study is usually abnormal, but not a specific finding [13]. The average age at onset of symptoms is 38 years. Fewer than 10% of patients have such neurological symptoms at 10 years old, compared with 20% by the end of the teens and 80% by the 50s [1]. This delayed development of neurologic problems can be caused by size of the spinal canal, herniation of the nucleus pulposus, degenerative spurring of the vertebral facets, vertebral mal-alignment, vertebral instability, increasing lumbar lordosis, and hypertrophy of the ligamentum flavum [14]. Occasionally, neurologic complications may be abrupt in onset following undue physical exertion, like our case. Hamamci et al. reported spinal cord lesion occurred in 3 cases with achondroplastic dwarfism. All the 3 cases underwent surgical intervention, and obtained better recovery [14]. Wieting & Krach also described a case of a 12-year-old achondroplastic patient who incurred an apparently nontraumatic cervical cord infarction with resultant quadriplegia. The disable case was also treated by high dose steroid together with intensive multidisciplinary rehabilitation, and obtained better outcome, like our case. Coincidently, the 12-year-old boy did not have apparent cause, like ours, too [15]. Di Fabio et al. reported a case of achondroplasia suffered from acute paraparesis after lumbar bending as well [16].
Eligible treatments for patients with lumbar spinal stenosis vary. Conservative treatment is suitable for patients with mild to moderate symptoms [17]. Operation is generally indicated in cases of acute cauda equina syndrome or in condition with rapidly worsening neurologic deficits [18]. Our case had acute-onset paralysis, which is appropriate for surgical intervention. After refusal for operation, he started to receive methylprednisolone as the NASCIS II trial [8]. When some evidence of improvement appeared, rehabilitation programs were added. Four months after discharge from the hospital, the functional recovery revealed good.
Methylprednisolone, a synthetic glucocorticoid as gold standard in the management of acute spinal cord injury, is effective due to its inhibition of lipid peroxidation and hydrolysis preventing breakdown of the cell membrane [8,19]. It also increases blood perfusion of the spinal cord when given after an acute injury [8]. It is universally knowledge that administration of methylprednisolone has been confirmed for treatment of spinal cord injury, but not consensus in spinal cord lesion yet [20,21]. The above mechanisms might provide reasonable explanation for functional improvement observed in our case, no matter what radiculopathy/myelopathy or not.
The rehabilitation program for our patient mainly included stretching exercise, strengthening exercise, electrical muscular stimulation, and postural training. The goal of stretching exercise for hamstring and lumbar paraspinal muscles and strengthening exercises for the abdominal muscles were to decrease extension forces on the lumbar spine [17]. Extension of the spine can lead to posterior protrusion of an intervertebral disc and bulging of the ligamentum flavum, which can cause more narrowing of the spinal canal [17]. Therefore we stretched the patient’s hamstring and lumbar paraspinal muscles, the agonist muscles to lumbar extension, and strengthened the abdominal muscles, the antagonist muscles to lumbar extension. We also strengthened the patient’s gluteal and quadriceps muscles to enhance ambulation. Electrical muscular stimulation was given because it might prevent muscle atrophy. Finally we instructed the patient to keep proper posture and avoid lumbar extension to prevent further deterioration or recurrence of his neurological complications.
CONCLUSION
In summary, patients with achondroplasia and spinal stenosis sometimes develop acute neurologic deteriorating status, such as paralysis. If surgical intervention is unavailable, high dose steroid and comprehensive rehabilitation should be commenced as soon as possible, like our case. We attempt to bring attention to the role of methylprednisolone and rehabilitation for patients with spinal stenosis. Further study is needed to find any criteria that would identify patients most likely to benefit from conservative treatment.
REFERENCES
- Hunter AGW, Bankier A, Rogers JG, Sillence D, Scott CI Jr (1998) Medical complications of achondroplasia: a multicentre patient review. J Med Genet 35: 705-712.
- Bouali H, Latrech H (2015) Achondroplasia: Current options and future perspective. Pediatr Endocrinol Rev 12: 388-395.
- Unger S, Bonafé L, Gouze E (2017) Current care and investigational therapies in achondroplasia. Curr Osteoporos Rep 15: 53-60.
- Shirley ED, Ain MC (2009) Achondroplasia: manifestations and treatment. J Am Acad Orthop Surg 17: 231-241.
- Ain MC, Abdullah MA, Ting BL, Skolasky RL, Carlisle ES, et al. (2010) Progression of low back and lower extremity pain in a cohort of patients with achondroplasia. J Neurosurg Spine 13: 335-340.
- Thomeer RT, van Dijk JM (2002) Surgical treatment of lumbar stenosis in achondroplasia. J Neurosurg 96: 292-297.
- Hecht JT, Bodensteiner JB, Butler IJ (2014) Neurologic manifestations of achondroplasia. Handb Clin Neurol 119: 551-563.
- Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, et al. (1990) The national acute spinal cord injury study group: a randomized controlled trial of methylprednisolone or naloxone in the treatment of acute spinal injury. N Engl J Med 322: 1405-1411.
- Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P (1996) Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 84: 911-921.
- Cohen MM Jr (2002) Some chondrodysplasias with short limbs: molecular perspectives. Am J Med Genet 112: 304-313.
- Schkrohowsky JG, Hoernschemeyer DG, Carson BS, Ain MC (2007) Early presentation of spinal stenosis in achondroplasia. J Pediatr Orthop 27: 119-122.
- Fernandes JA, Devalia KL, Moras P, Pagdin J, Jones S, et al. (2014) Screening for spinal stenosis in achondroplastic patients undergoing limb lengthening. J Pediatr Orthop B 23: 181-186.
- Sinaki M, Mokri B (2000) Low back pain and disorders of the lumbar spine, in Braddom RL ( ed) Physical medicine and rehabilitation, ed 2. Philadelphia, W. B. Saunders, pp 853-93.
- Hamamci N, Hawran S, Biering-Sørensen F (1993) Achondroplasia and spinal cord lesion: Three case reports. Paraplegia 31: 375-379.
- Wieting JM, Krach LE (1994) Spinal cord injury rehabilitation in a pediatric achondroplastic patient: case report. Arch Phys Med Rehabil 75: 106-108.
- Di Fabio R, Giugni E, Vadalà R, Pierelli F, Bastianello S (2014) Acute paraparesis as consequence of lumbar bending in achondroplasia. Neurol Sci 35: 105-107.
- Bodack MP, Monteiro M (2001) Therapeutic exercise in the treatment of patients with lumbar spinal stenosis. Clin Orthop 384: 144-152.
- Fritz JM, Delitto A, Welch WC, Erhard RE (1998) Lumbar spinal stenosis: A review of current concepts in evaluation, management, and outcome measurements. Arch Phys Med Rehabil 79: 700-708.
- Gerndt SJ, Rodriguez JL, Pawlik JW, Taheri PA, Wahl WL, et al. (1997) Consequences of high-dose steroid therapy for acute spinal cord injury. J Trauma 42: 279-824.
- Wu YT, Chiang SL, Lai MH, Lu SC, Chang CC, et al. (2010) Methylprednisolone worsening neuropathic pain in non-traumatic thoracic myelopathy. J Clin Pharm Therap 35: 491-496.
- Wu YT, Li TY, Chu HY, Chen LC, Chiang SL, et al. (2011) Relationship between the interval before high-dose methylprednisolone administration and chronic pain in traumatic spinal cord injury. Neurosciences (Riyadh) 16: 324-328.
Citation: Kuo FC, Chiang SL, Chang ST (2019) Successful Treatment of Acute Paralysis of Lower Limbs with High Dose Steroids and Rehabilitation in Achondroplasic Dwarf: A Case Report. J Med Stud Res 2: 008.
Copyright: © 2019 Shin-Tsu Chang, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!

