
The Effect of Cyclic Pressure on Integrin β1-Hippo/YAP Signaling Pathway
*Corresponding Author(s):
Zhanying TangDepartment Of Rehabilitative Medicine, Longhua Hospital Affiliated To Shanghai University Of Traditional Chinese Medicine, Xuhui, Shanghai, China
Tel:+86 02151322222,
Fax:+86 02151322459
Email:tzy79@126.com
Abstract
Objectives: To clarify the mechanism of traditional Chinese medicine treatment of Knee Osteoarthritis (KOA), this study would further study the changes of integrin and Hippo/YAP signaling pathways.
Methods: The study of differential expression proteomics of chondrocytes after mechanical stimulation intervention was to explore to clarify the mechanism of KOA.
Results: The proteomic analysis identified 1697 proteins, and there were 333 up-regulated and 194 down-regulated differentially expressed proteins in pressure group and normal group. The protein level in hippo signaling pathway implied that ITG-β1, CYR61, MOB1and Smad2 were differentially expressed proteins in the three groups. The expression of ITG-β1 mRNA and protein in chondrocytes and cartilage was significantly up-regulated after cyclic stress or the intervention of reinforcement manipulation. Compared with the model group, the expression of YAP1 protein, mRNA and TEAD mRNA were significantly increased in the pressure group, and the expression of YAP1 protein, mRNA and TEAD mRNA were significantly increased in the manipulation group. Logistic linear regression analysis in chondrocytes and cartilage showed that there was a significant correlation between ITG-β1 and MST1 gene expression, and a significant correlation between ITG-β1 and YAP gene and protein expression.
Conclusion: The manipulation of regulating tendons could delay the degeneration of chondrocytes and that mechanical stimulation to chondrocytes was partly mediated by ITG-β1 involved in the classical Hippo/YAP signaling pathway, and there were other unknown signaling pathways involved in the regulation of YAP protein by ITG-β1.
Keywords
Cyclic Pressure; Integrin β1; Hippo/Yap Pathway; Knee Osteoarthritis; Regulating Tendon Manipulation
Introduction
Knee Osteoarthritis (KOA) is a common disease caused by multiple factors, which seriously affects the health and quality of life of elderly people [1,2]. With the increasing age, the incidence of the disease is on the rise [3,4]. Epidemiological research results show that the incidence rate of KOA reaches 50% for the middle-aged and elderly over 60 years old, while the incidence rate of KOA rises to 80% for those over 75 years old [5]. At present, the prevention and rehabilitation of KOA has become a worldwide problem [6,7]. The pathogenesis of KOA is caused by the imbalance of synthesis and degradation of chondrocytes, extracellular matrix and subchondral due to the interaction and joint action of two factors of knee joint mechanics and biology. Many basic and clinical studies have been carried out. Integrin, as a transmembrane protein, is mainly located on the cartilage cell membrane [8,9]. Its main function is to mediate the mechanical stimulation signals from outside the cell to enter the cell, and then regulate the signal pathway changes in the cell, thus regulating the metabolism of cartilage cells. Therefore, integrin-related signal pathways have been widely concerned as a hot topic in many researches.
Traditional Chinese medicine manipulation is one of the basic methods for the treatment of osteoarthritis, and has its unique features in the treatment of knee osteoarthritis [10,11]. It is based on the principles of relaxing muscles and tendons, dredging collaterals, promoting blood circulation, relieving pain, releasing adhesion and smoothing joints, promoting blood circulation around joints, improving cell metabolism, promoting chondrocyte proliferation and inhibiting cell apoptosis, thus achieving the aims of relieving inflammation, loosening adhesion and improving joint function. The previous research preliminarily confirmed that the Flexcell system was applied to simulate the different degrees of periodic pressure, and it could promote or inhibit chondrocyte proliferation through the regulation of integrin [12]. The hippo signaling pathway was one of the highly conserved signal pathways in mammals [13,14]. Transcription factor TAZ/YAP played an important biological function in regulating cell growth, proliferation and apoptosis [15,16]. Current research has preliminarily confirmed that the hippo signaling pathway not only played a role in maintaining the internal environment stability and tumor development, but also played an important role in signal transduction of mechanical stimulation.
Chondrocyte degeneration is the basis of the pathogenesis of KOA, and the mechanism of mechanical factors in the degeneration of chondrocytes has been a hot topic. To clarify the mechanism of traditional Chinese medicine treatment of KOA, based on the study of differential expression proteomics of chondrocytes after mechanical stimulation intervention, this study would further study the changes of integrin and Hippo/YAP signaling pathways closely related to mechanical stimulation, and the changes of these signaling pathways after intervention by rational manipulation.
Materials And Methods
- Samples
The chondrocytes derived from the two-week-old male SD rats were randomly divided into normal group, model group and pressure group. The chondrocytes in normal group were cultured under normal pressure and environment. The chondrocytes in model group were cultured in the medium with TNF-α reagent to simulate the OA inflammatory environment under the condition of normal pressure. The chondrocytes in pressure group were stimulated by TNF-α to simulate the inflammatory environment of OA in the medium, and the Flexcell-5000 cells were loaded with the tensile stress loading system of 172 KPa, and the cyclic compressive stress of 2Hz was given for 3 days continuously for 60 min/d.
- Proteomics
Total protein was subjected to proteomic analysis. Tryptic peptides from each sample (200 ug) were injected with an EASY-nLC1000 HPLC (Thermo Fisher Scientific), which was equipped with an EASY column SC200 150 um *100 mm (RP-C18), trapped at 300 μL/min on a Thermo column. The mass spectrometer was operated in a data-dependent mode. Full-scan MS spectra were obtained in an quadrupole Orbitrap mass spectrometer (Q Exactive) at a mass resolution of 70,000 (mass range 300-1800 m/z). For label-free analysis, the ten most abundant tandem mass spectrum peaks were obtained in a linear ion trap by peptide cleavage. The isolation width was 2 m/z. Proteins were identified using Maxquant software (1.5.5.1). The Raw file was imported and used to search in the UniProt KB /Swiss-Prot database (36088 sequences, version 2018.06.14).
- Proteomics data analysis
For database searches, the peptide mass tolerances were set to 20 ppm. The following parameters were used: trypsin as enzyme specificity; maximum two missed cleavage permitted; variable modifications: Cys carbamidomethylation, Oxidation (M); precursor ion mass tolerance of 20 ppm. Peptides with a false positive rate ≤ 0.01 were selected. Proteins that met the following criteria were differentially expressed proteins: mean change in protein ≥ 1.5 or ≤ 0.667 (Student's t-test, p < 0.05). GO and KEGG pathway enrichment analysis of differentially expressed proteins were performed.
- TUNEL and ELISA assays
The chondrocytes were detected by using a TUNEL kit (Boster, Wuhan, China) according to the manufacturer’s instructions. The chondrocytes were collected, and Bcl-2 and Bax was quantified by using a commercially available ELISA kit (eBioscience) according to the manufacturer’s instructions.
- RT-PCR
Total RNA of chondrocytes was isolated using Trizol (Invitrogen, Carlsbad, USA), and was reverse-transcribed into cDNA using a Super RT cDNA kit. The SYBR green Realtime PCR Master Mix was used for RT-qPCR amplification. The primers used for PCR are shown in table 1. The cycling conditions were 95°C for 5 min, followed by 35 cycles of 90°C for 10 s, 50°C for 35 s, and 72°C for 30 s, and 72°C for 5 min. The RT-qPCR analysis was performed with the Light Cycler 480 RT-qPCR System (Roche, Basel, Switzerland). Fold-changes in gene expression were estimated using the CT comparative method normalizing GAPDH CT values and relative to control samples as follows: ΔCt=Ct (assayed samples)-Ct(β-actin); ΔΔCt=ΔCt-ΔCt control; fold difference = 2-(ΔΔCT).
|
Genes |
Forward (5’–3’) |
Reverse (5’–3’) |
|
MST1 |
GCCTTCCACTACAATATGAGCAGC |
ATCAGCTGTCCTGGCCACAGTG |
|
YAP |
CCACCCCTGCTGCTCAACAT |
TTGGGAGAGCATGGCCTTCC |
|
TAED |
GCCCTGTGGGAGGAGGAAAA |
CACGAGATTTCCTTCTGGCAAG |
|
ITGB1 |
ATTCAAGAGGGCTGAAGACTACCC |
GCCAAAGCCAATGCGGAAG |
|
GAPDH |
AATGGATTTGGACGCATTGGT |
TTGCACTGGTACGTGTTGAT |
Table 1: Primers used for quantitative RT-PCR.
- Western Blot Analysis
The total protein was extracted with lysis buffer, and 50 mg protein was resolved on a 5% sodium dodecyl sulfate polyacrylamide gel. The fractionated proteins were electrophoretically transferred to an immobilon polyvinylidene difluoride membrane and probed with antibodies of GAPDH, YAP1 and ITGβ1 (ProteinTech Group, Chicago, IL, USA).
- Statistical analysis
SPSS 18.0 was used for statistical analysis. All data were expressed as means ± SD. Statistical analysis was performed using one-way analysis of variance, and followed by the Wilcoxon test for comparison of two groups. Statistical significance was considered at P < 0.05.
Results
- Protein analysis of chondrocytes
The proteomic analysis identified 7874 peptides and 1697 proteins. Among all the identified proteins, there were 81 up-regulated and 128 down-regulated differentially expressed proteins in the model group and the normal group, and there were 333 up-regulated and 194 down-regulated differentially expressed proteins in the pressure group and the normal group. After that, the function and pathway annotation of differentially expressed proteins were performed (Figure 1). In the model group and the normal group, the differentially expressed proteins were enriched in RNA processing, mRNA metabolic process, mRNA processing, RNA splicing, mRNA splicing, via spliceosome, intracellular part, membrane-bounded organelle, intracellular, organelle, intracellular organelle part, RNA binding, poly(A) RNA binding, organic cyclic compound binding, binding and heterocyclic compound binding (Figure 1A). Meanwhile, the differentially expressed proteins were enriched in those pathways, such as Spliceosome, Valine, leucine and isoleucine degradation, Proteasome, Fatty acid degradation and Adherens junction (Figure 1B).
In the pressure group and the normal group, the differentially expressed proteins were enriched in organic substance metabolic process, cellular metabolic process, cellular component organization or biogenesis, macromolecular complex subunit organization, metabolic process, intracellular part, cytoplasm, intracellular, intracellular organelle, cytoplasmic part, poly(A) RNA binding, RNA binding, protein binding, binding and nucleotide binding (Figure 1C). Meanwhile, the differentially expressed proteins were enriched in those pathways, such as spliceosome, carbon metabolism, citrate cycle (TCA cycle), pyruvate metabolism, RNA transport, proteasome, Glycolysis / Gluconeogenesis, valine, leucine and isoleucine degradation, fatty acid degradation, biosynthesis of amino acids, protein processing in endoplasmic reticulum, fatty acid metabolism, mRNA surveillance pathway, propanoate metabolism, regulation of actin cytoskeleton, tryptophan metabolism, metabolic pathways, endocytosis, glyoxylate and dicarboxylate metabolism, pentose phosphate pathway, bacterial invasion of epithelial cells, 2-Oxocarboxylic acid metabolism, oocyte meiosis, legionellosis, adherens junction, hippo signaling pathway, insulin signaling pathway, synaptic vesicle cycle, long-term potentiation and central carbon metabolism in cancer (Figure 1D).
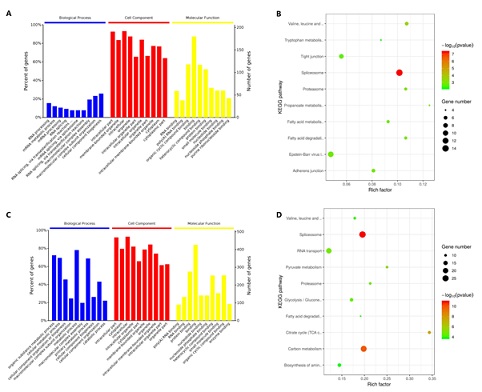 Figure 1: The results of the GO and pathway enrichment of differentially expressed proteins. A: the GO enrichment results of differentially expressed proteins in model group and normal group. B: the pathways enrichment results of differentially expressed proteins in model group and normal group. C: the GO enrichment results of differentially expressed proteins in pressure group and normal group. D: the pathways enrichment results of differentially expressed proteins in pressure group and normal group.
Figure 1: The results of the GO and pathway enrichment of differentially expressed proteins. A: the GO enrichment results of differentially expressed proteins in model group and normal group. B: the pathways enrichment results of differentially expressed proteins in model group and normal group. C: the GO enrichment results of differentially expressed proteins in pressure group and normal group. D: the pathways enrichment results of differentially expressed proteins in pressure group and normal group.
The previous research preliminarily confirmed that the Flexcell system was applied to simulate the different degrees of periodic pressure, and it could promote or inhibit chondrocyte proliferation through the regulation of integrin. The hippo signaling pathway was one of the highly conserved signal pathways in mammals. Transcription factor TAZ/YAP played an important biological function in regulating cell growth, proliferation and apoptosis. Current research has preliminarily confirmed that the hippo signaling pathway not only played a role in maintaining the internal environment stability and tumor development, but also played an important role in signal transduction of mechanical stimulation. In this study, the protein level in hippo signaling pathway in the normal group, the model group and the pressure group was shown in figure 2, implying that ITG-β1, CYR61, MOB1and Smad2 were differentially expressed proteins in the three groups.
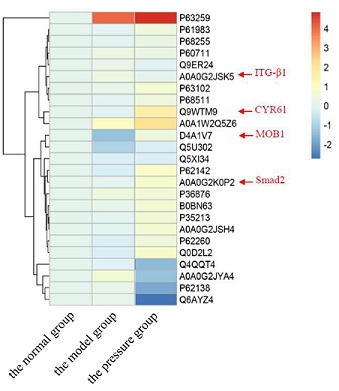 Figure 2: Heatmap based on the protein level in hippo signaling pathway in three groups. The colour from blue to red showed the protein level from high to low.
Figure 2: Heatmap based on the protein level in hippo signaling pathway in three groups. The colour from blue to red showed the protein level from high to low.
- The effect of cyclic stress on integrin β1-Hippo/YAP signaling pathway in chondrocytes
It was to observe the effects of cyclic compressive stress on the expression of ITG-β1 and Hippo signaling pathway related proteins in KOA chondrocytes. The apoptosis of chondrocytes in the model group was significantly lower than that in the pressure group (P < 0.01) (Table 2). Compared with the normal group, the expression of apoptosis-related protein Bcl-2 in the model group was significantly lower and the expression of Bax was significantly higher (P < 0.01) (Table 3). Compared with the model group, the expression of apoptosis-related protein Bcl-2 in pressure group was significantly higher than that in model group (P < 0.05), while the expression of Bax was decreased (P < 0.05) (Table 3). The expression of ITG-β1 mRNA in chondrocytes of model group was significantly lower than that of normal cartilage (P < 0.01) (Figure 3A). The expression of ITG-β1 mRNA and protein in chondrocytes was up-regulated after cyclic stress (P < 0.01 and P < 0.05) (Figure 3A and 3F). The expression of downstream transcription coactivator YAP1 and TEAD mRNA in the Hippo/YAP signaling pathway of chondrocytes in the model group was significantly lower than that in normal cartilage (P < 0.01) (Figure 3C and 3D). Compared with the model group, In the pressure group the expression of YAP1 protein, mRNA and TEAD mRNA were significantly increased (P < 0.01), but the expression of MST1 mRNA was not significantly increased (P > 0.05) (Figure 3). Logistic linear regression analysis showed that there was a significant correlation between ITG-β1 and MST1 gene expression (r = 0.864, P < 0.01). There was a significant correlation between ITG-β1 and YAP gene and protein expression (r = 0.779 and r = 0.851, P < 0.01).
|
Groups |
N |
Apoptosis rate (%) |
|
Normal group |
4 |
6.13 ± 0.01 |
|
Model group |
4 |
23.98 ± 0.10** |
|
Pressure group |
4 |
8.56 ± 0.05**## |
Table 2: Comparison of apoptosis rate of chondrocytes in each group.
Note: * represented p < 0.05 compared with the normal group; ** represented p < 0.01 compared with the normal group; # represented p < 0.05 compared with the model group; ## represented p < 0.01 compared with the model group.
|
Groups |
N |
Bcl-2 (ng/ml) |
Bax (ng/ml) |
|
Normal group |
4 |
5.86 ± 0.24 |
1.67 ± 0.03 |
|
Model group |
4 |
2.13 ± 0.17** |
3.92 ± 0.16** |
|
Pressure group |
4 |
4.15 ± 0.21**## |
2.41 ± 0.09**## |
Table 3: Apoptosis-related protein (Bcl-2 and Bax) level of chondrocytes in each group.
Note: * represented p < 0.05 compared with the normal group; ** represented p < 0.01 compared with the normal group; # represented p < 0.05 compared with the model group; ## represented p < 0.01 compared with the model group.
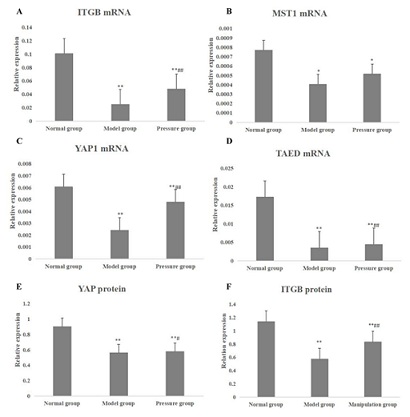 Figure 3: The mRNAs and proteins in Hippo/YAP pathway of chondrocytes in each group. A: the ITGB1 mRNA expression in normal group, model group and pressure group; B: the MST1 mRNA expression in normal group, model group and pressure group; C: the YAP1 mRNA expression in normal group, model group and pressure group; D: the TAED mRNA expression in normal group, model group and pressure group; E: the YAP protein expression in normal group, model group and pressure group; F: the ITGB1 protein expression in normal group, model group and pressure group. * represented p < 0.05 compared with the normal group; ** represented p < 0.01 compared with the normal group; # represented p < 0.05 compared with the model group; ## represented p < 0.01 compared with the model group.
Figure 3: The mRNAs and proteins in Hippo/YAP pathway of chondrocytes in each group. A: the ITGB1 mRNA expression in normal group, model group and pressure group; B: the MST1 mRNA expression in normal group, model group and pressure group; C: the YAP1 mRNA expression in normal group, model group and pressure group; D: the TAED mRNA expression in normal group, model group and pressure group; E: the YAP protein expression in normal group, model group and pressure group; F: the ITGB1 protein expression in normal group, model group and pressure group. * represented p < 0.05 compared with the normal group; ** represented p < 0.01 compared with the normal group; # represented p < 0.05 compared with the model group; ## represented p < 0.01 compared with the model group.
- Effect of regulating tendon manipulation on the signal pathway of integrin-Hippo/YAP in knee joint of KOA rat model
SPF grade SD rats were randomly divided into normal group, model group and manipulation group with 10 rats in each group. KOA model was established by medial collateral ligament resection combined with partial patellar ligament resection in rats. After 4 weeks of modeling, the rats in the manipulation group were treated with manipulation intervention for 7 minutes each time, once every other day, for 6 weeks. No intervention in normal group and model group. HE staining results showed that in the normal group, the cartilage of knee joint was smooth, the chondrocytes were spindle-shaped, horizontal arrangement, and the structure of tidal line was clear and complete (Figure 4). In the model group, there was moderate wear on the articular cartilage surface and the loss of chondrocytes was obvious in the model group. In manipulation group, the cartilage surface of knee joint was slightly worn, the morphology of chondrocytes was close to normal, and the phenomenon of chondrocyte clustering was less. The Mankin score of the model group was significantly higher than that of the normal group (P < 0.01) (Table 4). After manual therapy, the Mankin score of the manipulation group was significantly lower than that of the model group (P < 0.01), but it was still higher than that of the normal group (P < 0.01) (Table 4). There was a significant difference between the model group and the normal group (P < 0.01), and the apoptosis rate of chondrocytes in the manipulation group was significantly different from that in the model group (P < 0.05) (Table 5).
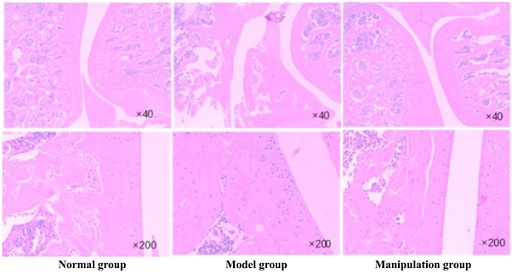 Figure 4: HE staining results of the cartilage of knee joint in the normal group, model group and manipulation group.
Figure 4: HE staining results of the cartilage of knee joint in the normal group, model group and manipulation group.
|
Groups |
N |
Mankin score |
|
Normal group |
6 |
0.91 ± 0.27 |
|
Model group |
6 |
4.24 ± 0.83** |
|
Manipulation group |
6 |
2.57 ± 0.54**## |
Table 4: Comparison of Mankin score of knee joint of rats in each group.
Note: * represented p < 0.05 compared with the normal group; ** represented p < 0.01 compared with the normal group; # represented p < 0.05 compared with the model group; ## represented p < 0.01 compared with the model group.
|
Groups |
N |
Apoptosis rate (%) |
|
Normal group |
6 |
1.70 ± 0.01 |
|
Model group |
6 |
11.00 ± 0.03** |
|
Manipulation group |
6 |
7.56 ± 0.01**# |
Table 5: Comparison of apoptosis rate of of knee joint of rats in each group.
Note: * represented p < 0.05 compared with the normal group; ** represented p < 0.01 compared with the normal group; # represented p < 0.05 compared with the model group; ## represented p < 0.01 compared with the model group.
The expression of ITG-β1 mRNA in the cartilage of the model group was significantly lower than that of the normal group (P < 0.01) (Figure 5A). The expression of ITG-β1 mRNA and protein in the cartilage of the model group was obviously up-regulated after the intervention of reinforcement manipulation, compared with the model group (P < 0.01) (Figure 5A and 5E). RT-PCR and Western blot studies showed that the expression of MST1, YAP1 and TEAD mRNA and YAP1 proteins in the Hippo/YAP signaling pathway of cartilage in the model group were significantly lower than those in the normal control group (P < 0.01) (Figure 5). Compared with the model group, the expression of YAP1 protein, mRNA and TEAD mRNA were significantly increased in the manipulation group (P < 0.01), but the expression of MST1mRNA was not significantly increased (P > 0.05) (Figure 5). Logistic linear regression analysis showed that there was a significant correlation between ITG-β1 and MST1 gene expression (r = 0.748, P < 0.01). There was a significant correlation between ITG-β1 and YAP gene and protein expression (r = 0.926 and r = 0.858, P < 0.01).
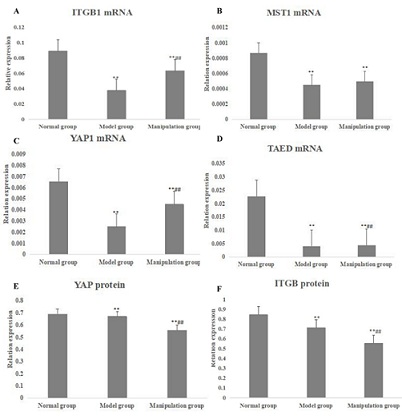 Figure 5: The mRNAs and proteins in Hippo/YAP pathway of knee joint of rats in each group. A: the ITGB1 mRNA expression in normal group, model group and manipulation group; B: the MST1 mRNA expression in normal group, model group and manipulation group; C: the YAP1 mRNA expression in normal group, model group and manipulation group; D: the TAED mRNA expression in normal group, model group and manipulation group; E: the YAP protein expression in normal group, model group and manipulation group; F: the ITGB1 protein expression in normal group, model group and manipulation group. * represented p < 0.05 compared with the normal group; ** represented p < 0.01 compared with the normal group; # represented p < 0.05 compared with the model group; ## represented p < 0.01 compared with the model group.
Figure 5: The mRNAs and proteins in Hippo/YAP pathway of knee joint of rats in each group. A: the ITGB1 mRNA expression in normal group, model group and manipulation group; B: the MST1 mRNA expression in normal group, model group and manipulation group; C: the YAP1 mRNA expression in normal group, model group and manipulation group; D: the TAED mRNA expression in normal group, model group and manipulation group; E: the YAP protein expression in normal group, model group and manipulation group; F: the ITGB1 protein expression in normal group, model group and manipulation group. * represented p < 0.05 compared with the normal group; ** represented p < 0.01 compared with the normal group; # represented p < 0.05 compared with the model group; ## represented p < 0.01 compared with the model group.
Discussion
Chondrocyte degeneration is the basis of the pathogenesis of KOA, and the mechanism of mechanical factors in the degeneration of chondrocytes has been a hot topic [17,18]. To clarify the mechanism of traditional Chinese medicine treatment of KOA, based on the study of differential expression proteomics of chondrocytes after mechanical stimulation intervention, this study would further study the changes of integrin and Hippo/YAP signaling pathways closely related to mechanical stimulation, and the changes of these signaling pathways after intervention by rational manipulation. The proteomic analysis identified 1697 proteins, and there were 333 up-regulated and 194 down-regulated differentially expressed proteins in pressure group and normal group. The protein level in hippo signaling pathway in the normal group, the model group and the pressure group implied that ITG-β1, CYR61, MOB1 and Smad2 were differentially expressed proteins in the three groups. MOB1, an important protein in Hippo signaling pathway [19,20], was significantly down-regulated in model group, while the protein expression was significantly up-regulated after periodic intervention. MOB1 protein is an important adaptor protein of Hippo signal pathway core kinase cascade reaction chain proteins MST1/2 and LAST1/2 [21,22], which can be combined with MST1/2 and LAST1/2 to promote phosphorylation of the above proteins [23,24]. At the same time, the clustering analysis results of Hippo differentially expressed proteins can also found that the target genes Cyr61 and Smad2 of downstream transcription coactivator TEAD in Hippo signaling pathway [25,26] had significant changed, especially after periodic pressure intervention [27,28]. The expressions of Cyr 61 and Smad 2, which were the target genes of TEAD [29,30], had a significant up-regulated trend.
It was to observe the effects of cyclic compressive stress on the expression of ITG-β1 and Hippo signaling pathway related proteins in KOA chondrocytes [31,32]. The expression of ITG-β1 mRNA and protein in chondrocytes and cartilage was significantly up-regulated after cyclic stress or the intervention of reinforcement manipulation. Compared with the model group, the expression of YAP1 protein, mRNA and TEAD mRNA were significantly increased in the pressure group, and the expression of YAP1 protein, mRNA and TEAD mRNA were significantly increased in the manipulation group. Logistic linear regression analysis in chondrocytes and cartilage showed that there was a significant correlation between ITG-β1 and MST1 gene expression, and a significant correlation between ITG-β1 and YAP gene and protein expression. The manipulation of regulating tendons could delay the degeneration of chondrocytes and that mechanical stimulation to chondrocytes was partly mediated by ITG-β1 involved in the classical Hippo/YAP signaling pathway, and there were other unknown signaling pathways involved in the regulation of YAP protein by ITG-β1.
Funding Statement
- This study is supported by 1.Scientific research plan of Shanghai Municipal Health Commission, No: 202040240
- Shanghai Jinshan expert workstation construction project, No.: jszjz2021017y
- Three year action plan no (ZY (2021-2023) - 0201-01) for Shanghai to further accelerate the inheritance, innovation and development of traditional Chinese medicine
- Original exploration project of Shanghai Science and Technology Commission, No: 20zr147340
References
- Xing D, Ma XL, Ma JX, Xu WG, Wang J, et al. (2013) Association between aspartic acid repeat polymorphism of the asporin gene and susceptibility to knee osteoarthritis: a genetic meta-analysis. 21: 1700-1706.
- Dell'Isola A, Allan R, Smith SL, Marreiros SS, Steultjens M (2016) 185 Identification of Clinical Phenotypes in Knee Osteoarthritis: A Systematic Review of the Literature. BMC Musculoskelet Disord 17: 425.
- Spira AP, Runko VT, Finan PH, Kaufmann CN, Bounds SC, et al. (2014) Circadian Rest/Activity Rhythms in Knee Osteoarthritis with Insomnia: A Study of Osteoarthritis Patients and Pain-Free Controls with Insomnia or Normal Sleep. Chronobiol Int 32: 242-247.
- Qiu L, Kan JW, Zheng X, Zhang M, Zhang J (2013) Observation on the long-term efficacy of knee osteoarthritis treated with warm needling and rehabilitation training. Zhongguo Zhen Jiu 33: 199-202.
- Peat G, McCarney R, Croft P (2001) Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis 60: 91-97.
- Fazaa A, Souabni L, Abdelghani KB, Kassab S, Chekili S, et al. (2014) Comparison of the clinical effectiveness of thermal cure and rehabilitation in knee osteoarthritis. A randomized therapeutic trial. Ann Phys Rehabil Med 57: 561-569.
- Rabago D, Nourani B (2016) Prolotherapy for Knee Osteoarthritis: A Descriptive Review. Curr Rheumatol Rep 4: 42-49.
- Shakibaei M, Merker HJ (1999) Research, β1-Integrins in the cartilage matrix. Cell Tissue Res 296: 565-573.
- Enomoto-Iwamoto M, Iwamoto M, Nakashima K, Mukudai Y, Boettiger D, et al. (2010) Involvement of α5β1 Integrin in Matrix Interactions and Proliferation of Chondrocytes. J Bone Miner Res 12: 1124-1132.
- Yan H, Su Y, Chen L, Zheng G, Lin X, et al. (2013) Rehabilitation for the management of knee osteoarthritis using comprehensive traditional Chinese medicine in community health centers: study protocol for a randomized controlled trial. Trials 14: 1-10.
- Yang M, Jiang L, Wang Q, Chen H, Xu G, et al. (2017) Traditional Chinese medicine for knee osteoarthritis: An overview of systematic review. PLoS One 12: 0189884.
- Gilbert H (2011) The response of human annulus fibrosus cells to cyclic tensile strain : evidence for an altered mechanotransduction pathway with intervertebral disc degeneration. The University of Manchester, UK.
- Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, et al. (2009) S21-03. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. 16: 398-410.
- Pan D (2010) The Hippo Signaling Pathway in Development and Cancer. Dev Cell 19: 491-505.
- Machado-Neto JA, Lazarini M, Favaro P, Franchi GC, Nowill AE, et al. (2014) ANKHD1, a novel component of the Hippo signaling pathway, promotes YAP1 activation and cell cycle progression in prostate cancer cells. Exp Cell Res 324: 137-145.
- Men T, Piao SH, Teng CB (2013) Regulation of differentiation of mesenchymal stem cells by the Hippo pathway effectors TAZ/YAP. Yi Chuan 35: 1283-1290.
- Wang X, Tang D, Shen P, Xu H, Qiu H, et al. (2017) Analysis of DNA methylation in chondrocytes in rats with knee osteoarthritis. BMC Musculoskelet Disord 18: 377.
- Guangwen WU (2017) Effect of Electroacupuncture on Chondrocyte Apoptosis and Cartilage Matrix of Experimental Rats with Knee Osteoarthritis. 27: 22.
- Sugimachi K, Nishio M, Aishima S, Kuroda Y, Iguchi T, et al. (2017) Altered Expression of Hippo Signaling Pathway Molecules in Intrahepatic Cholangiocarcinoma Oncology 93: 67-74.
- Grijalva JL, Huizenga M, Mueller K, Rodriguez S, Brazzo J, et al. (2014) Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. 307: 196-204.
- Couzens AL, Xiong S, Knight JDR, Mao DY, Guettler S (2017) MOB1 Mediated Phospho-recognition in the Core Mammalian Hippo Pathway*. Mol Cell Proteomics 16: 1098-1110.
- Lignitto L, Arcella A, Sepe M, Rinaldi L, Donne RD, et al. (2013) Proteolysis of MOB1 by the ubiquitin ligase praja2 attenuates Hippo signalling and supports glioblastoma growth. Nat Commun 4: 1822.
- Yang W, Han W, Qin A, Wang Z, Xu J, et al. (2018) The emerging role of Hippo signaling pathway in regulating osteoclast formation. J Cell Physiol 233: 4606-4617.
- Kulaberoglu Y, Lin K, Holder M, Gai Z, Gomez M, et al. (2017) Stable MOB1 interaction with Hippo/MST is not essential for development and tissue growth control. Nat Commun 8: 695.
- Lai D, Ho KC, Hao Y, Yang X (2011) Taxol Resistance in Breast Cancer Cells Is Mediated by the Hippo Pathway Component TAZ and Its Downstream Transcriptional Targets Cyr61 and CTGF. 71: 2728.
- Kim M, Kim T, Johnson RL, Lim DS (2015) Transcriptional Co-repressor Function of the Hippo Pathway Transducers YAP and TAZ. Cell Rep 11: 270.
- Shathasivam P, Kollara A, Ringuette MJ, Virtanen C, Wrana JL, et al. (2015) Human ortholog of Drosophila Melted impedes SMAD2 release from TGF-β receptor I to inhibit TGF-β signaling. Proc Natl Acad Sci USA 112: 3000-3009.
- Grannas K, Arngården L, Lönn P, Mazurkiewicz M, Blokzijl A, et al. (2015) Crosstalk between Hippo and TGFβ: Subcellular Localization of YAP/TAZ/Smad Complexes. J Mol Biol 427: 3407-3415.
- Chen L, Loh PG, Song H (2010) Structural and functional insights into the TEAD-YAP complex in the Hippo signaling pathway. Protein Cell 1: 1073.
- Qu Y, Zong L, Xu M, Dong Y, Lu L (2014) Effects of 18α-glycyrrhizin on TGF-β1/Smad signaling pathway in rats with carbon tetrachloride-induced liver fibrosis. Int J Clin Exp Pathol 8: 1292-1301.
- Liang CX, Guo Y, Tao L, Xiao H, Liu QG, et al. (2015) Effects of acupotomy intervention on regional pathological changes and expression of carti- lage-mechanics related proteins in rabbits with knee osteoarthritis. Zhen Ci Yan Jiu 40: 119-124.
- Yang B, Sun H, Song F, Yu M, Wu Y, et al. (2017) YAP1 negatively regulates chondrocyte differentiation partly by activating the β-catenin signaling pathway. Int J Biochem Cell Biol 87: 104-113.
Citation: Li X, Zhang H, Tang Z, Xiao J, Cui JW, et al. (2023) The Effect of Cyclic Pressure on Integrin β 1-Hippo/YAP Signaling Pathway. J Altern Complement Integr Med 9: 429.
Copyright: © 2023 Xia Li, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

