Testicular Assessment of (Okra) Abelmoschus Esculentus Treated Wistar Rats
*Corresponding Author(s):
Obeten KEDepartment Of Anatomy, Faculty Of Biomedical Sciences, Kampala International University, Uganda
Email:fredobeten@yahoo.com
Abstract
This study shows the testicular assessment of Abelmoschus esculentus treated animals. Twenty-one animals weighing between 100g-120g were grouped into three groups, seven animals for each group. The rats in group A served as the control and fed with normal rat chew and water while the animals in group B treated with 1.0mg/KgBw and C 3.0 mg/KgBw aqueous extract of Abelmoschus esculentus orally for 14 days. All the animals were sacrificed a day after the administration and the testes removed for histological tissues processing using Hematoxylin and Eosin (H&E) stain. The caudal part of the epididymis was harvested for collection of semen for analysis. Result of the study shows a significant (p < 0.05) increase in final body weight of all the animals compared with their initial body weight while result from semen analysis shows a significant increase (p < 0.05) in percentage sperm motility in the control and high dose group, the highest percentage of sperm morphology was observed in the control group while the sperm vitality reveals a significant decrease (p < 0.05) in the low dose and high dose. Results of the histology of the control group showed a normal histology of the seminiferous tubules (round cells), low dose showed seminiferous tubules with acute loss of Germinal cell area (G) and high dose showed testicular regression (loss of interstitial space, IT), tubular architecture and Germ layer (G).
Keywords
Abelmoschus esculentus; Seminiferous tubules; Testis; Testicular regression; Wistar rat
Introduction
Male infertility is the inability of the male to cause pregnancy (conception) after one year of regular unprotected intercourse with a fertile female. It accounts for 50% of the causative factors among of couples and a leading indication that Assisted Reproductive Techniques (ART) is required. Male infertility caused by testicular failure is the most prevalent cause of infertility in men, and it has long been thought to be irreversible. In the absence of successful pharmacological medicines, lifestyle counseling could be a valuable treatment alternative [1].
Medicinal plants have always played an important role in the history of human health. However, the populations and sustainable use of medicinal plants have been severely affected by human activities and climate change. Little is known about the current conservation status and distribution pattern of medicinal plants [2]. Since prehistoric times, medicinal plants (also known as medicinal herbs) have been identified and employed in traditional medicine. In the history of human health, medicinal plants have always played a significant role in defense against pests, fungus, diseases. Recent studies show that the usage of medical plants has increased in different areas as a way to treat and prevent some epidemics especially during COVID-19 [3, 4]. Plants produce dozens of chemicals numerous of which have potential or established biological activity. However, because a single plant encompasses such a wide variety of phytochemicals, the impacts of using the entire plant as medicine are unknown. Additionally, the phytochemical content and pharmacological effects of many plants having medicinal potential remain unassessed by rigorous scientific research to define efficacy and safety [5]. Medicinal plants are presently in demand and their acceptance is increasing progressively. Undoubtedly, plants play an important role by providing essential services in ecosystems. Without plants, humans and other living organisms cannot live in a way living should be. Anyway, herbals especially medicinal herbs have constantly acted as an overall indicator of ecosystem health [6].
The official definition of traditional medicine can be considered as “the sum total of the knowledge, skills and practices based on the theories, beliefs and experiences indigenous to different cultures, whether explicable or not, used in the maintenance of health, as well as in the prevention, diagnosis, improvement or treatment of physical and mental illnesses” [7].
One of the plants used in traditional medicine is the African okra. The plant Okra (Abelmoschus esculentus L.), belonging to the family Malvaceae, is commonly known as Lady’s finger, as well as by several vernacular names, including okra, bhindi, okura, quimgombo, bamia, gombo, and lai long ma, in the different geographical regions of its cultivation [8]. Okra is believed to have originated near Ethiopia, where it was frequently cultivated by the Egyptians during the 12th century, and thereafter spread throughout the Middle East and North Africa [9-11]. Okra is an annual shrub that is cultivated mostly within tropical and subtropical regions across the globe and represents a popular garden crop, as well as a farm crop. It is a widely cultivated food crop and is globally known for its palatability. The immature green pods of okra are usually consumed as vegetables, while the extract of the pods also serves as a thickening agent in numerous recipes for soups, as well as sauces, to augment their viscosity [12, 13]. Another noteworthy application of okra fruit is their wide use in the pickle industry. The polysaccharides present in okra are used in sweetened frozen foods such as ice-creams, as well as bakery products, due to their health benefits and longer shelf-lives [14-16]. The fruits, stem, and leaves of okra are covered with minute soft, hairy structures. Although the flowering of the okra plant is perennial, it is highly dependent on various biotic and abiotic factors. The leaves of okra are polymorphous, characterized by hairy upper and lower surfaces, whereas the petioles are around 15 cm long. The flowers of okra can be easily recognized due to their slight yellowish color with a crimson center. The edible part of okra or its capsule (pod) measures approximately 15-20 cm in length and has a pyramidal-oblong, pentagonal, hispid appearance. Historically, okra pods were utilized for various purposes, such as in food, appetite boosters, astringents, and as an aphrodisiac. Furthermore, okra pods have also been recommended to cure dysentery, gonorrhea, and urinary complications [10]. Extracts of young okra pods have also been reported to display moisturizing and diuretic properties, whereas the seeds of this plant have been reported to possess anticancer and fungicidal properties [16].
The testes are reproductive organs in male which develop in relation to the lumber region of the posterior abdominal wall [17]. During fetal life they gradually descend to the scrotum. They reach the iliac fossa during the 3rd month and at the site of the deep inguinal ring to the 7th month of intrauterine life. They pass through the inguinal canal during the 7th month and are normally in the scrotum by the end of the 8th month [18]. The testis is subdivided into approximately 250 lobules by septal extensions from the tunica albuginea. Each lobule contains 1 to 4 seminiferous tubules embedded in a stroma of “loose interstitial connective tissue which contains blood and lymphatic vessels and macrophages, but lacks typical fibroblast and other loose connective tissue cells. Seminiferous tubules fill most of the volume in the testis. It is lined with a complex stratified epithelium; it is about 150- 250cm in diameter and 30-70cm long. The seminiferous tubules narrow to form the straight tubule or tubule recti that connect the seminiferous tubule to anastomosing rete testis [19]. The seminiferous consists of tunics of fibrous connective tissue, a well-defined basal lamina and a complex germinal and seminiferous epithelium. The fibrous tunica propria enveloping the seminiferous tubule consist a several layer of fibroblast.
The interstitial connective tissues of the testis play a major role in the function of the testis viz., mechanical support to the seminiferous tubules and blood vessels, production of testosterone by interstitial cells (Leydig cells), participation in the sustentacular cell barrier and regulation of the sustentacular cell (Sertoli cell) functions.
Materials and Methods
Extract preparation
Powdered Okra was dispensed in 15,000mls of distilled water in a plastic container; the mixture was vigorously stirred intermittently with a stick and then left for 24 hours before it was filtered with a cloth sieve. The filtrate was evaporated at 45°C with water bath to obtain the crude solid extract and the extract obtained was stored in the refrigerator until the commencement of the administration.
Experimental animals
Twenty-one (21) adults male wistar rats were purchased from the animal house of the department of Human Anatomy, University of Cross River State (UNICROSS), Okuku campus and were used for this study. The animals were randomly distributed into three (3) groups: Control, Low Dose and High Dose, seven (7) animals for each group. The animals were acclimatized for two (2) weeks. They were housed in Perspex cages under controlled light (12 hours daylight cycle and 12 hours dark cycle) and were fed with standard growers vital feed and water before the start of the administration.
Experimental design
The twenty one (21) animals were allotted into three groups consisting of seven animals in each group.
Group A (Control) animals received food and water only.
Group B (Low Dose) animals received food, water and extract of Abelmoschus esculentus at a dose of 1.0mg/KgBw.
Group C (High Dose) animals received food, water and extract of Abelmoschus esculentus at a dose of 3.0mg/KgBw.
Termination of the experiment
At the end of administration which lasted for two weeks, the animals in all the groups were sacrificed a day after the administration using cervical dislocation. The testes of each animal were removed and the caudal epididymis separated from the testis and processed immediately for epididymal sperm parameters.
Results
Morphological observations
Morphological observation from the study shows an observable significant (p < 0.05) increase in the final mean body weight when compared with the initial body weight observable in control vs low dose, control vs high dose and low dose vs control but not observable in low dose vs high does and high dose vs control dose. The final body weight of the control animals (133.9 ± 7.058) was significantly (p < 0.05) higher than its initial body weight (111.4 ± 8.162). However, the mean final body weight of the low dose group (142.3 ± 4.716) and high dose group (145.7 ± 4.786) were significantly (p < 0.05) higher than their initial body weights (128.0 ± 6.856) and (130.3 ± 6.157) respectively.
Semen profile
From this study, there was significant increase (p < 0.05) in percentage sperm motility in all the experimental animals. This could be suggested that of Abelmoschus esculentus may increase sperm motility of sperm. This finding opposed [18], who documented a decrease in sperm motility using methanolic extract of Abelmoschus esculentus fruit in mice. Sperm vitality refers the percentage of living and healthy sperm present in the semen [20]. This study presented a significant (p < 0.05) decrease in sperm vitality treated animals when compared to the control group. Sperm morphology serves as an important and sensitive indicator of chemical toxicity on the reproductive cells. They can be used to evaluate the spermatogenic damage, fertility and heritable genetic changes, which provide a direct measure of the quality of sperm production in chemically treated animals.
The present studies shows a significant (p < 0.05) decrease in percentage sperm morphology in the control group when compared with the high dose and low dose group.
Histological observation of the testis
The control group revealed a normal histology of the seminiferous tubules (round cells) (table 1), the germinal area appears normal and sertoli cells, the spermatid and Interstitial Layer (IT) appears normal with no observable pathology (figure1). The low dose group showed seminiferous tubules with loss of germinal cell area (G) (figure 2). While the high dose group showed testicular regression (loss of Interstitial Space, (IT), tubular architecture and germ layer (G) (Figures 3 & 4).
Initial and final body weights of animals in various experimental groups
|
Body Weights |
||
|
Groups |
Initial |
Final |
|
Control |
111.4 ± 8.162 |
133.9 ± 7.058 |
|
Low dose |
128.0 ± 6.856 |
142.3 ± 4.716 |
|
High dose |
130.3 ± 6.157 |
145.7 ± 4.786 |
Table 1: Values are presented as Mean ± SEM.
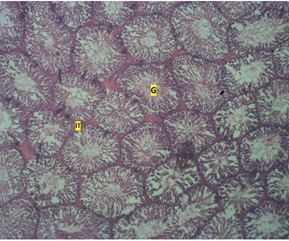 Figure 1: Photomicrograph of the testis of control group showing normal seminiferous tubules (round cells) and interstitial layer (IT) H & E. X40.
Figure 1: Photomicrograph of the testis of control group showing normal seminiferous tubules (round cells) and interstitial layer (IT) H & E. X40.
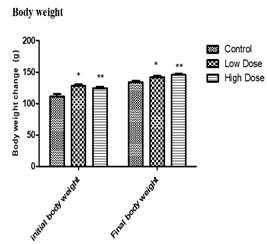 Figure 2: Showing effect of daily adm inistration of values are expressed in mean+SEM, n=7, p < 0.05**=P < 0.05 VS control.
Figure 2: Showing effect of daily adm inistration of values are expressed in mean+SEM, n=7, p < 0.05**=P < 0.05 VS control.
Abelmoschus esculentus on male wistar rats.
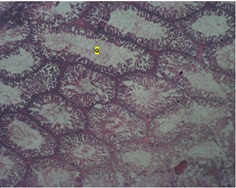 Figure 3 : Photomicrograph of the testis of low dose group showing seminiferous tubules with acute loss of Germinal cell area (G) H & E. X40.
Figure 3 : Photomicrograph of the testis of low dose group showing seminiferous tubules with acute loss of Germinal cell area (G) H & E. X40.
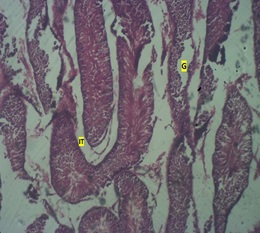 Figure 4: Photomicrograph of the testis if high dose group showing testicular regression (loss of Interstitial space (IT), tubular architecture and Germ layer (G).
Figure 4: Photomicrograph of the testis if high dose group showing testicular regression (loss of Interstitial space (IT), tubular architecture and Germ layer (G).
Discussion
This study affirm that Abelmoschus Esculentus administration causes significant increase in body weight of treated animals as previously reported by [21-25]. Natural healthy diets are essential in human’s ability to produce offspring, diets rich in vitamins such as E and C, antioxidants such as selenium, β-carotene, lycopene and cryptoxanthin, omega 3 fatty acid, and zinc are positively correlated with increased fertility. Meanwhile, diets rich in gossypol such as okra, caffeine, saturated fatty acid and trans-fatty acids negatively correlated with increased fertility. Despite the inadequate studies on the effect of okra on male infertility, few studies confirmed its effect on male infertility, having showed that long term consumption could lead to decrease in sperm count, decrease in sperm motility, abnormality in sperm morphology, impaired testicular tissues, decrease in testosterone levels and ultimately impaired spermatogenesis. Findings from this present study through semen analysis showed observable significant reductions in sperm vitality and sperm morphology. This was in consonance with the reports by [26], who found out that oral administration of 70 mgkg-1 body weight per day of the methanolic extract of okra fruit for 28 days causes significant loss of testes weight and significant (P < 0.05) increase in body weight of the experimental mice (treated groups) compared to control group. In the present study, negative histomorphological changes were observed in the testicular tissue in form of loss geminal cell area in seminiferous tubules, testicular regression and loss of interstitial space, tubular architecture and germ layer. This was in conformity with findings by [23,26], who reported degenerating testicular tissue, reduction in testicular weight, reductions in sperm mortality, sperm count, increase in abnormal sperm cells, deterioration in testicular cytoarchitecture and reductions in testicular parameters in rats treated with various extracts of Abelmoschus Esculentus. The negative histomorphological changes seen in the testicular tissue and seminal analytical parameters of treated rats in this study, suggests that the active phytochemicals (carotenoids, polyphenols and flavonoids) found in Abelmoschus. Esculentus have the potentials to impair the optimal functioning of the testis and could be implicated in male infertility. This is in conformity with the previous suggestions made by [27]. Testicular tissue regression may occur as a result of the depletion of androgenic hormone. Estrogens decrease the volume of the glandular epithelium in reproductive glands. Treatment with anti-androgenic substances also leads to negative histomorphological changes in male reproductive organs and the deficiency of the Egr family of zinc finger transcription factors like Egr4 and Egr1 as in Egr4-Egr1double-mutant mice leads to atrophy of seminal vesicle, prostate, epididymis and testis [28]. The overall effect of the above histomorphological changes can lead to infertility [27]. The observations from the present study compared with previous studies are suggestive of the fact that A. esculentus is of rich nutritional value observable in the weight gain in the treated group but could also be implicated negatively in male fertility [29-38].
Conclusion
Concisely, this result is positive evidence that A. Esculentus extracts might be used to enhance sperm motility, morphology and vitality reproductive hormones and increase body weight especially at lower doses of 1.0mg/kgBw. However, might be disruption of biosynthetic pathways administering the extracts at higher doses. It thus implies that caution should be exercised during consumption.
References
- Sharma A, Minhas S, Dhillo WS, Jayasena CN (2021) Male infertility due to testicular disorders. In Journal of Clinical Endocrinology and Metabolism.
- Xia C, Huang Y, Qi Y, Yang, Xue T, et al. (2022) Developing long-term conservation priority planning for medicinal plants in China by combining conservation status with diversity hotspot analyses and climate change prediction. BMC Biol 20: 89.
- Joppa LN, Roberts DL, Myers N, Pimm SL (2011) Biodiversity hotspots house most undiscovered plant species. Proceedings of the National Academy of Sciences of the USA 108: 13171-13176.
- Khadka D, Dhamala MK, Li F, Aryal PC, Magar PR, et al. (2021) The use of medicinal plants to prevent COVID-19 in Nepal. Journal of Ethnobiology and Ethnomedicine 1-17.
- Chhetri VT, Jha P, Maharjan SK (2021) Medicinal Plants Used in COVID-19 Pandemic in Buddhabhumi Municipality, Kapilvastu, Nepal. Ethnobotany Research and Applications 22: 1-19.
- Garg AK, Faheem M, Singh S (2021) Role of Medicinal Plant in Human Health Disease. Asian Journal of Plant Science & Research 12: 14695-14697.
- Salmerón-Manzano E, Garrido-Cardenas JA, Manzano-Agugliaro F (2020) Worldwide research trends on medicinal plants. Int J Environ Res Public Health 17: 3376.
- Jain N, Jain R, Jain V, Jain S (2012) A review on: Abelmoschus esculentus. Pharmacia 1: 84-89.
- Lamont WJ (1999) Okra-A versatile vegetable crop. Hort Technology 9: 179-184.
- Islam MT (2019) Phytochemical information and pharmacological activities of Okra (Abelmoschus esculentus): A literature-based review. Phytother Res 33: 72-80.
- Tindall HD (1983) Vegetables in the Tropics; Macmillan Publishers Limited: London 37-45.
- Dhaliwal MS (2010) Okra (Abelmoschus esculentus) L. (Moench); Kalyani Publishers: New Delhi, India. Molecules 2021, 26, 696 16 of 21.
- Kumar DS, Tony DE, Kumar AP, Kumar KA, Rao DBS, et al. (2013) A review on: Abelmoschus esculentus (Okra). International Research Journal of Pharmaceutical and Applied Sciences 3: 129-132.
- Archana G, Babu PAS, Sudharsan K, Sabina K, Raja RP, et al. (2015) Evaluation of Fat Uptake of Polysaccharide Coatings on Deep-Fat Fried Potato Chips by Confocal Laser Scanning Microscopy. International Journal of Food Properties 19: 1583-1592.
- Costantino A, Romanchik-Cerpovicz J (2004) Physical and sensory measures indicate moderate fat replacement in frozen dairy dessert is feasible using okra gum as a milk-fat ingredient substitute. Journal of the American Dietetic Association 104: 44.
- Yuennan P, Sajjaanantakul T, Kung B (2014) Effect of Okra Cell Wall and Polysaccharide on Physical Properties and Stability of Ice Cream. J Food Sci 79: 1522-1527.
- Durazzo A, Lucarini M, Novellino E, Souto EB, Daliu P, et al. (2018) Abelmoschus esculentus (L.): Bioactive Components’ Beneficial Properties-Focused on Antidiabetic Role-For Sustainable Health Applications. Molecules 24: 38.
- Singh A, Singh SK (2009) Evaluation of antifertility potential of Brahmi in male mouse. Contraception 79: 71-79.
- Singh SK, Chakravarty S (2000) Histologic changes in the mouse testis after bilateral vasectomy. Asian J Androl 2: 115-120.
- Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, et al. (2001) National cooperative reproductive medicine Network. Sperm morphology, motility and concentration in infertile men. N Engl J Med 345: 1388-1393.
- Kyrian UN, Godswill NK, Izuchukwu CI (2014) Effects of the methanolic extract of Abeloschus esculentus (L) Moench (Okro) fruit on the testes and sperm characteristics of male albino wistar rats. CASRP Publishing Company 2: 2686-2690.
- Sabitha V, Ramachandran S, Naveen KR, Pannerselvam K (2011) Antidiabetic and Antihyperlipidemic Potential of Abelmoschus esculentus (L) Monech. in streptozotocin induced Diabetic Rats. J Pharm Bioallied Sci 3: 397-402.
- Umoh I, Oyebadejo S, Bassey E, Udoh N (2013) Histomorphological Study of the Effect of Chronic Consumption of Abelmoschus Esculentus and Piper Guineense on the Gastric Mucosa of Albino Wistar Rats. In J of Pharm Res and All Sci 2: 31-37.
- Uchenna NK, Nweze KG, Charles II (2014) Effects of the Methanolic Extract of Abeloschus Esculentus (L) Moench (Okro) Fruit on the Testes and Sperm Characteristics of Male Albino Wistar Rats. IJABBR.
- Dubey P, Mishra S (2017) A Review on Diabetes and Okra (Abelmoschus Esculentus). Journal of Medicinal Plants Studies 5: 23-26.
- Majd NE, Azizian H, Tabandeh MR, Shahriari A (2019) Effects of Abelmoschus Esculentus Powder on Ovarian Histology, Expression of Apoptotic Genes and Oxidative Stress in Diabetic Rats Fed with High Fat Diet. Iranian Journal of Pharmaceutical Research 18: 369.
- Olatunji-Bello II, Ijiwole TF, Awobajo O (2009) Evaluation of the Deleterious Effects of Aqueous Fruit Extract of Abelmoschus Esculentus (Okro Fruit) on Some Male Reproductive Parameters in Sprague Dawley Rats. Journal of Phytology 1: 6.
- Christina LY, Julie Y, Zachary MF, John TA, Jonathan F, et al. (2015) The Effects of Dietary Polyphenols on Reproductive Health and Early Development. Human Reproduction Update 21: 228-248.
- Creasy DM, Maronpot RR, Giri DK (2002) Non Neoplastic Lesion Atlas; Section, Seminal Vesicle Atrophy, Pp. 1-4. National Toxicology Program (NTP).
- Grover JK, Yadav S, Vats V (2002) Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol 81: 81-100
- Gurib-Fakim A (2006) Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol Aspects Med 27: 1-93.
- Houghton PJ (1995) The role of plants in traditional medicine and current therapy. J Altern Complement Med 1: 131-143.
- Jones WP, Chin YW, Kinghorn AD (2006) The role of pharmacognosy in modern medicine and pharmacy. Current Drug Targets 7: 247-264.
- Kinghorn AD, Seo EK (2020) Plants as Sources of Drugs. ACS Symposium Series 647: 179-193.
- Kunle OF, Egharevba HO, Ahmadu PO (2012) Standardization of herbal medicines-A review. International Journal of Biodiversity and Conservation 4: 101-112.
- Pimm SL, Jenkins CN, Abell R, Brooks TM, Gittleman JL, et al. (2014) The biodiversity of species and their rates of extinction, distribution, and protection. Science 344: 1246752.
- Singh JS (2002) The biodiversity crisis: A multifaceted review. Current Science 82: 638-647.
- Fonnegra FG (2007) Plantas Medicinales Aprobadas en Colombia; University of Antioquia: Antioquia, Colombia.
Citation: Obeten KE, Olakunle OA, Etukudo E, Egwumba FC (2022) Testicular Assessment of (Okra) Abelmoschus Esculentus Treated Wistar Rats. Trends Anat Physiol 5: 014.
Copyright: © 2022 Obeten KE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.