
Takayasu's Arteritis and Ankylosing Spondylitis: Is it a Fortuitous Association?
*Corresponding Author(s):
Ilyas El KassimiDepartment Of Internal Medicine, Mohammed V Military Teaching Hospital, Mohammed V University, Rabat, Morocco
Tel:+212 614742828,
Email:dr.elkassimiilyas@gmail.com
Abstract
Takayasu's Arteritis (TA) and Ankylosing Spondylitis (AS) are two inflammatory diseases of pathophysiology that are imperfectly elucidated. Only a few cases of authentic TA and AS associations have been reported. We report the case of a woman followed for AS under Etanercept, admitted for diffuse myalgia and left upper limb weakness. The diagnosis of Takayasu's arteritis was made on the basis of clinical and radiological criteria required by the American College of Rheumatology. This association most probably reflects a link between these two diseases, and the anti-TNF induced vasculitis hypothesis is not to rule out.
Keywords
Ankylosing spondylitis; Anti-TNF; Induced vasculitis; Takayasu's arteritis
INTRODUCTION
Takayasu's Arteritis (TA) is an inflammatory arteritis of large vessels, affecting the aorta and its main branches. It is a rare vascular disease that usually affects women before the age of 40. Ankylosing Spondylitis (AS) is chronic inflammatory rheumatism characterized by a predictive impairment of axial structures (spine and sacroiliac joints). The combination of these two pathologies is very rare but not exceptional. We report a new case.
CASE PRESENTATION
A 40-year-old woman followed for AS for 3 years under Etanercept. She was admitted for diffuse myalgia and arms numbness. Anamnesis revealed presence of inflammatory low backache. There was no history of ocular, dermatological or gastrointestinal problems.
The clinical examination found a stiffness of the cervical spine, abolition of the left radial, ulnar and humeral pulses with homolateral muscle weakness; lower limbs pulses were equally palpable with normal force. Auscultation revealed an ipsilateral murmur over Left Subclavian Artery (LSA) region; abdominal and carotid bruits were absent. The difference in systolic blood pressure was (>10 mm Hg) between the two upper limbs; blood pressure measurement was at 123/61 mmHg and 105/54 mmHg in right and left arm respectively.
Doppler ultrasonography found a circumferential stenosis of the initial portion of the LSA as well as of the axillary and the humeral arteries of the left upper limb. CT angiography showed fusiform stenosis of the LSA (34 mm long) with weak opacification downstream; the rest of the aorto-arterial network was intact (Figures 1 and 2). Pelvis X-ray showed bilateral sacroiliitis (Figure 3). Biological assays showed an inflammatory syndrome: C-reactive protein at 80 mg/l (N < 6 mg/l) and erythrocyte sedimentation rate was at 35 mm/hr.
Blood count showed: hemoglobin at 10.5 g/dl (NR: 12-16), mean globular volume at 77 fl (NR: 80-100), white blood cells at 8630 elements/mm3 (NR: 4000-10000), platelets at 234000 elements/ mm3 (NR: 150000-450000), HLA-B27 test was negative. Oral corticosteroid (1 mg / kg / day) was started. The response to treatment was favorable.
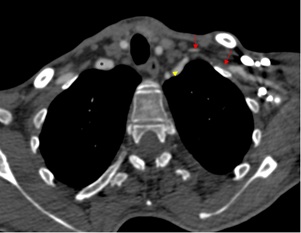 Figure 1: CT axial section with injection of contrast product, showing the small aspect of the left subclavian artery from its origin (arrowhead) with no opacification (→) and then recanalization downstream. Note the preserved caliber of the brachiocephalic arterial trunk on the right (*).
Figure 1: CT axial section with injection of contrast product, showing the small aspect of the left subclavian artery from its origin (arrowhead) with no opacification (→) and then recanalization downstream. Note the preserved caliber of the brachiocephalic arterial trunk on the right (*).
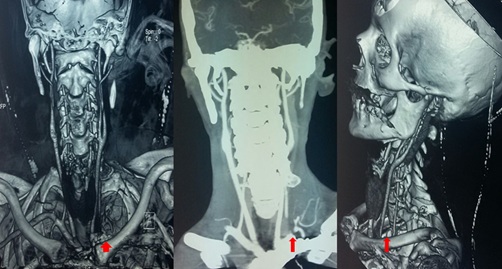 Figure 2: CT angiography reconstruction images showing left subclavian artery obstruction with no downstream opacification.
Figure 2: CT angiography reconstruction images showing left subclavian artery obstruction with no downstream opacification.
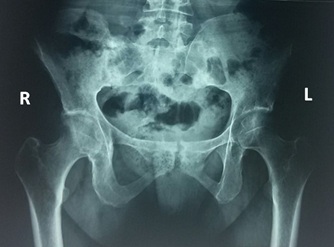 Figure 3: Pelvis X-ray suggestive of bilateral sacroiliitis.
Figure 3: Pelvis X-ray suggestive of bilateral sacroiliitis.
DISCUSSION
Association of TA and AS is very rare and may be accidental. In our case, the diagnosis of Takayasu's arteritis was made on the basis of clinical and radiological criteria required by the American College of Rheumatology Classification Criteria for Takayasu's arteritis (Table 1).
|
Criteria |
Definition |
|
Age at disease onset <40 years |
Development of symptoms or findings related to TA at age <40 years |
|
Claudication of extremities |
Development and worsening of fatigue and discomfort in muscles of 1 or more extremity while in use, especially the upper extremities |
|
Decreased brachial artery pulse |
Decreased pulsation of 1 or both brachial arteries |
|
Blood pressure difference >10 mmHg |
Difference of >10 mmHg in systolic blood pressure between arms |
|
Bruit over subclavian arteries or aorta |
Bruit audible on auscultation over 1 or both subclavian arteries or abdominal aorta |
|
Arteriograms abnormality |
Arteriography narrowing or occlusion of the entire aorta, its primary branches, or large arteries in the proximal upper or lower extremities, not due to arteriosclerosis, fibromuscular dysplasia, or similar causes; changes usually focal or segmental |
Table 1: The 1990 American college of rheumatology classification criteria for takayasu's arteritis [1].
Until nowadays, literature data showed 34 previously broadcasted cases of this association. Paloheimo et al., reported the first cases in 1966 [2]; since then, other authors have followed [3-7]. Most of these previously published cases concern women, spondyloarthritis usually precedes arteritis. This may be due to more predominant articular pain in pre-pulseless phase of Takayasu. Only in one case report, TA was the first presentation and AS developed later [8].
It’s believed that all said patients had a large vessels vasculitis with aorta and collaterals lesions. Conjunction with SA is well documented. All patients had radiological signs of bilateral sacroiliitis, which was indistinguishable from AS as in our case. In the series of cases reported by Mielnik P et al., peripheral arthritis was reported in half of these patients. Moreover, two patients with AS had an Inflammatory Bowel Diseases (IBD). Psoriasis was concomitant in two patients [7].
TA is defined by an inflammation of the aorta and its collaterals. Clinical symptoms consist of pain caused by inflammation in the arterial wall and, in a later phase, ischemic symptoms due to reduction of the arteries. Computed tomography, magnetic resonance and ultrasound imaging can show signs of the inflammation such as thickening and stenosis of the arteries. Classical angiography portrays stenosis or even occlusion of concerned arteries.
AS has an insidious onset and it is not associated with systemic symptoms [3]. The impairment of the lumbar spine is less severe (lower back pain without bone lesions) as in our case, which has already been reported in some series. This particularity characterizes the female AS.
The association between AS and aortitis is well established. Anatomically, AS often involves the ascending aorta, mainly the aortic root and subaortic structures, but distal aortic root involvement has rarely been reported [3,9-11]; Hull et al. defined this arteritis as TA [12].
As seen above, SA symptoms can be hardly apparent due to severity of vasculitis. Although some authors say that pathological pattern of different arteritis in large vessels can be told apart, descriptions of features are quite similar for all of them and environ around others: intima thickness, lymphocytes infiltration with presence of CD4+ cells, and inflammation in vasa vasorum. In addition, granuloma formation and giant cells can be found in all sorts of large vessels vasculitis [13]. Similar results are described in patients with aortitis amid SA without TA diagnosis [14-17].
In our patient, Takayasu’s development was characterized by subclavian artery involvement. In other cases, they all had aortitis with the same subclavian arteries involvement. Also, others had cervical arteries inflammation. One patient had mesenteric and renal arteries lesions [7].
High doses of prednisone (1 mg/kg/day) were an initial treatment of our patient. She had a good response to the corticotherapy which allowed her to reach arteritis remission. Treatment protocols varied fairly as the reports come from a long duration. The majority of the patients were treated by corticosteroids. Immunosuppressive treatment such as methotrexate, azathioprine, leflunomide, cyclophosphamide and cyclosporine was used much less frequently, only in some cases. The Outcome has been hard to follow, but only one fatal case was reported [7].
The appearance of vascular signs a few years after the joint signs may suggest a vascular complication of AS, but features including laboratory findings, ultrasonographic and angiographic characters, and clinical course argue more for a proven TA.
The possibility of an anti-TNF-induced vasculitis in our patient is also not to be ruled out, the first manifestations of the disease having occurred under etanercept, but these generally affect the arteries of small and medium caliber. It is interesting to note that some authors reported cases of patients who developed arteritis classified as TA during anti-TNF therapy [18-20]. Several hypotheses are formulated to explain this vasculitis. One calls up either the role of immune complexes or a modification of the cytokine balance. It has been suggested that TNFα-anti-TNFα complexes can be deposited on the vascular walls and locally activate the complement system (type III hypersensitivity reaction). A work on B-cells populations during rheumatoid arthritis demonstrates that anti-TNFα inhibitors reduce CD23 expression on activated B cell surfaces [21]. This downward regulation is associated with the presence of TNFα-anti-TNFα immune complexes that are captured by Fc?RIIb1 of the B-lymphocyte. The hypothesis most often mentioned is that of a modification of the cytokine balance. Thus, by changing a TH1-mediated disease (IL-1, TNF, interferon gamma, etc.,) towards a TH2 disease (production of IL-4, IL-5, IL-6, IL-10, IL-5 and IL-13...), Anti-TNFα is promoting the occurrence of manifestations of antibody-mediated immunity [22].
In our case, the vascular symptomatology persisted despite Etanercept discontinuation. This may be an argument that opposes the anti-TNFα-induced vasculitis hypothesis in which the occurrence of vasculitis manifestations after the introduction of the product is quite fast; these manifestations disappear quickly with the cessation of treatment and reappear in case of reintroduction of the incriminated product.
There isn’t enough evidence to link AS and TA, but in the etiology of associated spine and joint involvement in TA, autoimmunity and genetic factors may play a role [4,5,23]. This association most likely reflects a link between these two conditions, a specific HLA marker and a common autoantibody, but only a more in-depth study of these cases could ever answer these questions.
Recent studies imply that IL12 can be one of the major inflammation mediators involved both in TA and SA. A correlation was established between serum levels of IL12 and TA activity status [24], IL12 gene polymorphism can be reported both in TA and SA patients [25,26]. All these epidemiological and pathologic findings lead the authors to hypothesis that patients with both TA and SA diagnoses can have extreme arterial involvement due to SA which could be mistaken for TA.
CONCLUSION
The secrets of AS and TA are not yet revealed. Some authors believe that Takayasu’s like arteritis could take part in SPA family with IBD, psoriasis and chronic uveitis.
Their association is rare, but more studies can allow us to understand more of the mysterious common pathological mechanisms in order to improve the treatment efficiency.
CONSENT
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
DISCLOSURE
This case report was written based on clinical observation without any funding.
CONFLICTS OF INTEREST
There are no conflicts of interest between the authors and between the authors and the patient.
REFERENCES
- Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, et al. (1990) The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 33: 1129-1134.
- Paloheimo JA, Julkunen H, Siltanen P, Kajander A (1966) Takayasu’s arteritis and ankylosing spondylitis. Report of four cases. Acta Med Scand 179: 77-85.
- Cowley ML, Hickling P, Wells IP, Marshall AJ (1987) Takayasu’s disease and bilateral sacroiliitis. Clin Exp Rheumatol 5: 67-70.
- Magaro M, Altomonte L, Mirone L, Zoli A, Corvino G (1988) Seronegative spondarthritis associated with Takayasu’s arteritis. Ann Rheum Dis 47: 595-597.
- Cherin P, Blétry O, Ziza JM, Kieffer E, Arfi S, et al. (1990) [The association of ankylosing spondylitis and Takayasu's disease. 3 new cases]. Rev Rhum Mal Osteoartic 57: 33-37.
- Soubrier M, Dubost JJ, Demarquilly F, Therre T, Boyer L, et al. (1997) Maladie De Takayasu Et Spondylarthrite Ankylosante: Une Association Probablement Non Fortuite. Presse Med 26: 610.
- Mielnik P, Hjelle AM, Nordeide JL (2018) Coexistence of Takayasu's arteritis and ankylosing spondylitis may not be accidental - Is there a need for a new subgroup in the spondyloarthritis family? Mod Rheumatol 28: 313-318.
- Wilson WA, Morgan O, Bain B, Taylor JE (1979) Takayasu’s arteritis: association with Still’s disease in an adult. Arthritis Rheum 22: 684-688.
- Bergfeldt L, Edhag O, Rajs J (1984) HLA-B27-associated heart disease. Am J Med 74: 961-967.
- Bulkley BH, Roberts WC (1973) Ankylosing spondylitis and aortic regurgitation. Description of the characteristic cardiovascular lesion from study of eight necropsy patients. Circulation 18: 1014-1027.
- Tucker CR, Fowles RE, Calin A, Popp RL (1982) Aortitis in ankylosing spondylitis: early detection of aortic root abnormalities with two dimensional echocardiography. Am J Cardiol 49: 680-686.
- Hull RG, Asherson RA, Rennie JA (1984) Ankylosing spondylitis and an aortic arch syndrome. Br Heart J 51: 663-665.
- Slobodin G, Naschitz JE, Zuckerman E, Zisman D, Rozenbaum M, et al. (2006) Aortic involvement in rheumatic diseases. Clin Exp Rheumatol 24: 41-47.
- Eder L, Sadek M, McDonald-Blumer H, Gladman DD (2010) Aortitis and spondyloarthritis--an unusual presentation: case report and review of the literature. Semin Arthritis Rheum 39: 510-514.
- Hoogland YT, Alexander EP, Patterson RH, Nashel DJ (1994) Coronary artery stenosis in Reiter's syndrome: a complication of aortitis. J Rheumatol 21: 757-759.
- Stamp L, Lambie N, O’Donnell J (2000) HLA-B27 associated spondyloarthropathy and severe ascending aortitis. J Rheumatol 27: 2038-2040.
- Palazzi C, Salvarani C, D’Angelo S, Olivieri I (2011) Aortitis and periaortitis in ankylosing spondylitis. Joint Bone Spine 78: 451-455.
- Gan FY, Fei YY, Li MT, Wang Q, Xu D, et al. (2014) The characteristics of patients having ankylosing spondylitis associated with Takayasu's arteritis. Clin Rheumatol 33: 355-358.
- Mariani N, So A, Aubry-Rozier B (2013) Two cases of Takayasu’s arteritis occurring under anti-TNF therapy. Joint Bone Spine 80: 211-213.
- Souabni L, Ben Abdelghani K, Jradi S, Zakraoui L (2014) Takayasu's arteritis occurring under TNF-α blockers: a new paradoxical effect? Case Rep. 2014: bcr2014204226.
- De Miguel S, Jover J, Vadillo C, Judez E, Loza E, et al. (2003) B cell activation in rheumatoid arthritis patients under infliximab treatment. Clin Exp Rheumatol 21: 726-732.
- Saint Marcoux B, De Bandt M (2006) Vasculitides induced by TNFalpha antagonists: a study in 39 patients in France. Joint Bone Spine 73: 710-713.
- Sanders ME, Fischbein LC (1987) Hypertrophic osteoarthropathy with Takayasu’s arteritis. Clin Exp Rheumatol 5: 71-74.
- Verma DK, Tripathy NK, Verma NS, Tiwari S (2005) Interleukin 12 in Takayasu's arteritis: plasma concentrations and relationship with disease activity. J Rheumatol 32: 2361-2363.
- Saruhan-Direskeneli G, Biçakçigil M, Yilmaz V, Kamali S, Aksu K, et al. (2006) Interleukin (IL)-12, IL-2, and IL-6 gene polymorphisms in Takayasu's arteritis from Turkey. Hum Immunol 67: 735-740.
- Zhang L, Fan D, Liu L, Yang T, Ding N, et al. (2015) Association study of IL-12B polymorphisms susceptibility with ankylosing spondylitis in mainland Han population. PLoS One 10: 0130982.
Citation: EL Kassimi I, Sahel N, El Aoufir O, Rkiouak A, Sekkach Y (2020) Takayasu's Arteritis and Ankylosing Spondylitis: Is it a Fortuitous Association? J Angiol Vasc Surg 5: 039.
Copyright: © 2020 Ilyas El Kassimi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

