
The Combined Role of Serum Talin-1 with Traditional Liver Biomarkers in Diagnosis of Hepatocellular Carcinoma
*Corresponding Author(s):
Mohamed S AttiaGaballahDepartment Of Biochemistry And Molecular Biology, Faculty Of Pharmacy, Helwan University, Ain Helwan, Cairo, Egypt
Tel:+20 55421891695,
Email:Mohamed.gaballah@pharm.helwan.edu.eg
Abstract
Hepatocellular carcinoma (HCC) is the most severe and frequent complication of chronic liver diseases. It represents a crucial public health problem in Egypt, where up to 90% of HCC cases are attributed to hepatitis C virus (HCV) infection. The outcome of HCC depends mainly on its early diagnosis. Objective: assess the role of serum Talin-1 with a panel of biomarkers that can significantly differentiate between HCC and non-HCC patients with chronic liver diseases. Patients and methods: A total of 120 adult subjects were divided into 3 main groups, 40 HCC patients without evidence of cirrhosis (HCC), 40 cirrhotic HCC free liver cirrhosis patients (LC), and 40 healthy controls (HC). Liver functions, HCV antibodies, HBsAg, alpha-fetoprotein (AFP), Talin-1 (TLN1) were assayed. Results: Talin-1, AFP, AST/ALT ratio, and albumin were significantly different in HCC group with the optimum cut-off values as 33.75, 9 ng/ml, 1.65 and 2.65 g/dl respectively. By using these cut-off values combined; 100% of HCC patients showed two abnormal markers, corresponding to only 37.5% of the LC group. Conclusion: The use of combined Talin-1, AFP, AST/ALT ratio and albumin cut-off values improved the positive predictive value for HCC from 80% to 100%.
Keywords
Alpha-fetoprotein; Hepatocellular carcinoma; Liver cirrhosis; Talin-1
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most known cancer in the world, and the third most common cause of cancer-related deaths [1,2]. Annually, it kills more than 650,000 people all over the world [3]. In Egypt, The burden of HCC has been increasing with a doubling in the incidence rate in the last10 years [4]. This has been attributed mainly to viral infection (hepatitis B and C viruses), and dietary factors (e.g. aflatoxin B1) [5]. HCV causes HCC through promoting continuous inflammation and hepatocyte regeneration in the setting of chronic hepatitis leading to subsequent progression to cirrhosis, which is thought to cause chromosomal damage and possibly to initiate hepatic carcinogenesis [6,7].
HCC has wide regional differences in the pathology and epidemiology, which is mainly influenced by the disease etiology. The major contributing risk factors for HCC development are chronic viral hepatitis and liver cirrhosis [8,9]. Early detection of HCC is of great importance in order to offer the possibility of curative treatment [10]. Surveillance and screening programs have been conducted in different countries to detect HCC at an early stage.
Talin was the firstly identified cytoplasmic protein partner of integrins [11]. Vertebrates have two talin genes, TLN1 and TLN2, which encode Talin-1 and Talin-2, respectively. Talin-1 is essential for cell adhesion and motility and is the primary talin component of focal adhesions. Talin-1 is expressed mainly in the kidney, liver, spleen, stomach, lung and vascular smooth muscle and its overexpression can promote prostate cancer cell adhesion, migration and invasion [12-14]. A Previous study has identified talin-1 by differential Tissue Proteome as a novel molecular marker for HCC progression [15]. Moreover, talin-1 has been identified in HCC serum samples obtained from Egyptian patients [16].
Usually, Alpha-fetoprotein (AFP) and ultrasonography are used as usual diagnostic tools [17]. Despite the recent scientific advances and the implementation of measures for early HCC diagnosis in patients at risk, the overall survival rate of patients has not yet significantly improved during the last decades. The reason for that is the late diagnosis of most HCC patients [18] leading to extensive involvement of the liver, invasion of the hepatic or portal vein and/or the presence of metastasis [19], so curative treatment is not possible. Accordingly, the development of tumor markers that can detect HCC at earlier stages is essential.
The functions of tumor markers include diagnosis or screening of cancer as well as prediction of prognosis or therapeutic response [20]. As markers predicting HCC occurrence are still very scarce, and early detection of HCC increases the chance of treatment, consequently, the objective of the present study was to assess the diagnostic value of serum talin-1 the novel tumor marker with a panel of liver markers that can significantly increase both the specificity and sensitivity to recognize HCC.
MATERIALS AND METHODS
Subjects and samples
The present study included 120 subjects and they were classified into three groups:
Hepatocellular carcinoma group (HCC) included 40 liver patients (mean age ± SEM, 51.13 ± 1.216 years; male: female ratio, 1.3:1) diagnosed with hepatocellular carcinoma at Liver and Endemic diseases department of Al-Kasr Al-Ainy educational hospital, Cairo, Egypt. HCC diagnosis was based on elevated AFP levels and presence of hepatic focal lesion(s) detected by liver ultrasound and confirmed by computed tomography and/or magnetic resonance.
Liver cirrhosis group (LC) included 40 patients with cirrhosis (mean age ± SEM, 49.98 ± 1.37 years; male: female ratio, 1.2:1). Diagnosis of cirrhosis was based mainly on clinical, biochemical, ultrasonographic and/or histological criteria.
The healthy control (HC) included 40 healthy individuals (mean age ± SEM 48.55 ± 1.53 years; male: female ratio, 1.2:1). Patients with rheumatoid arthritis, alcohol abuse, autoimmune liver diseases and active infection or other malignancies were not included. The study protocol conformed to the ethical guidelines of the 1975 Helsinki Declaration and all patients gave informed consent to participate in the present study [16].
Samples collection, preparation and storage
Five milliliters blood were collected from each patient and control. Samples were collected between 9:00 am and 11:00 am to avoid circadian variation. Serum was separated by centrifugation at 3000 rpm for 15 minutes using centrifuge. Each serum sample was divided into three aliquots. One for measuring AFP, the second for determination of TLN1 and the last for other biochemical parameters determination. Sera were stored at -80°C and thawed only at the time of assay.
Detection of Hepatitis B and C viruses (HBV, HCV)
Serum samples had been screened for viruses using one step test device which is a qualitative membrane based immunoassay for detection of antigens or antibodies for viruses in serum or plasma. The membrane is coated with recombinant antigens or antibodies on the test line region of the device which reacts specifically with viruses. Presence of a colored line indicates a positive result while its absence indicates a negative one (Abon Biopharm (Hangzhou) Co.Ltd China).
Biochemical parameters
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), total proteins, albumin and total bilirubin levels were determined using commercially available kits for all serum samples. Determination of serum AFP
Serum AFP levels were measured using the CALBIOTECH human AFP ELISA kits. Briefly, 25 µl of AFP standards, control and patient’s sera were added to the plates pre-coated with AFP antibodies first, then 100 µl of AFP antibody conjugated to horseradish peroxidase were added to all wells, covered and incubated for 60 min at room temperature (18-26º C). After 3 washes using automatic microplate washer (Stat Fax® 2600), 100 µl of tetramethylbenzidine substrate solution were added to all wells, and incubated for 15 min at room temperature in dark, followed by the addition of 50 µl of stop solution. Absorbances were measured within 15min after adding stop solution at 450 nm using Microplate reader (Sunrise TM tecan group ltd.).
Determination of serum Talin-1
Serum Talin-1 levels were measured using human talin-1 ELISA kits (Wekea medical supplies corp, NEW YORK, USA). Briefly, 50µl of serially diluted standards, 5-fold diluted patient’s sera (10µl serum: 40µl sample diluents) were added to the plates pre-coated with talin-1 antibodies first and incubated for 30 min at 37º C. After five washes with the diluted buffer concentrate reagent, 50µl of talin-1 antibody conjugated to horseradish peroxidase were added to all wells except blank wells. After incubation for 30 min at 37ºC and five washes, 50µl of substrate A followed by 50µl of substrate B were added to each well and incubated for 15 min at 37ºC followed by the addition of stop solution to each well. Absorbances were measured within 15 min after adding stop solution at 450 nm using microplate reader (Sunrise TM tecan group ltd.).
Statistical analysis
All data are presented as mean values ± SEM. Associations between categorical variables were tested by the use of contingency tables and the calculation of chi-squared test. Differences between groups were evaluated by the calculation of one-way ANOVA. Correlations between biochemical markers and other continuous variables were tested using the Spearman or the Pearson's correlation coefficients. Sensitivity, specificity, positive and negative predictive values, and diagnostic accuracy were calculated [21]. Receiver operating characteristics (ROC) analysis was used to evaluate the diagnostic value for biomarkers to identify the optimal cut off values. Sensitivity and specificity, positive and negative predictive values were profiled by curves. The Statistical Package for the Social Sciences version 16 (SPSS, Inc., Chicago, IL, USA) was used for ROC curve.
RESULTS
Patients’ characteristics
(Table 1) shows the demographic and clinical characteristics of all study groups expressed as the mean ± SEM. A significant difference has been shown among all studied groups with regard to measured liver function tests. Levels of markers used in differentiation of hepatocellular carcinoma in the studied groups are also shown in (Table 2).
|
Parameters |
Hepatocellular carcinoma (HCC) |
Liver cirrhosis (LC) |
Healthy Controls (HC) |
|
No. |
40 |
40 |
40 |
|
Age |
51.13 ± 1.216 |
49.98 ± 1.37 |
48.55 ± 1.53 |
|
Gender m (%) |
23(57.5%) |
22 (55%) |
22 (55%) |
|
BMI (kg/m2) |
31.30 ± 0.73 |
30.35 ± 0.79 |
28.40 ± 0.70 |
|
ALT (U/ml) |
70.13 ± 2.65a,b |
47.63 ± 1.39a |
21.55 ± 1.12 |
|
AST (U/ml) |
137.4 ± 5.30a,b |
75.30 ± 2.30a |
16.60 ± 0.70 |
|
ALP (IU/L) |
125.00 ± 1.58a,b |
98.95 ± 4.10a |
64.15 ± 3.27 |
|
GGT (U/L) |
102.80 ± 1.47a,b |
56.60 ± 4.06a |
25.95 ± 1.82 |
Table 1: Demographic and clinical characteristics of the studied grou
Data are expressed as Mean ± SEM. Significantly different from: aHC, bLC group (p<0.05). m: male, BMI: Body Mass Index, ALT: Alanine Aminotransferase, AST: Aspartate aminotransferase, ALP: Alkaline Phoaphatase, GGT: Gamma Glutamyl Transferase, (Kg/m2) Kilogram/meter2, (U/ml): Unit/milliliter, (IU/L): International Unit/Liter. Age, gender and BMI are matched (not significantly different).
|
Parameters |
Hepatocellular carcinoma (HCC) |
Liver cirrhosis (LC) |
Healthy Controls (HC) |
|
Talin-1 (ng/ml) |
61.63 ± 2.47a,b |
17.24 ± 4.78a |
1.1 ± 0.32 |
|
AFP (ng/ml) |
123.3 ± 35.59a,b |
80.2 ± 26.60a |
14 ± 12.47 |
|
AST/ALT ratio |
2.023 ± 0.08a,b |
1.59 ± 0.03a |
0.80 ± 0.02 |
|
Albumin (g/dl) |
2.29 ± 0.06a,b |
2.77 ± 0.11a |
3.97 ± 0.05 |
Table 2: Comparative HCC biomarker levels in different studied groups.
Data are expressed as Mean ± SEM. Significantly different from: acontrol, bLC group (p<0.05). AFP; Alpha feto protein, AST; Aspartate Aminotransferase, ALT; Alanine Amino transferase. (ng/ml) nanogram/milliliter, (g/dl) gram/deciliter.
Receiver operating characteristic (ROC) curve plots serum TLN1, AFP, AST/ALT ratio and Albumin in discriminating HCC patients from those with LC(Figure 1). It shows AUC 0.90, 0.67, 0.77 and 0.72 for each biomarker respectively. AST: Aspartate Aminotransferase, ALT: Alanine Aminotransferase, AFP: Alpha Feto Protein (Table 3).
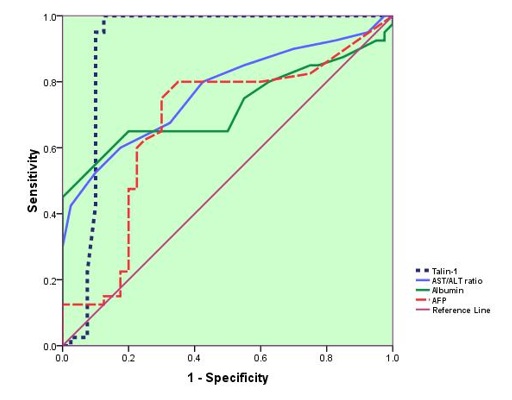 Figure 1: ROC Curve analysis
Figure 1: ROC Curve analysis
|
Marker |
AUC ± SEM |
Asymptotic 95% CI |
|
|
Lower bound |
Upper bound |
||
|
Talin-1 (ng/ml) |
0.908 ± 0.044 |
0.823 |
0.994 |
|
AFP (ng/ml) |
0.677 ± 0.063 |
0.553 |
0.8 |
|
AST/ALT ratio |
0.772 ± 0.053 |
0.668 |
0.876 |
|
Albumin (g/dl) |
0.724 ± 0.060 |
0.607 |
0.842 |
Table 3: AUC for HCC biomarkers.AUC; Area under curve, SEM: Standard Error of Mean, AFP: Alpha Feto Protein, AST: Aspartate Amino Transferase, ALT; Alanine Aminotransferase, CI; Confidence interval.
Performance of biomarkers
(Table 4) shows cut-off values, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of serum Talin-l, AFP, AST/ALT ratio and albumin in hepatocellular carcinoma (HCC) patients relative to the liver cirrhosis (LC) group. These results reveal the highest sensitivity and specificity for serum talin-1 biomarker compared to other biochemical parameters when used lonely (Figures 2-5).
|
Marker |
Cut-off value |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
Accuracy (%) |
|
Talin-1 (ng/ml) |
33.75 (ng/ml) |
100 |
87 |
88 |
100 |
93.75 |
|
AFP (ng/ml) |
9 (ng/ml) |
80 |
65 |
69 |
76 |
72.5 |
|
AST/ALT ratio |
1.65 |
80 |
57 |
65 |
74 |
68.75 |
|
Albumin (g/dl) |
2.65 (g/dl) |
80 |
65 |
69 |
76 |
72.5 |
Table 4: Observed sensitivity, specificity, PPV, NPV and accuracy for the chosen cut-off values.
PPV: Positive predictive value, NPV: Negative predictive value, AFP: Alpha Feto Protein, AST: Aspartate Aminotransferase, ALT: Alanine Aminotransferase, (ng/ml) nanogram/milliliter, (g/dl) gram/deciliter.
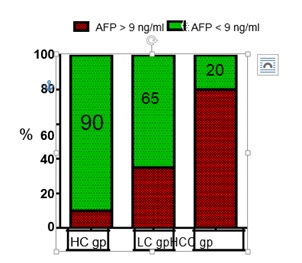 Figure 2: Columns representing the percentage of subjects with high risk of developing HCC by using AFP level ≥ 9 ng/ml, AFP gp: Alpha Feto Protein, HC gp: Healthy Control group, LC gp: Liver Cirrhosis group, HCC gp: Hepatocellular carcinoma group.
Figure 2: Columns representing the percentage of subjects with high risk of developing HCC by using AFP level ≥ 9 ng/ml, AFP gp: Alpha Feto Protein, HC gp: Healthy Control group, LC gp: Liver Cirrhosis group, HCC gp: Hepatocellular carcinoma group.
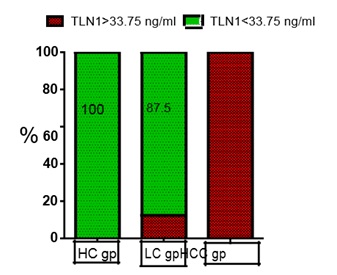 Figure 3: Columns representing the percentage of subjects with high risk of developing HCC by using Talin-1 level ≥ 33.75 ng/ml. TLN1: Talin1, HC gp: Health Control group, LC gp:Liver Cirrhosis group, HCC gp: Hepatocellular group.
Figure 3: Columns representing the percentage of subjects with high risk of developing HCC by using Talin-1 level ≥ 33.75 ng/ml. TLN1: Talin1, HC gp: Health Control group, LC gp:Liver Cirrhosis group, HCC gp: Hepatocellular group.
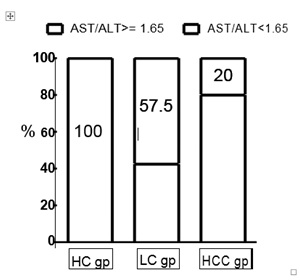 Figure 4: Columns representing the percentage of subjects with high risk of developing HCC by using AST/ALT level ≥ 1.65. AST: Aspartate Aminotransferse, ALT: Alanine Aminotransferase, HC gp: Healthy control group, LC gp: Liver Cirrhosis group, HCC gp: Hepatocellular group.
Figure 4: Columns representing the percentage of subjects with high risk of developing HCC by using AST/ALT level ≥ 1.65. AST: Aspartate Aminotransferse, ALT: Alanine Aminotransferase, HC gp: Healthy control group, LC gp: Liver Cirrhosis group, HCC gp: Hepatocellular group.
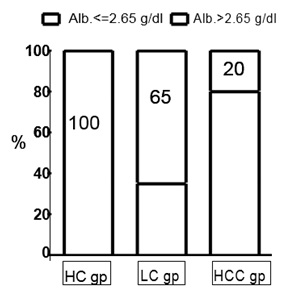 Figure 5: Columns representing the percentage of subjects with high risk of developing HCCby using Albumin level ≤ 2.65 g/dl. Alb: Albumin, HC gp: Healthy Control group, LC gp:Liver Cirrhosis group, HCC gp: Hepatocellular group.
Figure 5: Columns representing the percentage of subjects with high risk of developing HCCby using Albumin level ≤ 2.65 g/dl. Alb: Albumin, HC gp: Healthy Control group, LC gp:Liver Cirrhosis group, HCC gp: Hepatocellular group.
The cross tabulations using two and four markers are illustrated in (Figure 6) shows the percent of subjects differentiated when talin-1 used alone or combined with AFP. In HCC group 80 of subjects have both markers higher than normal while only 20 have only one marker is high and the other is normal. In cirrhotic patients 5% had two abnormal markers with high levels and 37.5% had one abnormal marker. (Figure 7) shows the combined use of serum Talin-1, AFP, AST/ALT ratio and albumin, 100% of HCC patients had at least two abnormal markers compared to 37.5 % of LC patients.
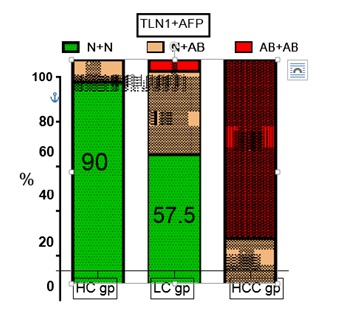 Figure 6: Percent of subjects with abnormal levels of either one or two markers in the different groups.TLN1: Talin1, AFP: Alpha Feto Protein, N+N both markers are within normal range, N+AB one marker is within normal range and the other one is abnormal, AB+AB both markers’ levels are higher than normal level, HC gp: Healthy Control group, LC gp: Liver Cirrhosis group, HCC gp: Hepatocellular Carcinoma group.
Figure 6: Percent of subjects with abnormal levels of either one or two markers in the different groups.TLN1: Talin1, AFP: Alpha Feto Protein, N+N both markers are within normal range, N+AB one marker is within normal range and the other one is abnormal, AB+AB both markers’ levels are higher than normal level, HC gp: Healthy Control group, LC gp: Liver Cirrhosis group, HCC gp: Hepatocellular Carcinoma group.
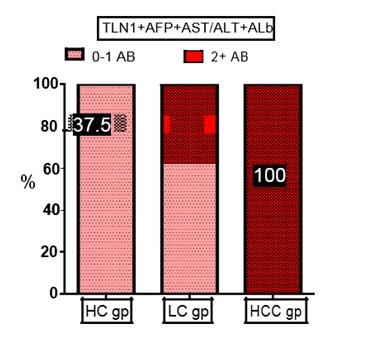 Figure 7: Percent of subjects with abnormal levels of more than two markers in the different groups. TLN1: Talin1, AFP: Alpha Feto Protein, AST: Aspartate Aminotransferase, ALT: Alanine Aminotransferase, Alb, Albumin, (0-1 AB) no or one marker is within abnormal level, (2+ AB) more than two markers’ levels are higher than normal, HC gp: Healthy Control group, LC gp: Liver Cirrhosis group, HCC gp: Hepatocellular group.
Figure 7: Percent of subjects with abnormal levels of more than two markers in the different groups. TLN1: Talin1, AFP: Alpha Feto Protein, AST: Aspartate Aminotransferase, ALT: Alanine Aminotransferase, Alb, Albumin, (0-1 AB) no or one marker is within abnormal level, (2+ AB) more than two markers’ levels are higher than normal, HC gp: Healthy Control group, LC gp: Liver Cirrhosis group, HCC gp: Hepatocellular group.
DISCUSSION
Liver cancer is the sixth most common cancer all over the world and the third most common cause of cancer mortality, with more than 500,000 deaths annually [22-24]. A previously published study indicated that the incidence rates of HCC had been increased in the United States three times from 1975 through 2005 [24]. In Egypt, HCC incidence rates have been doubled over the last ten years and that is attributed to the growing HCV incidence [25-29]. However, recently developed direct-acting antivirals (DAAs), which directly target the viral protease, polymerase, or non-structural proteins, have enabled interferon-free anti-HCV therapies with a revolutionary improvement of sustained virologic response (SVR) rate, approaching or surpassing 90%, and this may limit HCC incidence rate[30].
The ideal marker for HCC would be specific for HCC and not be detected in cirrhosis. From a practical point of view, it is very important to find noninvasive biomarkers to identify early HCC cases within the liver cirrhosis (LC) patients. With comparison to normal healthy controls, the serum concentrations of various biomolecules change in LC patients and may lead to a discovery of biomarkers [31]. The ideal marker should be easily accessed, easily measurable, reproducible, minimally invasive, accurate, and acceptable to patients and physicians. The current tests do not meet these criteria and an urgent need for newer ones are needed [32].
The ideal study design would be to compare cirrhosis that progresses into HCC and cirrhosis that does not progress into HCC in the follow-up of the disease, but such samples were very scarce and very difficult to collect as the follow-up among cirrhotic patients would be extremely long and difficult. Therefore, we compared the levels of these markers, single or combined, in two groups of patients suffering from chronic HCV infection, chronic HBV infection or other risk factors for liver disease with a third healthy control group; these groups represented HCC-free/liver cirrhosis (LC), and HCC patients. Study groups are of matched age, sex and body mass index, however, body mass index showed a significant negative correlation with our marker talin-1.
In this study, after assessing a panel of blood markers for HCC, we developed a predictive model consisting of Talin-1 and three routine laboratory tests. Talin-1 was the firstly assessed marker. Talin-1 is easily measurable and requires only serum samples from patients and a microplate colorimetric reader. However, there are few studies on the efficiency of Talin-1 as a marker for HCC. Our previous publication clearly proved the potential use of serum talin-1 as HCC biomarker [16].
The level of AFP, the second marker in our study increases in HCC patients due to the re-expression of the related gene, which is usually repressed in adult subjects. In the present study, the sensitivity and specificity of AFP “the gold standard marker” were 80% and 65% at cutoff 9 ng/ml. Our study agrees with those of Bessa et al., and Attallah et al., concerning AFP AUC (AUC 0.71 and 0.7) in predicting HCC in Egyptian population [33,34]. Thus there is a need for the enhancement of the diagnosis of HCC using AFP.
Serum aspartate aminotransferse to alanine aminotransferase ratio was the third marker that can aid in HCC discrimination. Our results showed values of sensitivity, specificity, cut off value and AUC for AST/ALT ratio as 80, 57, 1.65, and 0.77 respectively. By using this marker 20% of HCC were negatively diagnosed and 42.5% of LC were positively diagnosed. Pohl et al and Giannini et al. reported comparable observations[35,36]. However, other studies found that the AST/ALT had no correlation with the presence of HCC [37,38], these controversial results could be attributed to lower sensitivity and specificity for this liver marker.
Furthermore, albumin was the fourth marker. Albumin produced only by the liver, which is the major serum protein that circulates in the bloodstream. The serum albumin concentration is usually normal in chronic liver disease, until cirrhosis and HCC develop. In HCC patients, the serum albumin level may be less than 3.5 g/dl [39]. In the present study, AUC was 0.72 for albumin with the best cutoff value of ≤2.65g/dl. The diagnostic value of albumin is not significantly worse and may be easier to measure as it is included in routine liver function tests in many cases. However, hypoalbuminemia is not specific for liver diseases and can be caused by various conditions, including nephrotic syndrome, heart failure, liver disease and malnutrition [40-43].Our results agrees with that obtained by Masuzaki et al. concerning albumin AUC value (AUC 0.71)[44]. Several studies used the concept of combining several markers in order to support the diagnosis of HCC using AFP [45-47].
From the present study we have concluded that serum talin-1 is a novel sensitive marker for HCC. However, HCC traditional markers AFP, AST/ALT ratio and albumin cannot be considered as ideal markers for HCC when measured lonely. Furthermore, their combination could serve as a good predictive model for HCC. Finally, the combined use of our novel marker serum talin-1, with AFP, AST/ALT ratio and albumin improves the positive predictive value for HCC from 80% to 100%.
ACKNOWLEDGEMENT
We are grateful for the staff members at the National cancer institute (NCI) and at Al-Kasr Al-Ainy educational hospital, for their expert technical help during subject recruitment, samples collections, practical and data analyses.
REFERENCES
- Lau W, Lai E (2008) Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int 7:237-257.
- ZekriA,Alam El-Din H,BahnassyA,Zayed N, Mohamed W et.al (2010) Serum levels of soluble Fas, soluble tumor necrosis factor-receptor II, interleukin-2 receptor and interleukin-8 as early predictors of hepatocellular carcinoma in Egyptian patients with hepatitis C virus genotype-4. Comp Hepatol 9:1.
- Mazzanti R, Gramantieri L, Bolondi L (2008) Hepatocellular carcinoma: epidemiology and clinical aspects. Mol Aspects Med 29:130-143.
- Lehman EM, Wilson ML (2009) Epidemiology of hepatitis viruses among hepatocellular carcinoma cases and healthy people in Egypt: A systematic review and meta-analysis. Int J Cancer 124:690-697.
- Anwar W, KhaledH,Amra H, El-Nezami H, Loffredo CA (2008) Changing pattern of hepatocellular carcinoma (HCC) and its risk factors in Egypt: possibilities for prevention. Mutat Res659:176-184.
- But D, Lai CL,Yuen MF (2008) Natural history of hepatitis-related hepatocellular carcinoma. World J Gastroenterol14:1652-1656.
- Gomaa A, Khan S, Toledano M, Waked I, Robinson SD (2008) Hepatocellular carcinoma: Epidemiology, risk factors and pathogenesis. World J Gastroenterol 14:4300-4308.
- DeugnierY (1997)Other causes of hepatocellular carcinoma. Churchill-Livingstone, USA.
- SolimanA, Hung C, Tsodikov A, Seifeldin I, Ramadan M et al (2010) Epidemiologic risk factors of hepatocellular carcinoma in a rural region of Egypt. HepatolInt 4:681-690.
- RaphaelSW,Yangde Z (2012) Hepatocellular carcinoma: focus on different aspects of management. ISRN Oncol 01-12.
- Horwitz A, Duggan K, Buck C (1986) Interaction of plasma-membrane fibronectin receptor with talin - a transmembrane linkage. Nature 320:531-533.
- TsujiokaM, Yoshida K, Inouye K, Talin B (2004) is required for force transmission in morphogenesis of dictyostelium. EMBO J 23:2216-2225.
- Senetar MA, McCann RO (2005) Gene duplication and functional divergence during evolution of the cytoskeletal linker protein talin. Gene 362:141-152.
- Senetar M, Moncman C, McCann R (2007) Talin2 is induced during striated muscle differentiation and istargeted to stable adhesion complexes in mature muscle. Cell Motil Cytoskeleton 64:157-173.
- Kanamori H, Kawakami T, Effendi K, Yamazaki K, Mori T, et al. (2011) Identification by differential tissue proteome analysis of talin-1 as a novel molecular marker of progression of hepatocellular carcinoma. Oncology 80:406-415.
- Youns M, Wahab AH, Hassan Z, Attia MS (2013) Serum talin-1 is a potential novel biomarker for diagnosis of hepatocellular carcinoma in Egyptian Patients.Asian Pac J Cancer Prev 14:3819-3823.
- Giardina M, Matarazzo M, Morante R, Lucariello A, Varriale A (1992)Guardasole, V. Serum alpha-L-fucosidase activity and early detection ofhepatocellular carcinoma: A prospective study of patients with cirrhosis. Cancer 83:2468-2474.
- Blum HE, Spangenberg HC (2007) Hepatocellular carcinoma: an update. Arch Iran Med 10:361-371.
- Kaneko S,Unoura M (1997)Early detection of hepatocellular carcinoma. Churchill-Livingstone, USA.
- Yoon SK (2008) Recent advances in tumor markers of human hepatocellular carcinoma. Intervirology51:34-41.
- Sox H, Blatt M, Higgins M, Marton K (1989) Medical Decision Making. Butterworth, UK.
- Parkin D, Bray F,Ferlay J, Pisani P (2001) Estimating the world cancer burden: Globocan 2000. Int J Cancer 94:153-156.
- Parkin D, BrayF,Ferlay J (2005) Pisani, P. Global cancer statistics.CA Cancer J Clin 55:74–108.
- Altekruse S, McGlynnK,Reichman M (2009) Hepatocellular carcinoma incidence,mortality, and survival trends in the United States from 1975 to 2005. J ClinOncol 27:1485-1491.
- El-Zayadi AR, BadranHM,Barakat EM, Attia Mel-D, Shawky S et al (2005) Hepatocellular carcinoma in Egypt: a single center study over a decade. World J Gastroenterol 11:5193-5198.
- Abdel-Aziz F, Habib M, Mohamed MK, Abdel-Hamid M, Gamil F et al. (2000) Hepatitis C virus (HCV) infection in a community in the Nile Delta: population description and HCV prevalence. Hepatology 32:111-115.
- Khattab MA, Eslamm M, Sharwae MA, Hamdy L (2010)Seroprevalence of hepatitis C and B among blood donors in Egypt: Minya Governorate. Am J Infect Control 38:640-641.
- Hassan MM, Zaghloul AS, El-Serag HB, Soliman OC, Patt YZ et al. (2001) The role of hepatitis C in hepatocellular carcinoma: a case control study among Egyptian patients. J ClinGastroenterol33:123-126.
- Freedman L, Edwards B, Ries L (2006) Cancer incidence in four member countries (Cyprus, Egypt, Israel, and Jordan) of the middle east cancer consortium (MECC) compared with US SEER. National Cancer Institute.
- Baumert TF, Jühling F, Ono A, Hoshida Y (2017) Hepatitis C-related hepatocellular carcinoma in the era of new generation antivirals. BMC Medicine.
- Ang IL, Poon TC,LaiPB, Chan AT, Ngai SM et al. (2006) Study of serum haptoglobin and its glycoforms in the diagnosis of hepatocellular carcinoma: a glycoproteomic approach. J Protozool Res 5:2691-2700.
- Block TM, Marrero J, Gish RG, Sherman M, London WT et al. (2008)The degree of readiness of selected biomarkers for the early detection of hepatocellular carcinoma: notes from a recent workshop. Cancer Biomark4:19-33.
- Bessa S, Elwan N, Suliman G, El-Shourbagy S (2010) Clinical significance of plasma osteopontin level in Egyptian patients with hepatitis C virus-related hepatocellular carcinoma. Arch Med Res 41:541–547.
- Attallah AM, El-Far M, Abdel Malak C, Zahran F, Farid K et al. (2011) Evaluation of cytokeratin-1 in the diagnosis of hepatocellular carcinoma. ClinicaChimicaActa412:2310-2315.
- Pohl A, Behling C, Oliver D, Kilani M, Monson P (2001) Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. Am J Gastroenterol 96:3142-3146.
- Giannini E, Risso D, Botta F, Chiarbonello B, Fasoli A et al. (2003) Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch Intern Med163:218-224.
- Sebastini G, Alberti A (2006) Non-invasive fibrosis biomarkers reduce but not substitute the need for liver biopsy. World J Gastroenterol12:3682-3694.
- Lin CS, Chang CS, Yang SS, YehHZ,LinCW (2008) Retrospective evaluation of serum markers APRI and AST/ALT for assessing liver fibrosis and cirrhosis in chronic hepatitis B and C with hepatocellular carcinoma. Intern Med 47:569-575.
- Nagao Y, Sata M (2010) Serum albumin and mortality risk in a hyperendemic area of HCV infection in Japan. Virol J 31:375–379.
- Fliser D, Zurbruggen I (1999)Coadministration of albumin and furosemide in patients with the nephrotic syndrome. Kidney Int 55:629–634.
- Djousse L, Rothman KJ, Cupples LA, Levy D, Ellison RC (2002) Serum albumin and risk of myocardial infarction and all-cause mortality in the Framingham Offspring Study. Circulation 106:2919–2924.
- Faloon WW, Eckhardt RD, Murphy TL, Cooper AM (1949)An evaluation of human serum albumin in the treatment of cirrhosis of the liver. J Clin Invest 28:583–594.
- Rothschild MA, Oratz M, SchreiberSS (1983) Effects of nutrition and alcohol on albumin synthesis. Alcohol ClinExp Res 7:28-30.
- MasuzakiR,Tateishi R, Yoshida H, Yoshida H, Sato S, Kato N et.al (2008) Risk assessment of hepatocellular carcinoma in chronic hepatitis C patients by transient elastography. J ClinGastroenterol 42:839–843.
- Tangkijvanich P, Chanmee T, Komtong S (2010) Diagnostic role of serum glypican-3 in differentiating hepatocellular carcinoma from non-malignant chronic liver disease and other liver cancers. J GastroenterolHepatol 25:129–137.
- Tangkijvanich P, Tosukhowong P, Bunyongyod P (1999) Alpha-L fucosidase as a serum marker of hepatocellular carcinoma in Thailand. Southeast Asian J Trop Med Public Health 30:110–114.
- Bachtiar I, Santoso JM, Atmanegara B (2009) Combination of alpha-1-acid glycoprotein and alpha-fetoprotein as an improved diagnostic tool for hepatocellular carcinoma. ClinChimActa399:97–101.
Citation: AttiaGaballah MS(2019) The Combined Role of Serum Talin-1 with Traditional Liver Biomarkers in Diagnosis of Hepatocellular Carcinoma. Adv Microb Res 3: 008.
Copyright: © 2019 Mohamed S AttiaGaballah, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

