
The Early Coasting Method during Ovarian Stimulation in Antagonist Protocols with Combined Use of Cabergoline is Useful in Patients with Polycystic Ovary Syndrome
*Corresponding Author(s):
Akira NakashimaSoranomori Clinic, Yaese, Shimajirigun, Japan
Tel:+81 0989980011,
Fax:+81 0989980022
Email:nakadiam@gmail.com
Abstract
Purpose: This study aimed to investigate the efficacy of withholding gonadotropins (coasting) and early administration of cabergoline in a flexible Gonadotropin-Releasing Hormone (GnRH) antagonist protocol for patients with Polycystic Ovarian Syndrome (PCOS).
Methods: From November 2014 to April 2019, we studied 30 patients with PCOS aged < 40 years who received their first assisted reproductive technology treatment on the basis of a flexible GnRH antagonist protocol. Sixteen patients received 1-4 days of coasting after the follicle size reached 13-15 mm, and cabergoline 250 mg per day was started simultaneously. Fourteen patients received the conventional antagonist protocol. Clinical outcomes were compared between the two groups.
Results: Coasting was performed for 1.65±1.08 days and it significantly reduced the amount of gonadotropin and GnRH antagonist used. The numbers of retrieved oocytes and morphologically good quality blastocysts were significantly higher in patients who received coasting and cabergoline than in those who received the conventional antagonist protocol (both P<0.05). The live birth rate was similar by thawed-embryo transfer cycles between the groups (92.9% vs 78.6%, p=0.298).
Conclusion: The combined use of coasting and cabergoline during ovarian stimulation in the GnRH antagonist protocol is effective for assisted reproductive technology treatment in patients with PCOS.
Keywords
Assisted reproductive technology; Cabergoline; Coasting; Gonadotropin-releasing hormone antagonist; Polycystic ovarian syndrome
Introduction
Polycystic Ovarian Syndrome (PCOS) has been reported in 6.1%-19.9% of infertile patients [1,2]. PCOS contains more than 10 follicles in the unilateral ovary and is characterized by an irregular menstrual cycle for more than 40 days, anovulation and hyperandrogenism [3]. In the criteria of the Japan Society of Obstetrics and Gynecology, PCOS is also diagnosed if there are high serum Luteinizing Hormone (LH) concentrations or a high LH/Follicle-Stimulating Hormone (FSH) ratio >1 [4].
Ovarian stimulation using clomiphene or an aromatase inhibitor is effective for these patients in the timing of intercourse and artificial insemination treatment [5]. A gonadotropin injection is required in patients with resistance to these oral medicines [6,7]. However, there is a risk of multiple pregnancies and Ovarian Hyperstimulation Syndrome (OHSS) owing to multiple follicular growth, and ovarian stimulation should be carefully performed [7,8]. In the treatment of Assisted Reproductive Technology (ART), which prevents the occurrence of severe OHSS in high responders, is also an important issue [9-12]. A Gonadotropin-Releasing Hormone (GnRH) antagonist protocol combined with a GnRH agonist trigger [10,12,13], coasting of gonadotropin injection [14-16], and administration of cabergoline after oocyte retrieval [15-21] is recommended to reduce the risk of OHSS in ovarian stimulation in patients with PCOS. Additionally, avoiding fresh embryo transfer and freezing all embryos greatly reduce the risk of the occurrence of late-onset OHSS [22-24].
In recent years, obtaining a large number of oocytes by controlled ovulation stimulation has been a goal to obtain multiple live births using only one time of oocyte retrieval [25-28]. Furthermore, making as many blastocysts as possible for preimplantation genetic screening has been attempted [29,30]. However, development of numerous follicles in the early follicular phase increases the potential of the occurrence of early-onset severe OHSS, even if the freeze-all strategy is considered [31]. Therefore, many physicians hesitate in continuing injection of gonadotropins because of early discomfort, abdominal pain, and accumulation of ascites when they observe a large number of follicles growing.
The early coasting method (withholding gonadotropins) controls follicular development during the mid to late follicular phase and has led to appropriate clinical outcomes in the GnRH agonist long protocol [14,32]. There have been a few reports on the early coasting method based on the GnRH antagonist protocol [16,33,34]. However, the cumulative pregnancy rate and clinical outcomes after thawed embryo transfer are unknown. Therefore, this study aimed to investigate the efficiency of early coasting in the GnRH antagonist protocol in patients with PCOS.
Materials and Methods
Patients
This study included women aged ≤40 years with a high ovarian reserve due to PCOS who received their first ART session at our clinic from November 2014 to April 2019. Patients with PCOS who matched the Japanese criteria were recruited in this study.
Ovarian stimulation
Ovarian stimulation was performed with daily subcutaneous gonadotropin injections of 225-300 IU (Gonal-F; Merck Biopharma, Tokyo, Japan/FOLYRMON-P; Fuji, Toyama, Japan/HMG-F; Fuji, Toyama, Japan/HMG-Ferring; Ferring Pharmaceuticals, Tokyo, Japan) without pituitary suppression by GnRH agonists. Measurement of serum LH and estradiol levels was started when the leading follicles reached approximately 14 mm, and a GnRH antagonist (Cetrotide; Merck Biopharma) was flexibly administered according to serum LH concentrations or follicular growth. Early coasting was performed when the leading follicles reached 13-15 mm in high responder patients with >15 developing follicles to prevent OHSS. At the same time, daily intake of cabergoline 250 mg (Sawai Pharmaceutical Co., Ltd., Osaka, Japan) was administered to prevent production of ascites.
Daily measurement of the size of these follicles and monitoring of serum estradiol, progesterone, and LH levels were continued during coasting. The duration of coasting days was adjusted depending on follicular development. Gonadotropin injection was added according to follicular development. After the leading follicles exceeded 18 mm in diameter, ovulation was triggered by any of the following methods: two times of 300 μg GnRH agonist nasal spray (Buserecure; Fuji, Toyama, Japan), human chorionic gonadotropin 3000-5000 IU (HCG Fuji; Fuji, Toyama, Japan) injection, or a combination of GnRH agonist nasal spray with human chorionic gonadotropin 1500-3000 IU injection.
Women who received the usual antagonist protocol without coasting between November 2014 and September 2019 were retrospectively compared as a control group with women who had coasting and cabergoline (CS group).
Oocyte retrieval, insemination, embryo culture and cryopreservation
Oocytes were picked up 36-37 hours after the trigger using a 20 G needle under cervical block with 1% xylocaine injection (xylocaine injection 1%; Aspen, Tokyo, Japan), a diclofenac sodium suppository (Voltaren; Novartis, Tokyo, Japan), and intravenous anesthesia with propofol (propofol intravenous injection 1%, 20 ml, Maruisi; Maruishi Phramaceutical, Osaka, Japan). In Vitro Fertilization (IVF), Intracytoplasmic Sperm Injection (ICSI), or split-ICSI (IVF+ICSI) were performed 1-3 hours after collection of oocytes. Fertilization was confirmed 20 hours after insemination and embryos were cultured until the blastocyst stage. The embryo quality was evaluated morphologically at day 3 and days 5-6. One or two good quality embryos were cryopreserved using a vitrification procedure on day 3 and blastocysts with much better quality than CC grade (Gardner’s classification) were additionally cryopreserved.
Cabergoline 250-500 mg and low-dose aspirin (Bayaspirin 100 mg; Bayer, Osaka, Japan) were used for preventing ascites production and thrombosis after oocyte retrieval. The severity of OHSS was evaluated by abdominal pain, discomfort and the estimated ascites volume. The necessity of hospitalization was assessed by each physician in each case.
Embryo transfer
Single frozen-thawed embryo transfers were conducted with a hormone replacement cycle or ovulation cycle after one or more menstruation cycles from oocyte retrieval. Hormone replacement cycles consisted of estrogen replacement using a transdermal estrogen patch (Estrana® Tapes 0.72 mg; Hisamitsu, Saga, Japan) or conjugated estrogen tablets (PREMARIN®; Pfizer, Tokyo, Japan) from days 3-5, and a sequential progestin vaginal suppository (lutinus; Ferring Pharmaceuticals, Tokyo, Japan). Ovulation cycle embryo transfers were conducted in spontaneous ovulation cycles or in ovarian stimulation cycles with an aromatase inhibitor. A urinary test was performed 2 weeks after embryo transfer to determine pregnancy. Clinical pregnancy was determined according to the appearance of a gestational sac, and hormone replacement therapy was continued until 10 weeks of gestational age.
Statistical analysis
The CS group was compared with the control group. Differences in the patients’ characteristics and clinical outcomes were compared using the Mann-Whitney U test and chi-square test. All statistical comparisons were performed with Ekuseru - Toukei (Ekuseru - Toukei 2012; Social Survey Research Information Co., Ltd., Tokyo, Japan). P values < 0.05 were considered significant.
Results
Thirty patients were diagnosed with PCOS according to the Japanese diagnostic criteria. Sixteen patients were in the CS group and 14 were in the control group. The clinical outcomes in each group are shown in tables 1 and 2.
|
CS group |
Control group |
P* |
|
|
N |
16 |
14 |
|
|
Age (years) |
33.6±3.6 |
35.1±3.4 |
0.276 |
|
AMH (ng/ml) |
11.4±5.0 |
8.9±2.9 |
0.124 |
|
Gravidity |
0.75±1.18 |
0.57±1.09 |
0.717 |
|
Parity |
0.31±1.01 |
0.07±0.27 |
0.604 |
|
Body mass index (kg/m2) |
23.2±4.30 |
23.5±4.12 |
0.803 |
|
Day 3 FSH (mIU/ml) |
6.17±1.37 |
6.38±1.58 |
0.618 |
|
Day 3 LH (mIU/ml) |
7.74±3.64 |
8.02±3.68 |
0.708 |
|
DHEA-s (μg/dl) |
209.1±156.8 |
221.7±89.5 |
0.800 |
|
Testosterone (ng/ml) |
0.38±0.18 |
0.52±0.24 |
0.203 |
|
Total amount of Gn (IU) |
1331.3±242.6 |
1682.1±345.2 |
0.004 |
|
Duration of Gn injection (days) |
7.25±1.06 |
9.14±1.29 |
<0.001 |
|
Total amount of GnRH antagonist (mg) |
0.132±0.155 |
0.58±0.29 |
<0.001 |
|
Duration of GnRH antagonist injection (days) |
0.69±0.79 |
2.43±1.02 |
<0.001 |
|
Duration of Gn coasting (days) |
1.69±1.08 |
0 |
<0.001 |
|
Number of follicles >14 mm |
14.63±5.08 |
11.21±3.02 |
0.036 |
|
Estradiol (pg/ml) on the day of ovulation induction |
10103.0±5132.9 |
4602.4±3436.1 |
0.001 |
|
LH (mIU/ml) on the day of ovulation induction |
3.52±1.78 |
4.07±5.58 |
0.114 |
|
Progesterone (ng/ml) on the day of ovulation induction |
1.24±0.69 |
0.68±0.29 |
0.012 |
Table 1: Characteristics of patients with polycystic ovarian syndrome in both groups.
Values are mean ± standard deviation. *Mann-Whitney U test.
Abbreviations: AMH, Anti-Müllerian Hormone; DHEA-s, Dehydroepiandrosterone Sulfate; FSH, Follicle-Stimulating Hormone; LH, Luteinizing Hormone; Gn, Gonadotropin; GnRH, Gonadotropin-Releasing Hormone; CS, patients with polycystic ovarian syndrome who received coasting.
|
CS group |
Control group |
P* |
|
|
Number of retrieved oocytes |
22.75±8.93 |
15.23±3.83 |
0.011 |
|
Number of oocytes for IVF |
10.25±8.99 |
8.36±5.52 |
0.079 |
|
Number of 2PN in IVF |
6.25±6.40 |
6.8±3.85 |
0.619 |
|
Number of oocytes for ICSI |
12.88±10.91 |
8.71±4.78 |
0.419 |
|
Number of MII oocytes in ICSI |
11.56±9.02 |
6.79±3.96 |
0.091 |
|
Number of 2PN in ICSI |
8.43±7.24 |
4.86±3.21 |
0.054 |
|
Total number of 2PN |
14.69±6.36 |
9.71±3.83 |
0.067 |
|
Number of good quality embryos at day 3 |
9.44±5.60 |
5.93±3.65 |
0.208 |
|
Number of cryopreserved embryos at day 3 |
1.63±0.5 |
1.93±0.27 |
0.054 |
|
Number of prolonged culture embryos |
13.06 |
7.78 |
0.055 |
|
Number of good quality expanded blastocysts (≥3BB) |
4.13±3.32 |
1.36±1.34 |
0.012 |
|
Number of expanded blastocysts (>3CC) |
6.63±4.91 |
2.36±2.53 |
0.016 |
|
Total number of blastocysts |
9.31±6.23 |
5.29±3.24 |
0.084 |
|
Number of cryopreserved blastocysts |
7.25±5.05 |
3.71±2.97 |
0.053 |
|
Total number of cryopreserved embryos |
8.38±4.86 |
5.14±2.77 |
0.082 |
|
Number of patients hospitalized due to severe OHSS |
0 |
0 |
1.000 |
|
Cumulative pregnancy rate, % (n)† |
92.9 (13/14) |
78.6 (11/14) |
0.298 |
|
Live birth rate, %(n)‡ |
92.9 (13/14) |
78.6 (11/14) |
0.298 |
Table 2: Clinical outcomes of assisted reproductive technology treatment in both groups.
Values are mean ± standard deviation. *Mann-Whitney U test.
Abbreviations: CS, patients with polycystic ovarian syndrome who received coasting; IVF, In Vitro Fertilization; 2PN, two Pronuclei; ICSI, Intracytoplasmic Sperm Injection; MII, Metaphase II; OHSS, Ovarian Hyperstimulation Syndrome. BB and CC indicate Gardner classification grades.
†The cumulative pregnancy rate per patient who underwent thawed embryo transfer. Two patients did not undergo embryo transfer for private reasons in the CS group. ‡The live birth rate per patient who underwent thawed embryo transfer.
Comparison between the groups using the Japanese diagnostic criteria
There were no significant differences in the women’s age, anti-Müllerian hormone levels, gravidity, parity, body mass index, and basal hormone levels between the CS and control groups. The mean (SD) coasting duration in the CS group was 1.69 (1.08) days. The dose of gonadotropin, the number of days of gonadotropin injection, the dose of the antagonist, and the number of days of GnRH antagonist injection were significantly lower in the CS group than in the control group (p=0.004, < 0.001, < 0.001, < 0.001). The number of follicles >14 mm, and serum estradiol and progesterone concentrations at the day of the ovulation trigger were significantly higher in the CS group than in the control group.
Figure 1 shows the changes in serum estrogen and LH concentrations at the start and end of coasting according to the duration of coasting. No patients showed a decrease in estradiol values after coasting. Additionally, LH concentrations after coasting in all patients were maintained at >1.0 mIU/ml, which were considered sufficient for the GnRH agonist trigger.
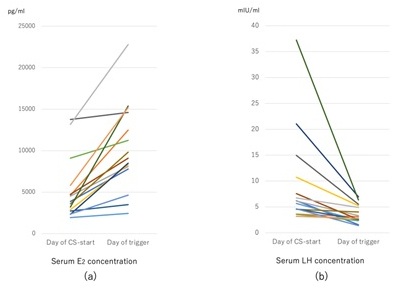 Figure 1: (a) Changes in serum estradiol concentrations in each patient in the coasting group from the day of starting coasting to the day of the ovulation trigger. None of the patients’ estradiol values decreased after 1-4 days of coasting. (b) Changes in serum LH concentrations in each patient in the coasting group from the day of starting coasting to the day of the ovulation trigger. All patients’ LH values decreased after coasting, but they were >1.0 mIU/ml in each patient on the day of the ovulation trigger.
Figure 1: (a) Changes in serum estradiol concentrations in each patient in the coasting group from the day of starting coasting to the day of the ovulation trigger. None of the patients’ estradiol values decreased after 1-4 days of coasting. (b) Changes in serum LH concentrations in each patient in the coasting group from the day of starting coasting to the day of the ovulation trigger. All patients’ LH values decreased after coasting, but they were >1.0 mIU/ml in each patient on the day of the ovulation trigger.
CS, patients with polycystic ovarian syndrome who received coasting; E2, Estradiol; LH, Luteinizing Hormone.
The number of retrieved oocytes was significantly higher in the CS group than in the control group, but the total number of normal fertilized embryos by IVF or ICSI was comparable between the two groups. The number of good quality blastocysts of ≥3BB (Gardner classification) was significantly higher in the CS group than in the control group. The blastocyst formation rate per embryos cultured additionally after day 3 and the rate of high-quality blastocyst formation (≥3BB) were significantly higher in the CS group than in the control group. There was no significant difference in the cumulative pregnancy rate per patient who received subsequent frozen-thawed embryo transfer or the live birth rate between the two groups. No patients required hospitalization for severe OHSS.
Discussion
In this study, we retrospectively investigated ovarian stimulation using a GnRH antagonist protocol with the early coasting method and cabergoline in patients who were hyper-responders and diagnosed with PCOS by the Japanese criteria. This protocol was useful in these women who had an extremely high ovarian reserve and it improved clinical outcomes without the occurrence of severe OHSS. The early coasting protocol reduced the amount and duration of using gonadotropin and a GnRH antagonist, increased the number of retrieved oocytes, and increased the number of high-quality blastocysts compared with the conventional GnRH antagonist protocol. This protocol might improve the cumulative pregnancy rate following embryo transfer cycles in patients with PCOS owing to the large number of good quality embryos.
In the GnRH agonist long protocol, even when coasting is performed in the mid to late follicular phase, the pregnancy and live birth rates are comparable with the conventional long protocol [14,35-38]. However, the rate of OHSS is decreased in the GnRH agonist long protocol, even in pregnant women [35-38]. There have been several controversial reports about the clinical effectiveness of coasting in the GnRH antagonist protocol [33,34]. In the antagonist protocol, serum estrogen concentrations decrease during coasting, but there is little effect on the embryo quality [33]. Benadiva et al., reported that serum gonadotropin concentrations had a sufficient effect on follicular development and serum estrogen level for 1.9±0.9 days (mean±SD), even after the injection was stopped in GnRH agonist protocol [39]. In our method, we started early coasting for 1-4 days in patients who had a relatively high number of large follicles when the leading follicle grew up to 13-15 mm in diameter. In this study, prolonged coasting for 3-4 days did not decrease serum estrogen concentrations. These patients had a sufficient number of oocytes and good quality embryos available for cryopreservation.
In this study, the rate of blastocyst formation per additional cultured embryos was increased in the CS group, especially the rate of high-quality blastocyst formation. Observing large numbers of follicles with ultrasound during ovarian stimulation may strongly indicate a risk of severe OHSS and may lead to premature trigger use without waiting for sufficient follicular development. Insufficient follicular development reportedly leads to oocyte immaturity and a low potential of blastocyst formation [40]. However, coasting may improve embryo quality due to waiting for oocyte maturation during withholding of additional gonadotropin and GnRH antagonist injection without the concern of early-onset severe OHSS occurring.
Cabergoline is usually used to suppress prolactin secretion from the pituitary gland in patients with hyperprolactinemia. Cabergoline also suppresses the Vascular Endothelial Growth Factor (VEGF)/vascular endothelial growth factor receptor pathway by inhibiting vascular endothelial growth factor receptor phosphorylation and reducing ascites retention after ovarian stimulation in the ART cycle [41]. The use of cabergoline is recommended to prevent severe OHSS with significantly reducing the production of ascites after oocyte retrieval [19,20]. Gaafar et al., reported that early administration of cabergoline in the middle follicular phase was useful for preventing the incidence of severe OHSS [18]. In our study, cabergoline was used from the day of coasting for 5-8 days. The use of cabergoline before oocyte retrieval did not adversely affect the embryo quality after oocyte retrieval. As a result, this method resulted in good clinical outcomes in patients without hospitalization for severe OHSS.
This was a retrospective case-control study with a small number of PCOS cases. A randomized, prospective study with a larger sample size is required to show the effectiveness of the coasting method in patients with PCOS with or without the combined use of cabergoline.
Conclusion
In controlled ovarian stimulation of patients with a high ovarian reserve, such as those with PCOS, the early coasting method may be useful in a GnRH antagonist protocol. This protocol may improve embryo quality and clinical outcomes without the occurrence of severe OHSS requiring hospitalization by the early combined use of cabergoline.
Acknowledgment
We thank Ellen Knapp, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Disclosure
Human rights statements and informed consent: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all patients for being included in the study.
Approval By Ethics Committee
This retrospective study was approved by our institutional review board (No. 2019-01).
Conflict of Interest
The authors declare no conflict of interest.
References
- Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, et al. (2016) Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril 106: 6-15.
- Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H (2012) Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod 27: 3067-3073.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81: 19-25.
- Mizunuma H, Irahara M, Kugu K, Takahashi K, Douchi T, et al. (2007) The Committee for Reproductive and Endocrine in Japan Society of Obstetrics and Gynecology. Annual Report (2005-2006) of the Revised Diagnostic Criteria for Polycystic Ovary Syndrome. Acta Obstet Gynaecol Jpn 59: 868-886.
- Franik S, Eltrop SM, Kremer JA, Kiesel L, Farquhar C (2018) Cochrane Gynaecology and Fertility Group. Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome. Cochrane Database Syst Rev 5: CD010287.
- Balen AH, Morley LC, Misso M, Franks S, Legro RS, et al. (2016) The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update 22: 687-708.
- Huang S, Du X, Wang R, Li R, Wang H, et al. (2018) Ovulation induction and intrauterine insemination in infertile women with polycystic ovary syndrome: A comparison of drugs. Eur J Obstet Gynecol Reprod Biol 231: 117-121.
- Weiss NS, Kostova E, Nahuis M, Mol BWJ, van der Veen F, et al. (2019) Gonadotrophins for ovulation induction in women with polycystic ovary syndrome. Cochrane Database Syst Rev 1: CD010290.
- Dale PO, Tanbo T, Abyholm T (1991) In-vitro fertilization in infertile women with the polycystic ovarian syndrome. Hum Reprod 6: 238-241.
- Gadalla MA, Norman RJ, Tay CT, Hiam DS, Melder A, et al. (2020) Medical and surgical treatment of reproductive outcomes in polycystic ovary syndrome: An overview of systematic reviews. Int J Fertil Steril 13: 257-270.
- Bosch E, De Vos M, Humaidan P (2020) The future of cryopreservation in assisted reproductive technologies. Front Endocrinol (Lausanne) 11: 67.
- Mourad S, Brown J, Farquhar C (2017) Interventions for the prevention of OHSS in ART cycles: an overview of Cochrane reviews. Cochrane Database Syst Rev 1: CD012103.
- Krishna D, Dhoble S, Praneesh G, Rathore S, Upadhaya A, et al. (2016) Gonadotropin-releasing hormone agonist trigger is a better alternative than human chorionic gonadotropin in PCOS undergoing IVF cycles for an OHSS free clinic: A randomized control trial. J Hum Reprod Sci 9: 164-172.
- Kailasam C, Griffith H, Wilson P, Gordon U (2018) The effect of early coasting on blastocyst development and outcome following blastocyst transfer in IVF/ICSI programme. JBRA Assist Reprod 22: 301-306.
- Bassiouny YA, Dakhly DMR, Bayoumi YA, Salaheldin NM, Gouda HM, et al. (2018) Randomized trial of combined cabergoline and coasting in preventing ovarian hyperstimulation syndrome during in vitro fertilization/intracytoplasmic sperm injection cycles. Int J Gynaecol Obstet 140: 217-222.
- Duvan ZCI, Kalem MN, Onaran Y, Keskin EA, Ayrim A, et al. (2017) The effect of coasting on intracytoplasmic sperm injection outcome in antagonist and agonist cycle. Int J Fertil Steril 11: 1-6.
- Shrem G, Steiner N, Balayla J, Volodarsky-Perel A, Tannus S, et al. (2019) Use of cabergoline and post-collection GnRH antagonist administration for prevention of ovarian hyperstimulation syndrome. Reprod Biomed Online 39: 433-438.
- Gaafar S, El-Gezary D, El Maghraby HA (2019) Early onset of cabergoline therapy for prophylaxis from Ovarian Hyperstimulation Syndrome (OHSS): A potentially safer and more effective protocol. Reprod Biol 19: 145-148.
- Inoue T, Hashimoto S, Iwahata H, Ito K, Nakaoka Y, et al. (2014) Cabergoline administration prevents development of moderate to severe ovarian hyperstimulation syndrome and it contributes to reduction in ovarian volume. Reprod Med Biol 14: 79-84.
- Alvarez C, Martí-Bonmatí L, Novella-Maestre E, Sanz R, Gómez R, et al. (2007) Dopamine agonist cabergoline reduces hemoconcentration and ascites in hyperstimulated women undergoing assisted reproduction. J Clin Endocrinol Metab 92: 2931-2937.
- Gomez R, Gonzalez-Izquierdo M, Zimmermann RC, Novella-Maestre E, Alonso-Muriel I, et al. (2006) Low-dose dopamine agonist administration blocks Vascular Endothelial Growth Factor (VEGF)-mediated vascular hyperpermeability without altering VEGF receptor 2-dependent luteal angiogenesis in a rat ovarian hyperstimulation model. Endocrinology 147: 5400-5411.
- Mizrachi Y, Horowitz E, Farhi J, Raziel A, Weissman A (2020) Ovarian stimulation for freeze-all IVF cycles: A systematic review. Hum Reprod Update 26: 118-135.
- Shin JJ, Jeong Y, Nho E, Jee BC (2018) Clinical outcomes of frozen embryo transfer cycles after freeze-all policy to prevent ovarian hyperstimulation syndrome. Obstet Gynecol Sci 61: 497-504.
- Griesinger G, Schultz L, Bauer T, Broessner A, Frambach T, et al. (2011) Ovarian hyperstimulation syndrome prevention by gonadotropin-releasing hormone agonist triggering of final oocyte maturation in a gonadotropin-releasing hormone antagonist protocol in combination with a "freeze-all" strategy: A prospective multicentric study. Fertil Steril 95: 2029-2033.
- Polyzos NP, Drakopoulos P, Parra J, Pellicer A, Santos-Ribeiro S, et al. (2018) Cumulative live birth rates according to the number of oocytes retrieved after the first ovarian stimulation for in vitro fertilization/intracytoplasmic sperm injection: a multicenter multinational analysis including ∼15,000 women. Fertil Steril 110: 661-670.
- Li Z, Wang AY, Bowman M, Hammarberg K, Farquhar C, et al. (2019) Cumulative live birth rates following a 'freeze-all' strategy: a population-based study. Hum Reprod Open 2019: hoz004.
- Zhao Z, Shi H, Li J, Zhang Y, Chen C, et al. (2020) Cumulative live birth rates according to the number of oocytes retrieved following the "freeze-all" strategy. Reprod Biol Endocrinol 18: 14.
- Vaughan DA, Leung A, Resetkova N, Ruthazer R, Penzias AS, et al. (2017) How many oocytes are optimal to achieve multiple live births with one stimulation cycle? The one-and-done approach. Fertil Steril 107: 397-404.
- Gutiérrez-Mateo C, Colls P, Sánchez-García J, Escudero T, Prates R, et al. (2011) Validation of microarray comparative genomic hybridization for comprehensive chromosome analysis of embryos. Fertil Steril 95: 953-958.
- Tan Y, Yin X, Zhang S, Jiang H, Tan K, et al. (2014) Clinical outcome of preimplantation genetic diagnosis and screening using next generation sequencing. Gigascience 3: 30.
- Lee KH, Kim SH, Jee BC, Kim YJ, Suh CS, et al. (2010) Comparison of clinical characteristics between early and late patterns in hospitalized patients with ovarian hyperstimulation syndrome. Fertil Steril 93: 2274-2280.
- Egbase PE, Al-Sharhan M, Grudzinskas JG (2002) 'Early coasting' in patients with polycystic ovarian syndrome is consistent with good clinical outcome. Hum Reprod 17: 1212-1216.
- Farhi J, Ben-Haroush A, Lande Y, Sapir O, Pinkas H, et al. (2009) In vitro fertilization cycle outcome after coasting in Gonadotropin-Releasing Hormone (GnRH) agonist versus GnRH antagonist protocols. Fertil Steril 91: 377-382.
- Aboulghar M (2012) Agonist and antagonist coast. Fertil Steril 97: 523-526.
- Ulug U, Ben-Shlomo I, Bahceci M (2004) Predictors of success during the coasting period in high-responder patients undergoing controlled ovarian stimulation for assisted conception. Fertil Steril 82: 338-342.
- Isaza V, García-Velasco JA, Aragonés M, Remohí J, Simón C, et al. (2002) Oocyte and embryo quality after coasting: the experience from oocyte donation. Hum Reprod 17: 1777-1782.
- Kovács P, Mátyás S, Kaali SG (2006) Effect of coasting on cycle outcome during in vitro fertilization/intracytoplasmic sperm injection cycles in hyper-responders. Fertil Steril 85: 913-917.
- Lukaszuk K, Liss J, Jakiel G (2011) "Internal coasting" for prevention of Ovarian Hyperstimulation Syndrome (OHSS) in IVF/ICSI. Ginekol Pol 82: 812-816.
- Benadiva CA, Davis O, Kligman I, Moomjy M, Liu HC, et al. (1997) Withholding gonadotropin administration is an effective alternative for the prevention of ovarian hyperstimulation syndrome. Fertil Steril 67: 724-727.
- McCulloh DH, Kutchukhidze N, Charkviani T, Zhorzholadze T, Barbakadze T, et al. (2020) Follicle size indicates oocyte maturity and blastocyst formation but not blastocyst euploidy following controlled ovarian hyperstimulation of oocyte donors. Hum Reprod 35: 545-556.
- Gómez R, Simón C, Remohí J, Pellicer A (2002) Vascular endothelial growth factor receptor-2 activation induces vascular permeability in hyperstimulated rats, and this effect is prevented by receptor blockade. Endocrinology 143: 4339-4348.
Citation: Nakashima A, Tokunaga Y, Hayata K, Yamashiro K, Terada Y, et al. (2021) The Early Coasting Method during Ovarian Stimulation in Antagonist Protocols with Combined Use of Cabergoline is Useful in Patients with Polycystic Ovary Syndrome. J Reprod Med Gynecol Obstet 6: 087.
Copyright: © 2021 Akira Nakashima, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

