
Successful Treatment of Refractory Fulminant Clostridioides Difficile Colitis after Ileocolostomy Reversal Using Faecal Microbiota Transplant
*Corresponding Author(s):
Gordon CarlsonDepartment Of Colorectal Surgery, Salford Royal Hospital, Stott Lane, Salford, United Kingdom
Email:Gordon.carlson@nca.nhs.uk
Abstract
Background
Clostridioides difficile (C.diff) colitis accounts for significant morbidity, mortality and inpatient bed days. Prevalence of C.diff associated diarrhoea (CDAD) in general surgical patients is high. Faecal Microbiota Transplant (FMT) is a recognised treatment for recurrent CDAD, but there is minimal evidence for its use in primary CDAD.
Case
A 72-year-old lady developed fulminant primary CDAD following elective reversal of an ileocolostomy. She deteriorated despite antibiotic, immunoglobulin and probiotic therapy. CT showed toxic megacolon, with gross oedema but no anastomotic leak. Due to previous colonic surgery (anterior resection and right hemicolectomy), severe illness (requiring mechanical ventilation and vasopressor support) and antiplatelet (clopidogrel) use, it was thought she would be unlikely to survive further surgical intervention. She received Faecal Microbiota Transplant (FMT), donated by a relative and screened for COVID-19 and subsequently made a full recovery, with apparent resolution of her C. Diff colitis, being discharged from hospital on the 79th day postoperatively.
Conclusion
There should be a low index of suspicion for CDAD following stoma reversal. FMT can be successfully used to treat primary, life-threatening CDAD and should be considered for patients with severe CDAD.
Keywords
Ileocolostomy; Metronidazole; Vancomycin
Background
There were 13,177 cases Clostridioides difficile colitis reported in England in the year ending March 2020 [1]. The prevalence of C. diff colitis infection in the UK has been reported to be as high as 0.47%, with increasing age, gastrointestinal surgery, malignancy and comorbidities conferring increased risk( [2]. C.diff infection is characteristically associated with prolonged antibiotic treatment [3], but even a single dose used as surgical prophylaxis may be causative [4]. Patients undergoing colorectal surgery are at particularly high risk of developing C. diff colitis postoperatively, with the condition affecting 0.9% of patients following bowel resection [5] and 3.04% after stoma reversal [6].
C.diff colitis ranges from mild to fulminant, life-threatening disease. Severe disease, defined as the need for colectomy or ITU admission, accounts for 4.1% of cases, with an inpatient mortality of 34.7%(3). Mild C.diff colitis may be treated with oral Vancomycin, adding IV Metronidazole for severe or refractory cases(5). Fidaxomicin has been advocated for recurrent disease or in those with the NAP1/B1/027 toxin-producing strain of C.diff [7]. Colectomy is usually a last resort for patients who fail to respond to antibiotic treatment(5), since post-op mortality may reach 50% in this setting(8). More conservative surgical options have been proposed, including loop ileostomy and colonic lavage [8].
The use of faecal enemas to treat C diff colitis was first reported in 1958 [9]. However, faecal microbiota transplant (FMT) has only recently been commonly utilised as a treatment for C. diff colitis. Current international guidelines recommend FMT in recurrent CDAD refractory to antibiotic therapy [10], but there is almost no published data to support its use in primary CDAD [10].
We present our experience of successfully using FMT to treat an episode of primary, severe, refractory C.diff colitis which developed after elective reversal of an ileocolostomy and for which surgical treatment was unlikely to be possible.
Clinical Case
A 72-year-old woman was admitted electively for reversal of an ileocolostomy. She had undergone an anterior resection seven years earlier for treatment of adenocarcinoma. A colonoscopy and polypectomy five years later resulted in a perforation of the ascending colon, for which she underwent an emergency right hemicolectomy and formation of an ileocolostomy.
The subsequent stoma reversal was undertaken via a peristomal incision, with a stapled side-to-side anastomosis between the ileum and ascending colon. The patient received a single dose of intravenous Co-Amoxiclav as antibiotic prophylaxis. No mechanical bowel preparation was administered. Within 48 hours of surgery she developed abdominal discomfort, diarrhoea and vomiting, progressing to left iliac fossa pain, peritonism and tachycardia. CT showed ileus, no anastomotic leak, but a hyperenhancing, fluid-filled colon, in keeping with ‘disuse colitis’.
She was commenced on IV Metronidazole, Amoxicillin and Gentamicin, then Tazocin because of suspicion of abdominal sepsis, but deteriorated further. Stool cultures were positive for C.diff toxin and gene expression, therefore antibiotics were converted to PO Vancomycin and IV Metronidazole. Due to continued clinical and biochemical deterioration, she was commenced on VSL#3 probiotics and transferred to ITU. Although there was an initial improvement, she developed respiratory failure by the 14th postoperative day due to pneumonia and diaphragmatic splinting secondary to massive colonic dilatation. She required intubation and mechanical ventilation with vasopressor (noradrenaline) therapy. A further CT scan showed toxic megacolon and bilateral pleural effusions, but no evidence of anastomotic leak as shown in Figures 1 & 2.
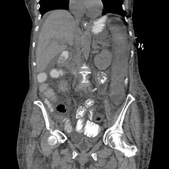 Figure 1. CT showing- Oedematous Descending Colon.
Figure 1. CT showing- Oedematous Descending Colon.
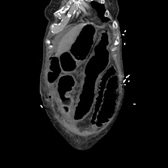 Figure 2. Toxic Dilatation of the Transverse Colon.
Figure 2. Toxic Dilatation of the Transverse Colon.
Despite nasogastric Vancomycin, Rifampicin and intravenous Metronidazole, she remained critically ill. She failed to respond to IV immunoglobulin therapy (20g normal immunoglobulin Privigen 10% infusion). Endoscopic decompression was attempted, but the procedure was abandoned due to the quantity of pseudomembranes and friable, oedematous mucosa. Colectomy was considered but the ligation of both inferior mesenteric and ileocolic pedicles at prior operations meant any colonic resection would likely have required a near total proctocolectomy (because resection would have resulted in ischaemia of the remaining large bowel) which she was felt unlikely to survive.
Following discussions with the family, a decision was taken on the 25th postoperative day to attempt FMT. Owing to the coronavirus pandemic the use of pre-donated, pre-screened, frozen FMT from the regional centre was prohibited. Following negative donor screening 24 hours prior to donation (serum tested for HIV, Hepatitis A, B, C and syphilis; nasal PCR swab for SARS-CoV2; stool for microscopy, culture and C.diff) a family member donated faeces (one motion in quantity) for FMT the day prior to administration. The donated faeces were prepared by technicians from the FMT centre, by mixing it with sterile saline to a slurry, then filtering it (using coffee filter paper) on the day of donation. The preferred administration route (enema) was precluded by profuse diarrhoea so 50ml was administered via nasogastric tube. Antibiotics and PPI were withheld for 48 hours and nasogastric feeding for 6 hours prior to administration.
Administration of FMT resulted in a slow but steady recovery. The albumin trend concurred with the clinical trend towards recovery, being 22(g/L) on the day of FMT, increasing to 28(g/L) 1 week later, and normalising 3 weeks after administration. A tracheostomy was performed to facilitate gradual respiratory weaning. The last dose of antibiotics (oral Vancomycin, restarted on day 33) was given on day 39. She was transferred to the ward on day 71 and discharged on the 79th postoperative day. By the time of clinic follow up, 6 weeks later, she was systemically well, her wound had healed and she had satisfactory bowel function.
Discussion
The prevalence of preoperative C. diff colonisation in patients admitted for elective colorectal surgery, particularly in those with pre-existing diversion of the faecal stream, is unclear. However, there is both decreased bacterial load and decreased biodiversity in the defunctioned limb of an ileostomy[11]. We hypothesise that our patient’s defunctioned colon had an altered preoperative microbiome, and may have been colonised with C. diff. Subsequent restoration of colonic continuity, combined with the dose of perioperative antibiotic prophylaxis, rapidly resulted in the development of C. diff colitis. Similar factors may explain the relatively high incidence of C. diff colitis reported by others after stoma closure [6].
Anastomotic leakage is a common source of sepsis following creation of a colorectal anastomosis. However, this patient rapidly developed sepsis, progressing to organ failure, not because of an anastomotic leak but because of the development of C. diff colitis. While the risk of postoperative C. diff colitis is probably not sufficiently high to justify preoperative C. diff colonisation screening for all patients undergoing stoma reversal, this case demonstrates the importance of including C. diff colitis, as well as anastomotic leakage, in the differential diagnosis of any patient who deteriorates with sepsis shortly after restoration of colonic continuity.
FMT has been widely advocated for treatment of recurrent C. diff colitis [12-13]. This case illustrates that it may also be effective when used to treat primary C.diff infection. Our patient’s previous vascular ligations would have made any surgery short of a panproctocolectomy unlikely to be technically possible and it seems unlikely this would have been survivable in the setting of critical illness. The use of FMT, when conventional therapy had failed, was associated with complete resolution of our patient’s colitis, despite the fact that it had progressed radiologically to toxic dilatation.
The method of delivery of FMT in this case was also noteworthy. The use of pre-prepared, frozen FMT was prohibited in this case, as MHRA approval had been withdrawn due to theoretical risk of COVID-19 transmission in stool microbiota [14]. Faeces were therefore donated by a relative, following satisfactory screening for transmissible infection. Although administration via the upper gastrointestinal tract has been less effective [10] and colonoscopic or enema administration is preferred, the severity of this patient’s colonic disease precluded this. However, a single dose of FMT via a nasogastric tube appears to have been dramatically effective.
Conclusion
A low threshold of suspicion is appropriate for the diagnosis of C. difficile colitis in patients who develop sepsis after restoration of colonic continuity, even after a single dose of prophylactic antibiotics. In this case a single dose of FMT using faeces donated by a family member proved effective in reversing severe primary pseudomembranous colitis which had been resistant to all other available treatment and in a setting where surgery would not have been technically feasible.
References
- Thelwall S, Nsonwu O, Rooney G, Hope R (2019) Annual epidemiological commentary: Gram-negative bacteraemia, MRSA bacteraemia, MSSA bacteraemia and C. difficile infections, up to and including financial year April 2019 to March 2020. UK Government National Statistics Website 2020. 54-63.
- Rodrigues MA, Brady RR, Rodrigues J, Graham C, Gibb AP (2010) Clostridium difficile infection in general surgery patients; identification of high-risk populations. International j of surgery 8: 368-372.
- Sailhamer E, Carson K, Chang Y, Zacharias N, Spaniolas K, et al. (2009) Fulminant Clostridium difficile Colitis: Patterns of Care and Predictors of Mortality. Archives of Surgery 144: 433-439.
- Rivitera G, Scarpellini P, Ortisi G, Nicastro G, Nicolin R, et al. (1991) Prospective study of Clostridium difficile intestinal colonization and disease following single-dose antibiotic prophylaxis in surgery. Antimicrobial Agents and Chemotherapy 35: 208-210.
- Sartelli M, Di Bella S, McFarland L, Khanna S, Furuya-Kanamori L, et al. (2019) Update of the WSES guidelines for management of Clostridioides (Clostridium) difficile infection in surgical patients. World J of Emergency Surgery 14: 8.
- Skancke M, Vaziri K, Umapathi B, Amdur R, Radomski M, et al. (2018) Elective Stoma Reversal Has a Higher Incidence of Postoperative Clostridium Difficile Infection Compared With Elective Colectomy: An Analysis Using the American College of Surgeons National Surgical Quality Improvement Program and Targeted Colectomy Databases. Diseases of the Colon and Rectum 61: 593-598.
- Ong G, Reidy T, Huk M, Lane F (2017) Clostridium difficile colitis: A clinical review. American J of Surgery 213: 565-571.
- Neal M, Alverdy J, Hall D, Simmons R, Zuckerbraun B (2011) Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease. Annals of Surgery 254: 423-429.
- Eiseman B, Silen W, Bascom GS, Kauvar AJ (1958) Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 44: 854-859.
- Gough E, Shaikh H, Manges A (2011) Systematic Review of Intestinal Microbiota Transplantation (Fecal Bacteriotherapy) for Recurrent Clostridium difficile Infection. Clinical Infectious Diseases 53: 994-1002.
- Beamish EL, Johnson J, Shaw EJ, Scott NA, Bhowmick A, et al. (2017) Loop ileostomy-mediated fecal stream diversion is associated with microbial dysbiosis. Gut microbes 8: 467-478.
- Lee C, Steiner T, Petrof E, Smieja M, Roscoe D, et al. (2016) Frozen vs Fresh Fecal Microbiota Transplantation and Clinical Resolution of Diarrhea in Patients With Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA : the journal of the American Medical Association 315: 142-149.
- Cammarota G, Ianiro G, Tilg H, Rajilic-Stojanovic M, Kump P, et al. (2017) European consensus conference on faecal microbiota transplantation in clinical practice. Gut 66: 569-580.
- Wang W, Xu Y, Gao R, Lu R, Han K, et al. (2020) Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA : J of the American Medical Association 323: 1843-1844.
Citation: Carlson G, Heard RSM, Singh S (2023) Successful Treatment of Refractory Fulminant Clostridioides Difficile Colitis after Ileocolostomy Reversal Using Faecal Microbiota Transplant. J Surg Curr Trend Innov 7: 058.
Copyright: © 2023 Rachel SM Heard, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

