
Superior Vena Cava Syndrome: A Clinical Case
*Corresponding Author(s):
Iveta TashevaDepartment Of Cardiology, Sofia University, Bulgaria
Email:igtasheva@uni-sofia.bg
Abstract
A clinical case of a 72-year-old male patient was admitted to the intensive care unit of the Cardiology Department with complaints of progressing dyspnea upon minimal physical effort and at rest, coughing with whitish expectoration, reddening and swelling of the neck and the face, headaches, and two-time loss of consciousness, with the onset of complaints dating back 20 days.He reported having a SARS-CoV-2 infection 3 months ago. Echocardiography was performed and revealed preservedejection fraction of the left and right ventricles (EF 57%, TAPSE 21 mm), without hemodynamicallysignificant valve lesions and a normal ECG. On the second day of the hospitalization, we performed a Computer Tomography (CT) scan of the lungs, which showed a tumour in the right lung compressing the superior vena cava.
Keywords
Superior vena cava; Superior vena cava syndrome; Venography
Introduction
External compression of the Superior Vena Cava (SVC) by a tumor mass in the mediastinum is the most common cause of Superior Vena Cava Syndrome (SVCS). In most cases, it is associated with a malignant condition, but there has also been an increase in the share of conditions related to the formation of an occlusive venous thrombus that compromises the venous flow back to the heart. SVCS is also encountered as a complication following implantation of intravasal devices, such as catheters, pacemakers, and implantable Cardioverter-Defibrillators (ICDs). Among tumours, the most common cause of SVCS are small cell bronchogenic carcinoma, non-Hodgkin lymphoma, and metastasizing tumours [1,2].
SVCS is a constellation of clinical signs and symptoms that derive from partial or total hindrance to the blood flow through SVC. As a result of the venous congestion, a clinical scenario emerges, which is related to increased venous pressure in the upper part of the body [3], most often charasterised by an oedema of the face and the neck, oedema of the upper extremities, oropharyngeal and nasal oedema, headache, and syncopes caused by the elevated intracranial pressure [4,5].
The careful physical examination is often sufficient to rule out a cardiogenic origin of the patient’s symptoms. In this article, we present a clinical case of SVCS treated successfully via interventional methods [6].
Description Of The Clinical Case
A 72-year-old male patient was admitted to the intensive care unit of the Cardiology Department with complaints of progressing dyspnea upon minimal physical effort and at rest, coughing with whitish expectoration [7], reddening and swelling of the neck and the face, headaches, and two-time loss of consciousness, with the onset of complaints dating back 20 days [8,9]. The patient had been known to have grade I arterial hypertension, and had been undergoing systemic antihypertensive treatment. He reported having a SARS-CoV-2 infection 3 months ago, which was treated in out-patient settings. The laboratory tests revealed the following: Tn 0.015 ng/ml (0.02-0.060; CPK 50 U/L; CPK-MB 8 U/L; CRP 47.86; D-Dimer 1.77 (<0.55); Blood gas analysis: pH 7.45; pCO2 33.10 mm Hg; pO2 51 mm Hg; SBC 24; BE 0.2; Sat. O2 88%. The emergency trans thoracic echocardiography (EchoCG) revealed evidence of preserved LV and RV systolic function, mild left ventricular hypertrophy without significant valve lesions (Figure 1) [10].
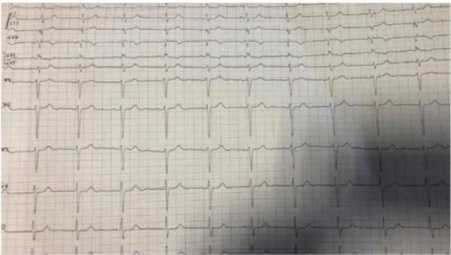 Figure 1: ECG upon admission.
Figure 1: ECG upon admission.
The 12-lead ECG performed upon admission displayed sinus rhythm with a frequency of 60 beats/minute, and an indifferent type of electrical axis without significant repolarization changes [11].
The transthoracic echocardiography manifested preserved LV and RV systolic function, mild left ventricular hypertrophy without significant valve lesions (Figure 2).
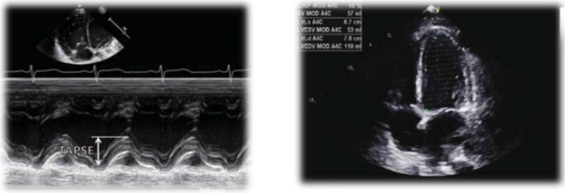 Figure 2: Transthoracic EchoCG.
Figure 2: Transthoracic EchoCG.
On the next day after the admission, a CT scan of the thorax was performed, which revealed a large conglomerate polycystic soft-tissue formation engaging predominantly the right half of the upper and middle mediastinum and the right hilus. There was evidence of separate formations that are difficult to demarcate, the largest of them measuring 30 and 50 mm. The process compressed to a significant extent SVC [12].
In conclusion, the finding corresponded to a solid space-engaging process in the right lung of a central type, a conglomerate space-engaging process in the mediastinum indicative of lymphadenomegaly, and nodular lesions in the right lung matching the characteristics of secondary dissemination (Figure 3) [13].
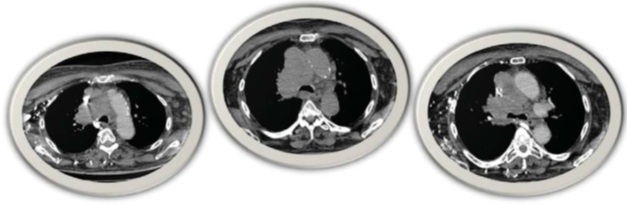 Figure 3: A solid tumour of the right lung compressing SVC to a significant extent.
Figure 3: A solid tumour of the right lung compressing SVC to a significant extent.
Description of the Interventional Procedure
On the second day after the hospitalization, the patient was subjected to a venography, which revealed the presence of a subtotal thrombosis of SVC. A Wall stent 16/60 mm was placed in SVC, and subsequent post-dilation with a balloon 10/30 mm was performed. The percutaneous venous access was via the right femoral vein, with the use of an 8 Fr introducer and a 0.35 STORQ guide. The intervention was successful, and good distal blood flow was achieved by the end of the procedure [14].
In the aftermath, the patient had no complaints and displayed stable hemodynamics, and soon after that a significant reduction in the dyspnea, the oedema and the cyanosis of the neck, the face and the upper extremities was observed. No rhythm or conduction disorders were registered during the hospitalization [15].
Treatment
The patient was discharged on the third day after the intervention, and was prescribed anticoagulant, antiaggregant, and antihypertensive therapy with Fraxiparine 2 x 0.6 ml, Clopidogrel 75 mg, and Lisinopril 10 mg [16].
Nowadays, the main possibilities for endovascular therapy are Percutaneous Transluminal Angioplasty (PTA) (with or without stenting), thrombolysis, and the combination of the two [17,18].
Discussion
The stenting of SVC leads to relieving of the severe symptoms prior to the histological verification of the tumour causing the obstruction. It is indicated in patients in whom chemotherapy or radiation therapy have not led to alleviation of the symptoms. There has been an increase in the support for recommending stenting as the first line of treatment that should be carried out early after establishing the diagnosis of SVCS. A 2008 study by Rizvi et al. came to the conclusion that stenting ought to be regarded as the first line of treatment for SVCS, with open surgical reconstruction still being a good option, if the endovascular approach proved unsuccessful or unsuitable.
Conclusion
Endovascular treatment with stenting in SVCS is a minimally invasive and safe procedure. The monitoring of patients subjected to percutaneous transluminal angioplasty (PTA) shows clinical improvement with considerable relieving of the symptoms. Interventional treatment of patients with SVCS caused by a malignant condition is of palliative nature and its objective is to improve the patient’s quality of life prior to the histological verification of the tumour process; it has a satisfactory mid-term effect and a favourable clinical outcome.
References
- Petrov I, Tasheva I (2018) UMBAL Ajibadem City Clinic. Cardiovascular Center, Sofia, Bulgaria
- Zimmerman S, Davis M (2018) Rapid Fire: Superior Vena Cava Syndrome. Emerg Med Clin North Am 36: 577-584.
- Carmo J, Santos A (2018) Chronic Occlusion of the Superior Vena Cava. N Engl J Med 379: 2.
- De Potter B, Huyskens J, Hiddinga B, Spinhoven M, Janssens A, et al. (2018) Imaging of urgencies and emergencies in the lung cancer patient. Insights Imaging 9: 463-476.
- Friedman T, Quencer KB, Kishore SA, Winokur RS, Madoff DC (2017) MalignantVenous Obstruction: Superior Vena Cava Syndrome and Beyond. Semin Intervent Radiol 34: 398-408.
- Kalinin RE, Suchkov IA, Shitov II, Mzhavanadze ND, Povarov VO (2017) Venous thromboembolic complications in patients with cardiovascular implantable electronic devices. Angiol Sosud Khir 23: 69-74.
- Ghorbani H, Vakili Sadeghi M, Hejazian T, Sharbatdaran M (2018) Superior vena cava syndrome as a paraneoplastic manifestation of soft tissue sarcoma. Hematol Transfus Cell Ther 40: 75-78.
- Labriola L, Seront B, Crott R, Borceux P, Hammer F, et al. (2018) Superior vena cava stenosis in haemodialysis patients with a tunnelled cuffed catheter: prevalence and risk factors. Nephrol Dial Transplant. 33: 2227-2233.
- Mansour A, Saadeh SS, Abdel-Razeq N, Khozouz O, Abunasser M, et al. (2018) Clinical Course and Complications of Catheter and Non-Catheter-Related Upper Extremity Deep Vein Thrombosis in Patients with Cancer. Clin Appl Thromb Hemost 24: 1234-1240.
- Khan UA, Shanholtz CB, McCurdy MT (2017) Oncologic Mechanical Emergencies. Hematol Oncol Clin North Am 31: 927-940.
- https://pubmed.ncbi.nlm.nih.gov/29781569/Nossair F, Schoettler P, Starr J, Chan AKC, Kirov I, et al. (2018) Pediatric superior vena cava syndrome: An evidence-based systematic review of the literature. Pediatr Blood Cancer 65: 27225.
- Ierardi AM, Jannone ML, Petrillo M, Brambillasca PM, Fumarola EM, et al. (2018) Treatment of venous stenosis in oncologic patients. Future Oncol 14: 2933-2943.
- Kalra M, Sen I, Gloviczki P (2018) Endovenous and Operative Treatment of Superior Vena Cava Syndrome. Surg Clin North Am 98: 321-335.
- Hague J, Tippett R (2010) Endovascular techniques in palliative care. Clin Oncol (R Coll Radiol). 2010 22: 771-80.
- Gradishar WJ, Magid D, Bitran JD (1990) Supportive care of the lung cancer patients. Hematol Oncol Clin North Am 4: 1183-1199.
- Lianos GD, Hasemaki N, Tzima E, Vangelis G, Tselios A, et al. (2018) Superior vena cava syndrome due to central port catheter thrombosis: a real life-threatening condition. G Chir 39: 101-106.
- Kuo TT, Chen PL, Shih CC, Chen IM (2017) Endovascular stenting for end-stage lung cancer patients with superior vena cava syndrome post first-line treatments - A single-center experience and literature review. J Chin Med Assoc 80: 482-486.
- Nakano T, Endo S, Kanai Y, Otani S, Tsubochi H, et al. (2014) Surgical outcomes after superior vena cava reconstruction with expanded polytetrafluoroethylene grafts. Ann Thorac Cardiovasc Surg 2: 310-315.
Citation: Tasheva I, Kafalieva S (2023) Superior Vena Cava Syndrome: A Clinical Case J Angiol Vasc Surg 8: 100.
Copyright: © 2023 Iveta Tasheva, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

