Telomeres Length Change in Peripheral Blood Lymphocytes of Psychotic Patients with Schizophrenia
*Corresponding Author(s):
Arkhipov Andrey YurevichRussian Academy Of Sciences Institute Of Higher Nervous Activity And Neurophysiology, Moscow, Russian Federation
Tel:+7 9031990909,
Email:arhip76@ihna.ru
Abstract
In patients with schizophrenia life expectancy is shorter compared to the general population and the main causes of early mortality are somatic diseases. Schizophrenia is a complex disorder with a high risk of inheritance and is increasingly recognized as a systemic disorder, presumably having a common genetic basis with various somatic disorders.
Modern research methods allow us to discover the mechanisms of etiology, pathogenesis and outcome of diseases. Changing the length of telomeres in the cells of organisms reflects the processes of development and degradation of cells, as well as the stages of its life cycle. The reduction in Telomere Length (TL) is considered one of the biological markers associated with age and can be accelerated by comorbid conditions in schizophrenia.
The present study is aimed at identifying differences in the telomere length in the peripheral blood cells of psychotic patients with schizophrenia in, as well as in identifying the relationship between telomere length and accelerated aging in schizophrenia.
The leukocyte telomere length was measured by a quantitative polymerase chain reaction to determine the ratio of telomere length to single copy gene (T/C) in blood samples of 32 patients with schizophrenia and 31 controls.
The results of the study suggest that the shorter telomere length of patients with schizophrenia aged 17 to 27 years may be the result of early aging. The telomere length indicator can be used as a marker of accelerated biological aging in the early stages of the disease.
Keywords
Premature aging; Schizophrenia; The length of telomeres
Introduction
Telomeres are simple tandem hexamer repeats localized at the ends of eukaryotic cell chromosomes that protect linear chromosomes from degradation and fusion and are involved in the process of cell division and in maintaining the stability of the genome. At each cell division, as a result of incomplete replication of the ends of the chromosomes, telomeres shorten [1]. In addition, telomeres are exposed to nucleases and other destructive factors. Accelerated telomere shortening promotes genetic instability of cells, replicating cell aging [2,3], arrest of the cell cycle or apoptosis and neoplastic transformation in vitro and in vivo [4,5]. Thus, telomeres are involved in the pathogenesis and clinical progression of various disorders (congenital diseases, acquired disorders of the hemopoietic system, age-related diseases) [6,7].
The literature presents works demonstrating the relationship between telomere shortening and pathological processes in the body. An increased risk of developing malignant tumors and aging processes are associated with a change in telomere length [8-11]. The shortening of telomeres of peripheral blood leukocytes accelerates with an increase in body weight and an increase in insulin resistance [12,13]. It was also found that not only an increase in body weight, but also leptin levels and smoking are significantly associated with shorter telomeres [8,14]. In addition, telomeres are shorter in individuals with coronary heart disease and dementia [15-18]. Schizophrenia is a progressive mental illness characterized by dissociative emotional and cognitive mental processes, most cases of schizophrenia occur in young people aged 15 to 35 years. Patients with schizophrenia have a shorter lifespan compared with the general population. It is believed that oxidative stress, mitochondrial dysfunction, and inflammatory processes play a leading role in accelerated aging in schizophrenia [19,20].
The reduction in Telomere Length (TL) is considered one of the biological markers associated with age and can be accelerated by comorbid diseases present in schizophrenia. In the literature there are conflicting data on the relationship of telomere length with schizophrenia. A number of works indicate the presence of shorter telomeres of peripheral blood leukocytes in patients with schizophrenia in comparison with groups of healthy people who are and are not members of the patient's family [21,22]. It was also found that telomere length is significantly reduced in patients with severe course, longer disease duration, frequent relapses of psychotic symptoms, and in patients with chronic schizophrenia who respond poorly to treatment [23]. The results of studies by Nieratschker et al., [24] Savolainen et al. show that schizophrenics have longer leukocyte telomeres than people who do not have this disease. According to other studies [25-29], telomere length shortening in schizophrenia was not detected.
Due to numerous discrepancies in the literature, we decided to conduct our own study of telomere length in blood samples of patients with schizophrenia and to identify a relationship between telomere length and accelerated aging in schizophrenia.
Materials And Methods
The study used blood samples from 32 patients with schizophrenia in psychosis, of which 14 were female samples (43·75%) and 18 were male samples (56·25%) from 17 to 51 years old (mean age was 27·75 ± 8·05 years) who were treated in the Psychiatric Clinic “Rosa” and Moscow City Psychiatric Clinical Hospital No.1. Blood samples of 31 people who had no acute diseases and exacerbations of chronic diseases at the time of the study were used as control, including 14 women (45·16%) and 17 men (54·84%) aged 16 to 51 years (average age the group was 34·12 ± 8·38 years). All respondents were native speakers of the Russian language and participated in the study voluntarily.
DNA isolation from venous blood was carried out by a standard method using proteinase K and phenol-chloroform. High molecular weight DNA was dried at room temperature and dissolved in TE buffer, in this form DNA was stored at -20°C. All DNA extractions were performed by only one researcher.
The amount of extracted DNA was estimated on a NanoDrop 1000 spectrophotometer, in the double-stranded DNA analysis mode - dsDNA-50. The quality of DNA was assessed by the ratio of the wavelength 260/280 when measuring the DNA concentration on a NanoDrop 1000 spectrophotometer in the double-stranded DNA analysis mode - dsDNA-50.
Analysis of telomere length in the lymphocyte fraction by the method of real-time polymerase chain reaction (qPCR) according to the original protocol taken from the literature [30], using specific primers that do not form dimers during qPCR, which is achieved due to their incomplete complementarity to telomeric repeats : Tel-1: 5'-GGTTTTTGA GGGTGAGGGTGAGGGTGAGGGTGAGGGTCCCGACTATCCCTATCCCTATCCCTATCCCTATCCCTA-3'.
The average telomere length in the sample was calculated according to the original method using the dilutions of the calibrator, an 84-membered synthetic oligonucleotide consisting of TTAGGG telomeric repeats. The average telomere length per genome was calculated by normalizing the number of copies of telomeric repeats to the number of copies of the myostatin gene (T/S), localized on chromosome 12 and presented in the cell in a single copy.
The following primers were used to amplify the myostatin gene: 36B4u: 5'-CAGCAAGTGGGAAGGTGTAATCC-3'; 36B4d: 5 'CCCATTCTATCATCAACGGGTACAA-3'.
The relative telomere length was assessed by the T/S index, which was calculated as the ratio of the number of copies of telomere repeats to the number of copies of the myostatin gene. The data were processed using MS Excel and Statistica 10.0 software packages, the level of confidence was taken at p<0·05.
Results
When assessing the normality of the distribution of telomere lengths in the general sample and groups, patients and healthy according to the Kolmogorov-Smirnov and Liliefors criteria, p>0·20 values were obtained; therefore, the distribution cannot be considered normal, therefore, the array was processed using non-parametric statistics methods.
In the total sample, the average telomere length was 0·83±0·066, while the Mann-Whitney test did not reveal differences for men and women (U=446·5, p=0·55). An assessment by the Spearman criterion showed the presence of a reliable weak direct relationship between the indicators of age and telomere length at R=0·26, p=0·04 (Figure 1).
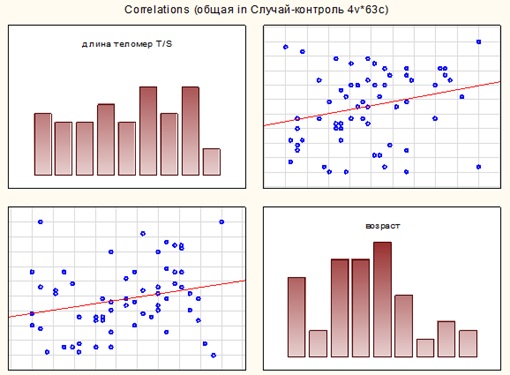 Figure 1: Correlation matrix for indicators “age” and “telomere length” in the total sample.
Figure 1: Correlation matrix for indicators “age” and “telomere length” in the total sample.
The average telomere length in the blood samples of patients with schizophrenia was 0·78±0·043, for men and women, according to the Mann-Whitney criterion, no significant differences were found (U=122, p=0·89). In the blood samples of the control group, the average telomere length was 0·89±0·029, for men and women, according to the Mann-Whitney criterion, no significant differences were found (U=75, p=0·08). Correlation analysis did not show the relationship between age and telomere length both in patients with schizophrenia and in the control group.
A comparison of the telomere lengths of patients with schizophrenia and the control group by the Mann-Whitney test revealed a significant difference (U=7·5, p=0·001), in the blood samples of the control group, the relative telomere length was on average higher than in patients with schizophrenia (Figure 2).
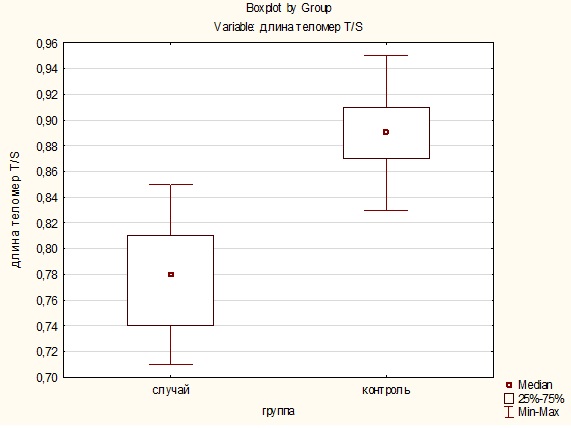
Figure 2: Distribution of telomere length in the “case” and “control” groups.
Considering that the majority of cases of schizophrenia occur in young people aged 15 to 35 years, an analysis of the relative length of telomeres in this age group was carried out and a significant shortening of telomeres was revealed in 55·6% of cases in men (0·74±0·030) and in 44·5% - in women (0·73±0·022) aged 17 to 27 years, which presumably may indicate early aging.
Discussion
A possible association of accelerated telomere shortening and schizophrenia is due to several factors. First, telomeres are shortened during aging, and the short length of the telomeres in leukocytes is observed in patients with neurodegenerative disorders, in particular with schizophrenia. Secondly, in a number of works, the relationship of inflammation accompanying the processes of neurodegeneration in schizophrenia with the length of telomeres is shown. Third, it was shown that oxidative stress leads to a shortening of the telomere length in vitro [19-24].
In addition, one of the hypotheses is based on the assumption of a common genetic basis for schizophrenia and various somatic disorders. Thus, an analysis of genetic studies indicated pleiotropy between schizophrenia and dyslipidemia, as well as a genetic relationship between schizophrenia and autoimmune diseases, cardiovascular diseases, and type 2 diabetes mellitus [31]. A recent study showed that a gene involved in the functioning of ion channels is associated with an increased risk of developing both schizophrenia and cardiovascular disease [32].
Intrauterine disorders are associated with adult mental disorders through dysregulation of the hypothalamic-pituitary-adrenal axis (GHN-axis), which leads to an increased level of glucocorticoids and inflammation. Disorders of the development of the nervous system (intrauterine infections, maternal starvation, intrauterine growth retardation, cesarean section, as well as pre- and perinatal hypoxia) are believed to lead to destruction of the nervous tissue and an increased risk of schizophrenia [33]. The hypothesis of intrauterine disorders suggests an important biological relationship between abnormal intrauterine development, the risk of schizophrenia and somatic disorders.
It is noted in the literature that telomere length reduction below a critical level leads to a cascade of metabolic processes leading to apoptosis, instability of the genome organization, and imbalance in the key links in the regulation of energy processes. In particular, activation of the p53 tumor suppressor gene, inhibition of the functions of PGC-1 alpha, which controls ATP biosynthesis during oxidative phosphorylation and antioxidant defense reactions in mitochondria, are observed.
Conclusion
The results of our study revealed a significant difference in the relative length in blood samples of patients with schizophrenia and the control group with predominance of telomere length in the control group. A significant shortening of the telomere length in blood samples of patients with schizophrenia aged 17 to 27 years suggests that this is caused by early aging. The telomere length indicator can be used as a marker of accelerated biological aging in the early stages of the disease.
References
- Lansdorp PM, Verwoerd NP, van de Rijke FM, Dragowska V, Little MT, et al. (1996) Heterogeneity in telomere length of human chromosomes. Hum Mol Genet 5: 685-691.
- Harley CB, Futcher AB, Greider CW (1990) Telomeres shorten during ageing of human fibroblasts. Nature 345: 458-460.
- Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, et al. (1990) Telomere reduction in human colorectal carcinoma and with ageing. Nature 346: 866-868.
- Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, et al. (1992) Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA 89: 10114-10118.
- Harley CB, Vaziri H, Counter CM, Allsopp RC (1992) The telomere hypothesis of cellular aging. Exp Gerontol 27: 375-382.
- Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, et al. (2001) The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature 413: 432-435.
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, et al. (2005) Increased body mass and cigarette smoking are associated with short telomeres in women. Lancet 366: 662-664.
- Spivak IM, Mikhelson VM, Spivak DL (2015) Telomere length, telomerase activity, stress and aging. Adv Gerontol 28: 441-448.
- Brouwers B, Hatse S, Dal Lago L, Neven P, Vuylsteke P, et al. (2016) The impact of adjuvant chemotherapy in older breast cancer patients on clinical and biological aging parameters. Oncotarget 21: 29977-29988.
- Endicott AA, Taylor JW, Walsh KM (2016) Telomere length connects melanoma and glioma predispositions. Aging 8: 423-424.
- Shay JW (2016) Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov 6: 584-593.
- Whisman MA, Robustelli BL, Sbarra DA (2016) Marital disruption is associated with shorter salivary telomere length in a probability sample of older adults. Soc Sci Med 157: 60-67.
- Gardner JP, Li S, Srinivasan SR, ChenW, Kimura M, et al. (2005) Rise in insulin resistance is associated with escalated telomere attrition. Circulation 111: 2171-2177.
- Huzen J, Wong LS, Veldhuisen DJ, Samani NJ, Zwinderman AH, et al. (2014) Telomere length loss due to smoking and metabolic traits. J Intern Med 275: 155-163.
- Ogami M, Ikura Y, Ohsawa M, Matsuo T, Kayo S, et al. (2004) Telomere shortening in human coronary artery diseases. Arterioscler Thromb Vasc Biol 24: 546-550.
- Panossian LA, Porter VR, et al. (2003) Telomere shortening in T cells correlates with Alzheimer's disease status. Neurobiol Aging 24: 77-84.
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, et al. (2005) Obesity, cigarette smoking, and telomere length in women. Lancet 366: 622-664.
- Okusaga OO (2013) Accelerated aging in schizophrenia patients: The potential role of oxidative stress. Aging Dis 5: 256-262.
- Okusaga O, Hamilton RG, Can A, Igbide A, Giegling I, et al. (2014) Phadiatop seropositivity in schizophrenia patients and controls: A preliminary study. AIMS Public Health 1: 43-50.
- Kirkpatrick B, Galderisi S (2008) Deficit schizophrenia: An update. World Psychiatry 7: 143-147.
- Kao HT, Cawthon RM, Delisi LE, Bertisch HC, Ji F, et al. (2008) Rapid telomere erosion in schizophrenia. Mol Psychiatry 13: 118-119.
- Polho GB, De-Paula VJ, Cardillo G , dos Santos B, Kerr DS (2015) A meta-analysis. Schizophr Res 165: 195-200.
- Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT-J, et al. (2004) Mitochondrial dysfunction in schizophrenia: Evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry 9: 684-697.
- Nieratschker V, Lahtinen J, Meier S, Strohmaier J, Frank J, et al. (2013) Longer telomere length in patients with schizophrenia. Schizophr Res 149: 116-120.
- Polho GB, De-Paula VJ, Cardillo G, dos Santos B, Kerr DS, et al. (2015) Leukocyte telomere length in patients with schizophrenia: A meta-analysis. Schizophr Res 165: 195-200.
- Rao S, Kota LN, Li Z, Yao Y, Tang J, et al. (2016) Accelerated leukocyte telomere erosion in schizophrenia: Evidence from the present study and a meta-analysis. J Psychiat Res 79: 50-56.
- Pawelczyk T, Szymanska B, et al. (2015) Telomere length in blood cells is related tothechronicity, severity, and recurrence rate of schizophrenia. Neuropsychiatr Dis Treat 11: 1493-1503.
- Nguyen T, Eyler L, V Jeste D (2018) Systemic Biomarkers of Accelerated Aging in Schizophrenia: A Critical Review and Future Directions. Schizophr bull 44: 398-408.
- Wu-Yang Y, Hsueh-Wen C, Lin C-H, Cho C-L (2008) Short telomeres in patients with chronic schizophrenia who show a poor response to treatment. J Psychiatry Neurosci 33: 244-24
- Cawthon RM (2009) Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 37: e21.
- Belsky DW, Caspi A, Arseneault L, Corcoran D, Domingue BW, et al. (2019) Genetics and the geography of health, behavior, and attainment. Nat Hum Behav 3: 576-586.
- Schizophrenia Working Group of the Psychiatric Genomics Consortium (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511: 421-427.
- Nielsen PR, Meyer U, Mortensen PB (2016) Individual and combined effects of maternal anemia and prenatal infection on risk for schizophrenia in offspring. Schizophr Res 172: 1-3.
Citation: Yurevich AA, Borisovna SV, Kubanichbekovich NM, Pavlovna DO, Nataliya SK (2021) Telomeres Length Change in Peripheral Blood Lymphocytes of Psychotic Patients with Schizophrenia. Trends Anat Physiol 4: 011.
Copyright: © 2021 Arkhipov Andrey Yurevich, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.