
The Correlation between CGB Gene Splice Variants in Women with Recurrent Spontaneous Abortions
*Corresponding Author(s):
Alexandros PsarrisDepartment Of Obstetrics And Gynecology, "Alexandra" General Hospital, National And Kapodistrian University Of Athens, Vasilissis Sofia 80 And Lourou Street, 11528 Athens, Greece
Tel:+0030 6979232977,
Email:Psarris.alexandros@gmail.com
Abstract
Background: Human Chorionic Gonadotrophin (hCG) is a heterodimer glycoprotein which is mainly produced by the syncytiotrophoblast cells of the placenta during pregnancy and it is involved in a variety of biological procedures. The β-subunit of hCG is coded by CGB, CGB5, CGB8 genes. Most of βCGB transcripts are produced by CGB5 and CGB8 genes, while CGB1 and CGB2 are considered to be pseudogenes, coding a hypothetical protein, involved in implantation stage. Discrepancies in the expression levels of these genes are associated with a higher risk of abortion or ectopic pregnancy. The presence of polymorphisms in these genes, could act as a prognostic marker in women at risk for recurrent miscarriage.
Objectives: The purpose of this study is to detect any correlation between CGB1 and CGB2 gene expression levels and the probability of recurrent miscarriage.
Method: 163 Caucasian women with recurrent miscarriages and 87 ethnically matched controls with at least one successful pregnancy were included in this study. DNA from cells of peripheral blood was isolated using and subjected to PCR amplification to determine the presence of specific for CGB1 and CGB2 splice variants using appropriate gene specific primers. The obtained product from each patient was visualized using agarose gel and acrylamide gel electrophoresis.
Conclusion: The splice variant of CGB1 (573bp) was not expressed neither in the RSA group nor in the control group. The CGB2 splice variant (627bp) was expressed in all the women of the study (both study group and control group).
Keywords
CGB splice variants; HCG genes; Recurrent miscarriage; Recurrent spontaneous abortions
INTRODUCTION
Human Chorionic Gonadotrophin (hCG) is a heterodimer glycoprotein which is mainly produced by the syncytiotrophoblast cells of the placenta during pregnancy and it is involved in a variety of biological procedures [1]. The synthesis of the β?subunit of Human Chorionic Gonadotropin (βhCG) begins shortly after fertilization (βhCG been detected in the 2-cell stage embryo [2]) and it reaches its peak in the maternal blood stream at 9-10 weeks of pregnancy. HCG has many functions during normal pregnancy as it is involved in delaying the apoptosis of the corpus luteum [3], modulating the implantation of the blastocyst [4,5], regulating the placentation and angiogenesis [6-8] and developing maternal immunotolerance [9].
HCG is composed of two subunits α and β. Subunit α is common while subunit β is hormone specific. Subunit α is encoded by a single gene located on chromosome 6q12-q21, while a cluster of genes on chromosome 19q13.3 are responsible for encoding the β subunit [10]. There are six Chorionic Gonadotropin Beta (CGB) genes all originating from the gene responsible for coding the β subunit of the Luteinizing Hormone (LH) via duplication during primate evolution [11]. The beta subunit of hCG is a 163 amino acid protein coded by CGB, CGB5, CGB7 and CGB8 genes which share 97-99% of their sequence identity [10].
CGB1 and CGB2 genes share an 85% identity with the other genes in the cluster and they encode a hypothetical protein of 132 amino acids that does not have any homology with the functional β subunit and does not correspond to any known protein from the GenBank database [10]. This change has been caused by an inserted DNA fragment (736 bp for CGB1 and 724 bp for CGB2) that replaced the 52-bp sequence at the proximal end of the promoter, and also the entire 5’-UTR of the ancestral hCG β-subunit coding gene fragment, which is still present in classical CGB genes [12,13]. Despite our lack of knowledge regarding the role of this protein, earlier studies have detected mRNA from CGB1 and CGB2 in the placenta as well as the pituitary proving the functionality of these genes [12,14].
In this study we aim to compare the presence of CGB1 and CGB2 gene splice variants in women with normal pregnancies and women with recurrent spontaneous abortions.
MATERIALS AND METHODS
The study group included 163 Caucasian women with at least two miscarriages of unexplained aetiology, before the 20th week of gestation who visited the recurrent miscarriage outpatient clinic of Alexandra Hospital. Women with a history of thromboembolic, infectious, autoimmune, endocrine or chromosomal disorders were excluded from the study. The control group included 87 Caucasian women of proven fertility, with no history of pregnancy loss. The study protocol was approved by the Alexandra Hospital scientific committee and the experiments were conducted in the Molecular Biology laboratory of the IVF unit of Alexandra Hospital.
Peripheral blood (2-3ml) was collected from all the women of the study. The DNA isolation was performed using the PureLink Genomic DNA kit by Invitrogen Life Technologies. The isolated DNA is stored in a buffer solution at -20oC. The DNA samples were subjected to PCR amplification to determine the presence of a specific for CGB1 and CGB2 splice variant, using appropriate gene specific primers which have been previously reported by Burczynska et al., [15]. The primers used for the detection of CGB1 and CGB2 were CGBF 5' CgTCCAACACCCTCACTCCCGBR ggCAgCCCTCCTTCTCCAC. The conventional PCR protocol used in our study included adding 10Χ PCR Buffer minus Mg2+, 10 mM dNTP mixture, 1μl 50 mM MgCl2, 1μl Primer Sense mix, 1μl Primer Antisense mix, 1μl Template DNA, 0,3μl Taq DNA polymerase, 17,2μl of distilled water. The PCR conditions are 95oC for 10 minutes and 95oC for 1 minute, 65oC for 1 minute, 72oC for 1 minute for 29 cycles, with a final extension step at 72oC for 10 minutes. Then the tubes are incubated at 72oC for 10 minutes. The splice variants were detected using agarose gel and acrylamide gel electrophoresis (Figure 1).
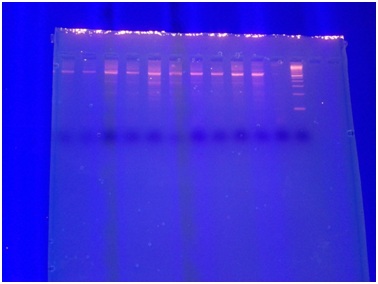 Figure 1: Detection of CGB splice variants.
Figure 1: Detection of CGB splice variants.
STATISTICAL ANALYSIS
The statistical analysis was performed via IBM SPSS Statistics for Windows, Version 25.0. Statistical significance was set to p<0.05. P-values were calculated using chi-squared and Mann-Whitney tests.
RESULTS
In our study the splice variant of CGB1 (573bp) was not expressed neither in the RSA group nor in the control group. However, the CGB2 splice variant (627bp) was expressed in all the women of the study (both study group and control group). Hence, this study failed to demonstrate a significant difference in the presence of either of these splice variants between women with recurrent spontaneous abortions and the control group. However, our study demonstrates the expression of CGB2 splice variant in the Greek women population as well as the absence of expression of the CGB1 splice variant. There was no statistically significant relationship between the expression pattern and the number of first or second trimester abortions, parity regardless of smoking habits, BMI and age (Tables 1 and 2).
|
|
Age |
|
ΒΜΙ |
|
|||
|
Recurrent Spontaneous Abortions Group |
Control Group |
p-value |
Recurrent Spontaneous Abortions Group |
Control Group |
p-value |
||
|
N |
Valid cases |
192 |
97 |
<0.001
|
180 |
88 |
0.129 |
|
Missing values |
12 |
3 |
24 |
12 |
|||
|
Mean |
32.60 |
43.16 |
24.85 |
25.39 |
|||
|
Median |
33.00 |
40.00 |
23.87 |
24.17 |
|||
|
SD |
5.03 |
14.81 |
4.91 |
4.18 |
|||
|
Minimum |
18.00 |
21.00 |
17.72 |
16.57 |
|||
|
Maximum |
42.00 |
94.00 |
45.78 |
36.36 |
|||
Table 1: Comparison of age and BMI between the study and the control group.
|
Study parameter |
Recurrent Spontaneous Abortions Group |
Control Group |
|
|||
|
|
|
Frequency |
% |
Frequency |
% |
p-value |
|
Smoking |
|
|
|
|
|
0.023 |
|
|
Νο |
99 |
48.5 |
59 |
59.0 |
|
|
|
Yes |
57 |
27.9 |
16 |
16.0 |
|
|
Parity |
<0.001 |
|||||
|
|
0 |
162 |
79.4 |
0 |
0.0 |
|
|
|
1 |
34 |
16.7 |
0 |
0.0 |
|
|
|
2 |
0 |
0.0 |
52 |
52.0 |
|
|
|
3 |
0 |
0.0 |
31 |
31.0 |
|
|
|
4 |
0 |
0.0 |
9 |
9.0 |
|
|
|
5 |
0 |
0.0 |
4 |
4.0 |
|
|
|
6 |
0 |
0.0 |
1 |
1.0 |
|
|
|
7 |
0 |
0.0 |
1 |
1.0 |
|
|
Chemical pregnancy |
<0.001 |
|||||
|
|
0 |
143 |
70.1 |
98 |
98.0 |
|
|
|
1 |
31 |
15.2 |
0 |
0.0 |
|
|
|
2 |
14 |
6.9 |
0 |
0.0 |
|
|
|
3 |
2 |
1.0 |
0 |
0.0 |
|
|
|
4 |
3 |
1.5 |
0 |
0.0 |
|
|
|
7 |
1 |
0.5 |
0 |
0.0 |
|
|
Ectopic pregnancy |
0.012 |
|||||
|
|
0 |
181 |
88.7 |
98 |
98.0 |
|
|
|
1 |
11 |
5.4 |
0 |
0.0 |
|
|
|
2 |
2 |
1.0 |
0 |
0.0 |
|
|
First trimester abortions |
<0.001 |
|||||
|
|
0 |
9 |
4.4 |
95 |
95.0 |
|
|
|
1 |
38 |
18.6 |
3 |
3.0 |
|
|
|
2 |
82 |
40.2 |
0 |
0.0 |
|
|
|
3 |
44 |
21.6 |
0 |
0.0 |
|
|
|
4 |
12 |
5.9 |
0 |
0.0 |
|
|
|
5 |
5 |
2.5 |
0 |
0.0 |
|
|
|
6 |
3 |
1.5 |
0 |
0.0 |
|
|
|
11 |
1 |
0.5 |
0 |
0.0 |
|
|
Second trimester abortions |
0.002 |
|||||
|
|
0 |
176 |
86.3 |
98 |
98.0 |
|
|
|
1 |
14 |
6.9 |
0 |
0.0 |
|
|
|
2 |
3 |
1.5 |
0 |
0.0 |
|
|
|
3 |
1 |
0.5 |
0 |
0.0 |
|
|
Sum of first trimester abortions, second trimester abortions and chemical pregnancies |
<0.001 |
|||||
|
|
0 |
0 |
0.0 |
96 |
96.0 |
|
|
|
1 |
0 |
0.0 |
3 |
3.0 |
|
|
|
2 |
108 |
52.9 |
0 |
0.0 |
|
|
|
3 |
60 |
29.4 |
0 |
0.0 |
|
|
|
4 |
17 |
8.3 |
0 |
0.0 |
|
|
|
5 |
5 |
2.5 |
0 |
0.0 |
|
|
|
6 |
3 |
1.5 |
0 |
0.0 |
|
|
|
7 |
2 |
1.0 |
0 |
0.0 |
|
|
|
11 |
1 |
0.5 |
0 |
0.0 |
|
|
Sum of recurrent first trimester abortions and chemical pregnancies |
<0.001 |
|||||
|
|
0 |
2 |
1.0 |
96 |
96.0 |
|
|
|
1 |
10 |
4.9 |
3 |
3.0 |
|
|
|
2 |
101 |
49.5 |
0 |
0.0 |
|
|
|
3 |
56 |
27.5 |
0 |
0.0 |
|
|
|
4 |
16 |
7.8 |
0 |
0.0 |
|
|
|
5 |
6 |
2.9 |
0 |
0.0 |
|
|
|
6 |
2 |
1.0 |
0 |
0.0 |
|
|
|
7 |
2 |
1.0 |
0 |
0.0 |
|
|
|
11 |
1 |
0.5 |
0 |
0.0 |
|
Table 2: Comparison of smoking, chemical pregnancy, parity, ectopic pregnancy, first and second trimester abortions between the study and the control group.
DISCUSSION/CONCLUSION
The possible association of recurrent spontaneous abortions with different CGB variants and polymorphisms has not been fully investigated. Rull et al., has demonstrated the presence of protective single nucleotide polymorphisms in the CGB5 and CGB8 genes in the Finish and Estonian populations [16]. Furthermore, Rull et al., discovered the protective effect of 4 CGB5 promoter variants [16]. The presence of a protective SNP in the CGB5 gene was also demonstrated by Yong Sun et al., in Chinese women with RSA [17]. CGB1 and CGB2 genes appear to have a role in implantation and early pregnancy. Rull et al., demonstrated that in the first trimester of pregnancy CGB1 and CGB2 genes provided only 1/1000 to 1/5000 of the CGB mRNA transcripts and during the second and third trimester of pregnancy their contribution was even lower (1/10.000) [18]. The expression profile of CGB genes in the trophoblastic tissue of women with ectopic pregnancies differs from normal pregnancies as it is characterized by higher expression levels of CGB1/CGB2 and lower transcriptional activity of the other CGB genes [18]. CGB1/CGB2 contributed 1/500 to 1/3000 of mRNA CGB transcripts [18]. Rull et al., demonstrated the higher transcriptional activity of CGB1/CGB2 in cases of ectopic pregnancy [10]. Moreover, Rull et al., found a complete lack of expression of CGB1 and CGB2 genes in women with RSA suggestive of an etiological relationship between expression failure of CGB genes and recurrent miscarriages [10].
CGB genes have also been associated with malignant disease. Expression of CGB genes has also been documented in cases of molar pregnancy [18]. In these cases, the transcription level of all CGB genes is at the highest level documented in normal first trimester pregnancies [18]. Furthermore, Kubiczak M et al., demonstrated an increased expression pattern of CGB1 and CGB2 genes in ovarian cancer tissue samples, while CGB1 and CGB2 genes were not active in normal ovaries [19]. CGB1 and CGB2 activity was demonstrated in 41% of ovarian tumor tissue and in none of the normal ovarian tissue samples [19]. In the same study, total CGB as well as CGB3-9 gene expression characterized both normal ovaries and ovarian cancer [19].
Expression of CGB genes has also been documented in non-trophoblastic non-malignant tissue samples. CGB gene transcripts have been detected in testis, prostate, muscle and lung samples [18]. CGB1/CGB2 genes were under-expressed in all these tissue samples (1/1000-1/10.000), just like in normal pregnancy, with the expression of the test is where CGB1/CGB2 mRNA accounts for 1/3 of the total CGB mRNA [18]. In our study CGB2 splice variant (627bp) was detected in all samples of both study and control group. On the other hand, splice variant CGB1 (573bp) was not detected in either group. Hence, our study did not reveal a significant correlation between these CGB splice variants and recurrent spontaneous abortions. However, it demonstrated the predominance of CGB2 (627pb) splice variant and the absence of CGB1 (573bp) splice variant in the Greek population, regardless of parity.The prospects of analyzing CGB gene expression patterns as well as CGB slice variants and SNPs are very promising. Furthermore, SNPs and splice variants of CGB genes have shown protective and/or aggravating effects on recurrent miscarriages. Hence, further studies are needed in order to clarify the role of CGB genes in cancerogenesis, in implantation and sustenance of pregnancies.
STATEMENT OF ETHICS
All subjects have given their written informed consent and the study protocol was approved by the Alexandra Hospital scientific committee.
DISCLOSURE STATEMENT
The authors have no conflicts of interest to declare.
REFERENCES
- Pierce JG, Parsons TF (1981) Glycoprotein hormones: Structure and function. Annu Rev Biochem 50: 465-495.
- Jurisicova A, Antenos M, Kapasi K, Meriano J, Casper RF (1999) Variability in the expression of trophectodermal markers beta-human chorionic gonadotrophin, human leukocyte antigen-G and pregnancy specific beta-1 glycoprotein by the human blastocyst. Hum Reprod 14: 1852-1858.
- King BF (1993) Development and structure of the placenta and fetal membranes of nonhuman primates. J Exp Zool 266: 528-540.
- Srisuparp S, Strakova Z, Fazleabas AT (2001) The role of Chorionic Gonadotropin (CG) in blastocyst implantation. Arch Med Res 32: 627-634.
- Cameo P, Srisuparp S, Strakova Z, Fazleabas AT (2004) Chorionic gonadotropin and uterine dialogue in the primate. Reprod Biol Endocrinol 2: 50.
- Toth P, Lukacs H, Gimes G, Sebestyen A, Pasztor N, et al. (2001) Clinical importance of vascular LH/hCG receptors--a review. Reprod Biol 1: 5-11.
- Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Münstedt K, et al. (2002) Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab 87: 5290-5296.
- Herr F, Baal N, Reisinger K, Lorenz A, McKinnon T, et al. (2007) HCG in the regulation of placental angiogenesis. Results of an in vitro study. Placenta 28: 85-93.
- Kayisli UA, Selam B, Guzeloglu-Kayisli O, Demir R, Arici A (2003) Human chorionic gonadotropin contributes to maternal immunotolerance and endometrial apoptosis by regulating Fas-Fas ligand system. J Immunol 171: 2305-2313.
- Rull K, Laan M (2005) Expression of beta-subunit of HCG genes during normal and failed pregnancy. Hum Reprod 20: 3360-3368.
- Talmadge K, Vamvakopoulos NC, Fiddes JC (1984) Evolution of the genes for the beta subunits of human chorionic gonadotropin and luteinizing hormone. Nature 307: 37-40.
- Bo M, Boime I (1992) Identification of the transcriptionally active genes of the chorionic gonadotropin beta gene cluster in vivo. J Biol Chem 267: 3179-3184.
- Hollenberg AN, Pestell RG, Albanese C, Boers ME, Jameson JL (1994) Multiple promoter elements in the human chorionic gonadotropin beta subunit genes distinguish their expression from the luteinizing hormone beta gene. Mol Cell Endocrinol 106: 111-119.
- Dirnhofer S, Hermann M, Hittmair A, Hoermann R, Kapelari K, et al. (1996) Expression of the human chorionic gonadotropin-beta gene cluster in human pituitaries and alternate use of exon 1. J Clin Endocrinol Metab 81: 4212-4217.
- Burczynska BB, Kobrouly L, Butler SA, Naase M, Iles RK, et al. (2014) Novel insights into the expression of CGB1 & 2 genes by epithelial cancer cell lines secreting ectopic free hCGβ. Anticancer Res 34: 2239-2248.
- Rull K, Nagirnaja L, Ulander VM, Kelgo P, Margus T, et al. (2008) Chorionic gonadotropin beta-gene variants are associated with recurrent miscarriage in two European populations. J Clin Endocrinol Metab 93: 4697-4706.
- Sun Y, Ji X (2014) Association of rs7260002 of chorionic gonadotrophin β5 with idiopathic recurrent spontaneous abortion in Chinese population. J Assist Reprod Genet 31: 1497-1500.
- Rull K, Hallast P, Uusküla L, Jackson J, Punab M, et al. (2008) Fine-scale quantification of HCG beta gene transcription in human trophoblastic and non-malignant non-trophoblastic tissues. Mol Hum Reprod 14: 23-31.
- Kubiczak M, Walkowiak GP, Nowak-Markwitz E, Jankowska A (2013) Human chorionic gonadotropin beta subunit genes CGB1 and CGB2 are transcriptionally active in ovarian cancer. Int J Mol Sci 14: 12650-12660.
Citation: Psarris A, Lourida C, Stavrou S, Mavrogianni D, Loutradis D, et al. (2019) The Correlation between CGB Gene Splice Variants in Women with Recurrent Spontaneous Abortions. J Reprod Med Gynecol Obstet 4: 027.
Copyright: © 2019 Alexandros Psarris, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

