
The Effect of Kinetic Oscillation Stimulation on Symptoms of Non-allergic Rhinitis: A Per-protocol Analysis of a Randomized Controlled Trial
*Corresponding Author(s):
Wytske J FokkensDepartment Of Otorhinolaryngology, Amsterdam UMC, Location Academic Medical Centre, Meibergdreef 9, 1105 AZ Amsterdam, Netherlands
Tel:+31 205666490,
Email:w.j.fokkens@amsterdamumc.nl
Abstract
Background: Non-allergic Rhinitis (NAR) afflicts a broad group of patients and lacks sufficient treatment options. Kinetic Oscillation Stimulation (KOS) of the nasal cavity might improve symptoms in NAR.
Objective: To evaluate the change from baseline in weekly mean Total Vasomotor Rhinitis Symptoms and Medication Score (TVRSMS) at week 4 after the treatment between KOS treatment compared to placebo.
Methods: Data from a previously published paper on KOS treatment in NAR patients were analyzed through a per-protocol analysis which included 124 subjects (64 treated, 60 placebo). The TVRSMS after 4 weeks was the primary outcome measure. A subgroup-analysis was performed based on Local Vasoconstrictor Abuse (LVA).
Results: KOS resulted in a significant reduction of TVRSMS after 4 weeks compared to placebo with change in baseline of -0.8 (p<0.05). In the subgroup analysis, stopping local vasoconstrictor abuse showed a significant reduction in TVRSMS (change from baseline: -1.1) which was additive to the KOS effect (change from baseline in those stopping LVA + KOS: -1.6). A mixed model analysis showed that KOS treatment significantly reduced TVRSMS in the NAR population, though the effect of LVA did not reach significance.
Conclusion: A single KOS treatment appears to reduce TVRSMS compared to placebo after 4 weeks. Stopping local vasoconstrictor abuse has a comparable effect. Future double-blind studies should be performed to ascertain these findings.
Keywords
Idiopathic rhinitis; Kinetic oscillation stimulation; Non-allergic rhinitis; Rhinitis medicamentosa; Vasomotor rhinitis
ABBREVIATIONS
CIP – Clinical investigator plan
EAACI – European Academy of Allergy and Clinical Immunology
ITT – Intention to treat analysis
KOS – Kinetic oscillation stimulation
LAR – Local allergic rhinitis
LVA – Local vasoconstrictor abuse
MCID – Minimal clinically important difference
NAR – Non-allergic rhinitis
NARES – Non-allergic rhinitis with eosinophilia syndrome
PNIF – Peak nasal inspiratory flow
PP – Per protocol
PPAS – Per protocol analysis set
SNOT-22 – Sino-nasal outcome test-22
TNSS – Total nasal symptom score
TRPV - Transient receptor potential vanilloid
TVRSS – Total vasomotor rhinitis symptom score
TVRSMS – Total vasomotor rhinitis symptom and medication score
BACKGROUND
Non-allergic Rhinitis (NAR) affects approximately 200 million people worldwide [1] and is characterized by the presence of nasal congestion, rhinorrhea, sneezing and itching in the absence of allergy and infection [2]. Two nasal symptoms should be present for at least 1 hour daily for a minimum of 12 weeks per year [1,2]. The only objective way to diagnose NAR is the use of cold dry air but this is not usually performed in daily practice [3-5].
There are several phenotypes of NAR: occupational, smokers, gustatory, hormonal (including pregnancy rhinitis), senile or elderly, drug-induced (e.g. by β-blockers, oral contraceptives, phosphodiesterase type 5 inhibitors), rhinitis medicamentosa, local allergic, and idiopathic rhinitis [2].
Rhinitis medicamentosa is caused by overuse of topical nasal decongestants. The exact duration of local decongestants usage that might lead to this condition is not conclusively determined yet, but it is commonly regarded that the risk of overuse increases after 7-10 days of continuous use [6]. With prolonged use, alpha-adrenergic receptors become refractory to stimulation and nasal obstruction becomes even worse with the use of local decongestants due to rebound swelling. Mucosal changes due to prolonged use of topical decongestants were demonstrated, including loss of ciliated cells in the nasal epithelium, and gaps and ruptures of basal lamina of vascular endothelium which may lead to an increased vascular permeability with consecutive interstitial edema [7]. The main treatment is the discontinuation of topical decongestants use, after which complaints normalize. There are, to our knowledge, no (sound) data on the reversibility of mucosal changes nor on the time-frame involved.
Inflammatory and neurogenic endotypes are described for NAR, although at the moment there is no tool to routinely differentiate between them. Inflammatory endotypes include Non-allergic Rhinitis with Eosinophilia Syndrome (NARES) and Local Allergic Rhinitis (LAR) [8,9]. An anti-inflammatory treatment (i.e., nasal corticosteroids) may be more effective in inflammatory subtypes, although currently there is no hard evidence [10].
Neurogenic endotypes include senile, gustatory, (some forms of) occupational, and Idiopathic Rhinitis (IR). The latter group is the largest, as it encompasses 50% of NAR patients [2]. More than half of IR patients experience nasal hyperreactivity [11] – overall hyperresponsiveness to non-specific stimuli in daily life (e.g. smoke, temperature changes, strong odors) or non-specific agents applied in the nose [12]. It is currently hypothesized that in neurogenic endotypes of NAR the balance between the sympathetic and parasympathetic innervation of the nasal mucosa is shifted towards the parasympathetic side [9,13]. Moreover, so-called neurogenic inflammation might play a role, [13] leading to increased release of inflammatory neuropeptides from unmyelinated sensory C-fibers, such as substance P [14], calcitonin gene related peptide [15], and neurokinin A and B [14].
A new treatment option that possibly influences neuro-regulation is called Kinetic Oscillation Stimulation (KOS). KOS is performed with a balloon that is inserted into the patient’s nose, is inflated and conducts mechanical oscillations with frequencies up to 68 Hz. This might influence the neurogenic balance at the level of the nasal mucosa, as it abundantly expresses Transient Receptor Potential Vanilloid (TRPV) channels [16,17], which are known to have mechanosensory properties (especially TRPV4) [18].
Indeed, a randomized control trial using KOS in NAR patients showed a positive effect on nasal obstruction compared to placebo, mainly in the first week after treatment [19].
METHODS
The current paper focuses on the Per-protocol (PP) analysis of the first study of KOS in NAR patients. Intention-to-treat analysis of the study was previously published [20]. Per-protocol analysis of the data is sensible due to the fact that a large proportion of patients (n=32 on a total of 207 subjects) that did not meet inclusion criteria (nasal congestion symptom score median ≥ 2) and were accidentally included in this study. The current Per-protocol Analysis Set (PPAS) includes subsets of patients that fulfilled the following criteria: do not have any other major Clinical Investigator Plan (CIP) violations which will affect the assessment of efficacy/performance (e.g. treatment not given according to CIP, nasal congestion score median was not ≥2, no week 4 assessments available); have available primary endpoint data, have full duration of study treatment (i.e. ten continuous minutes of treatment per nostril with a maximum of 5 minutes break between nostrils). Participants suffering from rhinitis medicamentosa were asked to discontinue local decongestants use after the first treatment. Participants, who used local decongestants on more than 4 days during the first four weeks after the treatment, were also removed from PPAS. Applying these criteria to the 207 patients included in the study, 124 could be included into the PPAS (for reasons of exclusion see Table 1).
|
Reasons for exclusion for PPAS |
Number of patients |
|
Did not meet inclusion criteria |
32* |
|
No week 4 assessment available |
5* |
|
Treatment not given according to CIP |
2* |
|
Low treatment group (originally planned as placebo) |
40 |
|
Failed to discontinue local vasoconstrictors |
6 |
|
Subjects randomized by mistake |
1 |
*two major violations were reported for two subjects. PPAS – Per-protocol analysis set, CIP – Clinical investigator’s plan
Table 1: Reasons for exclusion for PPAS.
Trial design
This was a multicenter, randomized study conducted in Sweden at six sites between April 2013 and August 2014. The original study design has been described in the Intention-to-treat (ITT) analysis [20]. For the PP analysis we only included patients receiving high frequency KOS treatment by means of mechanical pressure and oscillations at frequency of 68 Hz versus presumed placebo.
Participants
Participants were recruited from ENT practices and from advertisements. The study was approved by Regional Independent Ethical Committee in Stockholm on 20 March 2013. Informed consent was obtained from every participant prior to performing any investigation-related procedures.
Eligible participants were all adults between 18 and 65 years old who had symptoms of non-allergic rhinitis dominated by nasal congestion (median weekly score 2 or higher) ± secretion for an average of at least 1 h per day for at least 12 weeks per year [1,20]. Exclusion criteria were pregnancy, presence of allergic rhinitis or ongoing upper respiratory tract infections, the use of systemic steroids in less than 4 weeks before the inclusion in the study, a history of nasal surgery, nose bleeds or a condition that increases the risk of excessive bleeding; pronounced anterior septal deviation or other significant nasal pathology at endoscopic examination, current malignancy of any kind, known allergy to polyvinylchloride or medical liquid paraffin, any implant and/or neurostimulator device; radiotherapy in head and neck region in the past.
KOS treatment
The kinetic oscillation system consists of a single-use catheter with a silicone balloon and a base unit, which inflates the balloon and creates oscillations. The balloon was covered with medicinal paraffin or gel and inserted into participant’s nasal cavity subsequently in each nostril. For the verum treatment the balloon was inflated to create the pressure of 65 mbar and vibrated at frequency of 68 Hz (pressure amplitude of 100 mbar). In the placebo group the balloon was not inflated nor did it produce any oscillations. The duration of the treatment was 10 minutes for each side. No local anesthetic or vasoconstrictor was performed in connection to the treatment.
Randomization
Block randomization was performed using computer-generated list of random letters A-F (ViedocTM) with a block size of 6. Each subject received a randomization letter (A, B, C, D, E or F). The catheter was connected by an operator to a socket (A-F) according to randomization letter, which determined the treatment subject received and then dipped into paraffin or gel and placed in the participant’s nostril.
Rescue and concomitant medication
Participants of the study were allowed to use oral decongestant (phenylpropanolamine) as rescue medication. Subjects using concomitant medications (other than the rescue medication) for rhinitis, e.g. nasal steroids, were advised to remain on stable dose (no change in amount, dose or brand) from the screening visit up to the end of participation in the investigation.
Outcomes
Total vasomotor rhinitis symptoms score (TVRSS) and total vasomotor rhinitis and medication symptom score
Patients reported on the severity of their symptoms daily in the morning: nasal congestion, rhinorrhea, postnasal drip and sneezing (scale 0-3) in the previous 24 hours. The scores of these four symptoms were added up to a TVRSS (scale 0-12). If a participant used rescue medication, a score of 3 was added to the TVRSS, as rescue medication might lower the nasal congestion by a maximum of 3. In this way, the Total Vasomotor Rhinitis and Medication Symptom Score (TVRSMS) was composed, which was used for the final analysis.
The primary endpoint was the change from baseline in weekly mean TVRSMS at week 4 after the treatment between KOS treatment compared to placebo.
The first secondary endpoint was the change in weekly mean TVRSMS in the first 4 weeks after the treatment between KOS treatment and placebo.
Other secondary endpoints were the change in uncorrected for rescue medication TVRSS values, the change in nasal domain of the Sinonasal Outcome Test (SNOT-22) and PNIF at week 4 after the treatment between KOS treatment and placebo. Moreover, the same analysis was done with division in 4 groups based on treatment and the (stopping of) the abuse of local vasoconstrictors. Data on rescue medication use is presented as well.
Adverse events were registered and reported in the first paper [20]. Most common adverse events were temporary (increased tear and nasal secretion and sneezing). One participant had epistaxis and one had a serious adverse event (stroke), which was judged as being unrelated to the study.
Statistical analysis
Weekly mean TVRSMS and TVRSSwere calculated for baseline (week before KOS treatment) and in the four weeks after treatment. Change from baseline for TVRSMS, TVRSS, PNIF, and nasal domain of SNOT-22 were evaluated for normality when groups were smaller than 50 by graphical assessment of histograms and Q-Q plots. Some of the variables were not normally distributed. Therefore, both means with standard deviations and medians with interquartile ranges are reported for the subgroup analysis. Depending on normality non-parametric (Kruskall-Wallis/Mann-Whitney), or parametric (ANOVA/ t-test) testing was used. Participants who used rescue medication on 5 occasions or more after the treatment are presented in numbers and percentages. Finally, in order to account for repeated measures, TVRSMS was analyzed on the basis of a general linear mixed model with group (KOS or placebo) as a fixed effect and time as a repeated measure (weekly mean TVRSMS for patients for weeks 1-4 after the treatment). Vasoconstriction abuse and baseline TVRSMS were included as fixed covariates. We could not assume normality for the small groups (n = 13 and n=14) stopping local vasoconstriction abuse and placebo treatment, but we assumed this would have limited effect on the validity of the model. The model was used with unstructured covariance type and restricted maximum likehood estimation. A random intercept was not included in the model. P-values <0.05 were considered statistically significant.
Clinical meaningful differences have not been described for TVRS(M)S in idiopathic rhinitis. The best estimates are the differences measured in Total Nasal Symptoms Scores (TNSS) in allergic rhinitis. A difference of more than 0.55 units on the TNSS and a 5 L/min change in peak nasal inspiratory flow is considered clinically meaningful (de?ned as the Minimal Clinically Important Difference [MCID] [21]). For the nasal domain of the SNOT-22 a MCID of 3.8 was considered clinically meaningful in patients with chronic rhinosinusitis [22].
RESULTS
Patients
124 patients were included in this per protocol analysis, 64 in the KOS treatment group and 60 in the placebo group. Reasons for exclusion from per protocol analysis are given in table 1. Baseline characteristics of the study subjects are presented in table 2. There were no significant differences between the treatment and placebo group at baseline.
A significant subgroup of patients used Local Vasoconstrictors (LVA) when they entered the trial (13 in the verum and 14 in the placebo group). Patients were advised to stop the LVA treatment after receiving the KOS treatment and 27 of the patients abusing LVA indicated that they stopped the use of LVA after receiving the KOS treatment (2 reported using it 2-3 times in 4 weeks). Five subjects from LVA subgroup and one subject from non-LVA subgroup used local decongestants on 9-28 occasions after the first treatment, and were removed from per protocol analysis set.
|
|
KOS treatment (n=64) |
Placebo (n=60) |
||
|
Age (years) |
Mean, SD |
46.7±12.6 |
46.1±13.8 |
|
|
Gender (female) |
N, % |
35 (54.7%) |
27 (45.0%) |
|
|
TVRSS |
Mean, SD |
4.2±1.7 |
4.0±1.5 |
|
|
TVRSMS |
Mean, SD |
4.3±1.7 |
4.0±1.6 |
|
|
SNOT-22, nasal domain |
Mean, SD |
13.8±5.1 |
12.4±4.9 |
|
|
PNIF, [L/min] |
Mean, SD |
123.8±48.3 |
133.0±47.2 |
|
KOS – Kinetic oscillation stimulation, TVRSS – Total vasomotor rhinitis symptom score, TVRSMS – Total vasomotor rhinitis symptom and medication score, SNOT-22 – Sino-nasal outcome test 22, PNIF – Peak nasal inspiratory flow.
Table 2: Baseline characteristics.
Change in total symptoms scores: TVRSMS, TVRSS and nasal domain of the SNOT-22
There was a significant and clinical meaningful reduction in change from baseline of the TVRSMS, TVRSS and in the nasal domain of the SNOT-22 over time in the KOS treated group compared to placebo four weeks after the treatment (see Table 3). Regarding the TVRSS subscores, there was significant improvement of nasal congestion (p=0.005) and rhinorrhea (0.035).The number of rescue medication users was not significantly different between the groups.
|
|
KOS treatment (n=64) |
Placebo (n=60) |
p-value
|
|
|
TVRSS |
Mean, SD |
-0.9±1.4 |
-0.3±1.6 |
0.017 |
|
Number of rescue medication users |
N, % |
5 (8%) |
7 (12%) |
0.468 |
|
TVRSMS |
Mean, SD |
-0.8±1.6 |
-0.2±1.6 |
0.035 |
|
SNOT-22, nasal domain |
Mean, SD |
-3.7±4.3 |
-0.3±4.0 |
<0.001 |
|
PNIF, [L/min] |
Mean, SD |
7.6±34.5 |
8.4±44.2 |
0.903 |
KOS – Kinetic oscillation stimulation, TVRSS – Total vasomotor rhinitis symptom score, TVRSMS – Total vasomotor rhinitis symptom and medication score, SNOT-22 – Sino-nasal outcome test 22, PNIF – Peak nasal inspiratory flow
Table 3: Symptoms scores and PNIF change from baseline at 4 weeks.
A relatively large group of patients used local vasoconstrictors prior to treatment allocation (27/124 subjects; 22%). To check whether part of the reduction in TVRSMS could be caused by stopping the use of local vasoconstrictors, the TVRSMS change from baseline at 4 weeks was compared between LVA and non-LVA users. Interestingly, the TVRSMS change from baseline at 4 weeks wassignificantly bigger in the group that stopped LVA (-1.1±1.9) compared to non-LVA group (-0.4±1.6), irrespective of treatment allocation. Next, a subgroup analysis was performed by dividing the PPAS in 4 groups based on KOS and LVA use (Table 4). Figure 1 shows the mean values of TVRSMS for the four groups over time.
|
|
|
1. No local vasoconstrictor abuse KOS treatment (N=51) |
2. No local vasoconstrictor abuse Placebo (N=46) |
3. Stopping local vasoconstrictor abuse KOS treatment (N=13) |
4. Stopping local vasoconstrictor abuse Placebo (N=14) |
Kruskal-Wallis or ANOVA (p-values) |
|
TVRSMS |
Mean, SD |
-0.6±1.5 # |
- 0.1±1.4 # |
-1.6±1.4 $^ |
-0.6±2.1 |
ANOVA 0.022 |
|
Median, IQR |
-0.5 (-1.3, 0.14) |
-0.3 (-1.3, 0.6) |
-1.6 (-2.6, -0.5) + |
-0.2 (-1.0, 0.3) # |
K-W 0.029 |
|
|
SNOT, nasal domain |
Mean, SD |
-2.9±4.2 ^ |
0.2 ±3.5 $# |
-3.5±4.8 ^ |
-1.8±5.1 |
ANOVA 0.001 |
|
Median, IQR |
-3.0 (-6.0, 0.0) |
0.0 (-2.0, 2.3) |
-3.0 (-6.5, -2.0) |
-2.0 (-2.5, 2.0) |
K-W 0.001 |
|
|
PNIF |
Mean, SD |
3.2±29.9 |
9.8±36.1 |
24.6±46.3 |
3.8±66.9 |
ANOVA 0.349 |
|
Median, IQR |
0.0 (-10.0, 20.0) |
10.0 (-20.0, 32.5) |
30.0 (-25.0; 70.0) |
20.0 (-32.5, 30.0) |
K-W 0.449 |
$ p<0.05 vs Group 1; ^ p<0.05 vs Group 2; # p<0.05 vs Group 3; + p<0.05 vs Group 4. KOS – Kinetic oscillation stimulation, TVRSMS – Total vasomotor rhinitis symptom score, SNOT-22 – Sino-nasal outcome test 22, PNIF – Peak nasal inspiratory flow
Table 4: Symptoms scores and PNIF change from baseline at 4 weeks comparing KOS treatment and the use of local vasoconstrictors.
KOS – Kinetic oscillation stimulation, TVRSMS -Total vasomotor rhinitis symptom and medication score
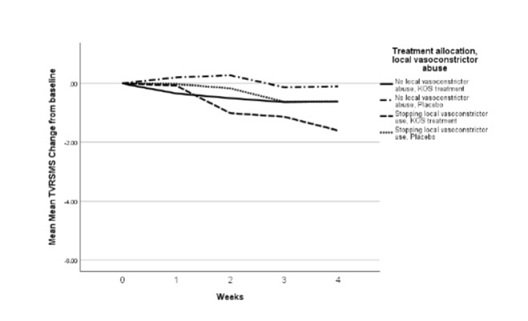
Figure 1: Mean weekly TVRSMS, change from baseline.
The KOS group that stopped LVA had a significantly larger reduction in TVRSMS compared to the rest of the subgroups. So, both the KOS treatment and stopping of LVA, and the combination of the two resulted in a significant reduction in TVRSMS.
The nasal domain of the SNOT-22 was significantly reduced 4 weeks after treatment in the KOS-treated group, with a clinically relevant mean change of -3.7 versus -0.3 in the placebo group (Table 3). In the subgroup analysis, there was also a significant reduction of nasal domain of SNOT-22 of both KOS subgroups compared to placebo-treated group that did not suffer abuse local decongestants (Table 4).
Finally, the linear mixed model showed a significant effect on TVRSMS of KOS treatment (p=0.02, SE=0,200, 95% CI -0.85, -0.0606), but no significant effect of cessation of local vasoconstrictor abuse. (p=0.114, SE=0.121, 95%CI -0.43, 0.05)
PNIF
Interestingly, despite the change in subjective feeling of obstruction, there was absolutely no effect of the KOS treatment on PNIF.
DISCUSSION
In this paper we performed a per-protocol analysis of data published earlier with an “intention-to-treat” analysis [20]. The earlier analysis found no significant difference inthe TVRSS mean score change from baseline at 4 weeks (p=0.053) for the group treated with high amplitude KOS compared to placebo, but did find a significant difference in the TVRSS mean score change for the group treated with low amplitude KOS compared to placebo (p < 0.01).
The CONSORT guidelines for reporting of "parallel group randomized controlled trials" recommend that both ITT and PP analyses should be reported for all planned outcomes to allow readers to interpret the effect of an intervention [23]. This per-protocol analysis shows a significant effect of a single treatment with KOS on TVRSMS after 4 weeks. The mean TVRSMS decrease from baseline was 0.8 versus 0.2 in the placebo group (Table 3). Whether this finding is clinically relevant, is hard to tell, as the Minimal Clinically Important Difference (MCID) for the TVRSS, and consequently TVRSMS, in NAR has not been established yet. With data from anchor-based and distribution-based models, Barnes et al. proposed a change of 0.55 units as the TNSS MCID for allergic rhinitis [21] which in this case would be reached. Another approach would be to use half the standard deviation as MCID. The mean TVRSMS score pre-treatment was ~4, with a standard deviation of ~1.7. The MCID would then be around 0.85. In light of the MCID in AR, and the SD-based MCID of the current data, we think that the KOS reduction in TVRSMS of 0.8 is clinically relevant. However, specific MCID studies still have to be performed in NAR. As far as we know, the TVRSMS is not validated to measure the severity of NAR symptoms. Also, in the EAACI position paper on non-allergic rhinitis no validated questionnaires are given [2]. The same applies for the nasal domain of the SNOT-22, a test only validated in chronic rhinosinusitis. So, there is an unmet need to have a properly validated set of outcome measures for NAR as has been provided for CRS [24]. Moreover, we couldn’t correct the nasal domain of SNOT-22 and PNIF for rescue medication use. To evaluate the potential effect of the rescue medication we also ran the analysis on the subset of patients that did not use rescue medication, but the outcomes did not change (data not shown).
Currently, treatment of NAR via neurogenic pathways has limited options. Capsaicin can be useful through its effects on the transient receptor potential vanilloid and ankyrin families [11,25-27] thus reducing nasal hyperreactivity [13]. However, this treatment is not commercially available and can only be performed in hospitals where a pharmacy can produce the medication. Influence on the parasympathetic shift can be achieved through locally applied ipratropium bromide, or by performing a vidianneurectomy [28,29]. But, despite the treatments mentioned above, a significant part of the NAR patients remains uncontrolled [30] and KOS might prove to be an additional tool here.
Unfortunately, the design of this study, as has been discussed in the first paper [20] is insufficient to draw any further conclusion. One of the issues is that the placebo treatment used was a non-inflated balloon. In the beginning of the study the control treatment was different (a low amplitude treatment): the balloon was inserted in the nose, inflated and vibrated at a much lower frequency than the verum treatment. After the interim analysis it turned out that both verum and low amplitude treatments might have a positive effect and that vibrations even on low frequencies could possibly improve the symptoms or that the fact that the patients felt something happening is a placebo-effect. That is why an uninflated and non-vibrating balloon was introduced as the new control treatment. One could argue that this is not a true placebo treatment and that both the caregiver and the patient were not really blinded. A new study with a really double blinded design is needed before firm conclusions can be drawn.
Although not primarily set up in this direction, the current study is suggesting that stopping with local vasoconstrictor abuse might have an effect on nasal complaints already after a short period of time of a few weeks.
Even though local vasoconstrictors give a reduction in nasal obstruction for a period up to 12 hours [31] it should not be used in NAR because of the risk of rhinitis medicamentosa [6] as has been shown in this study where a quarter of the patients (27/124) had this disease. Figure 1 shows that shortly upon the discontinuation of Local Vasoconstrictor Abuse (LVA), the TVRSMS decreases. Already after two weeks the effect is present and it further increased in later weeks. Moreover, the effect size is rather large with a mean TVRSMS decrease from baseline of 1.1 after 4 weeks. Most likely we were not able to show a significant effect of stopping local vasoconstrictor abuse due to a small subgroups size: a post-hoc analysis showed that a group of n=81 would have given a significant effect. To our knowledge, this is the first study to generate patient-reported outcome measures in the weeks after stopping LVA. Interestingly, the effect of KOS was additive to the effect of discontinuation of LVA. Therefore, the mean decrease of TVRSMS from baseline was largest in the KOS-treated LVA group (1.6 after 4 weeks). As such, KOS might be a supportive tool in rhinitis medicamentosa patients to help stop their LVA. The current data also suggests that studies in NAR should be aware of this phenomenon and either exclude LVA patients, make the LVA group large enough to perform sub-analyses, or include a wash-out period of at least several weeks before the actual trial is performed.
CONCLUSION
Through a per-protocol analysis, a single treatment with KOS was found to give a significant reduction in Total Vasomotor Rhinitis Symptom and Medication Score (TVRSMS) sustained over a period of 4 weeks in this not really placebo-controlled RCT. A new study with a true double-blinded design is needed before firm conclusions can be drawn.
Rhinitis medicamentosa is probably an independent factor, but studies with larger subgroups are needed to confirm the effect.
DECLARATIONS
Ethics approval and consent to participate
The clinical investigation was conducted in compliance with applicable regulatory requirements and with the ethical principles of the latest revision of the Declaration of Helsinki as adopted by the World Medical Association [32].
Approval of the Regional Independent Ethics Committee located in Stockholm, Sweden was obtained before enrolment of any subject into the investigation. Written informed consent is obtained from all participants before any trial related procedure is performed. The trial protocol was reviewed and approved by Regional Independent Ethics Committee. The trial was registered on ClinicalTrials.gov on the 1st of May, 2013. Registration number: NCT01844336.
Consent for publication
Not applicable.
Availability of data and material
The datasets analyzed during the current study are available from the corresponding author on reasonable request with permission of Chordate Medical AB.
Conflict of interest
All authors participate in a new trial with the Chordate apparatus funded by Chordate Medical AB.
Funding
Not applicable.
Authors’ contributions
KA analyzed the data and prepared the manuscript. WF and SR interpreted the data and prepared the manuscript. All authors read and approved the final manuscript.
REFERENCES
- Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, et al. (2008) Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 86: 8-160.
- Hellings PW, Klimek L, Cingi C, Agache I, Akdis C, et al. (2017) Non-allergic rhinitis: Position paper of the European Academy of Allergy and Clinical Immunology. Allergy 72: 1657-1665.
- Van Gerven L, Steelant B, Hellings PW (2018) Nasal hyperreactivity in rhinitis: A diagnostic and therapeutic challenge. Allergy 73: 1784-1791.
- Huang CC, Lee TJ, Huang CC, Chang PH, Fu CH, et al. (2019) Cold dry air provocation is a reliable diagnostic tool in nonallergic rhinitis. Rhinology 57: 225-230.
- Braat JP, Mulder PG, Fokkens WJ, van Wijk RG, Rijntjes E (1998) Intranasal cold dry air is superior to histamine challenge in determining the presence and degree of nasal hyperreactivity in nonallergic noninfectious perennial rhinitis. Am J Respir Crit Care Med 157: 1748-1755.
- Graf PM (2007) Rhinitis medicamentosa. Clin Allergy Immunol 19: 295-304.
- Knipping S, Holzhausen HJ, Goetze G, Riederer A, Bloching MB (2007) Rhinitis medicamentosa: electron microscopic changes of human nasal mucosa. Otolaryngol Head Neck Surg 136: 57-61.
- Hamizan AW, Rimmer J, Husain S, Alvarado R, Tatersall J, Sewell W, et al. (2018) Local specific Immunoglobulin E among patients with nonallergic rhinitis: a systematic review. Rhinology 57: 10-20.
- Papadopoulos NG, Bernstein JA, Demoly P, Dykewicz M, Fokkens W, et al. (2015) Phenotypes and endotypes of rhinitis and their impact on management: a PRACTALL report. Allergy 70: 474-494.
- Segboer CL, Fokkens WJ, Terreehorst I, van Drunen CM (2018) Endotyping of non-allergic, allergic and mixed rhinitis patients using a broad panel of biomarkers in nasal secretions. PLoS One 13: e0200366.
- Van Gerven L, Alpizar YA, Steelant B, Callebaut I, KortekaasKrohn I, et al. (2017) Enhanced chemosensory sensitivity in patients with idiopathic rhinitis and its reversal by nasal capsaicin treatment. J Allergy Clin Immunol 140: 437-446.
- Gerth van Wijk RG, de Graaf-in 't Veld C, Garrelds IM (1999) Nasal hyperreactivity. Rhinology 37: 50-55.
- Van Gerven L, Boeckxstaens G, Hellings P (2012) Up-date on neuro-immune mechanisms involved in allergic and non-allergic rhinitis. Rhinology 50: 227-235.
- Baraniuk JN, Lundgren JD, Okayama M, Goff J, Mullol J, et al. (1991) Substance P and neurokinin A in human nasal mucosa. Am J Respir Cell Mol Biol 4: 228-236.
- Baraniuk JN, Lundgren JD, Goff J, Mullol J, Castellino S, et al. (1990) Calcitonin gene-related peptide in human nasal mucosa. Am J Physiol 258: L81-88.
- Baraniuk JN, Merck SJ (2009) New concepts of neural regulation in human nasal mucosa. Acta Clin Croat 48: 65-73.
- Bhargave G, Woodworth BA, Xiong G, Wolfe SG, Antunes MB, et al. (2008) Transient receptor potential vanilloid type 4 channel expression in chronic rhinosinusitis. Am J Rhinol 22: 7-12.
- O'Neil RG, Heller S (2005) The mechanosensitive nature of TRPV channels. Pflugers Arch 451: 193-203.
- Juto JE, Axelsson M (2014) Kinetic oscillation stimulation as treatment of non-allergic rhinitis: an RCT study. Acta Otolaryngol 134: 506-512.
- Ehnhage A, Johnsson PS, Ahlstrom-Emanuelsson C, Andersson M, Knutsson J, et al. (2016) Treatment of idiopathic rhinitis with kinetic oscillations - a multi-centre randomized controlled study. Acta Otolaryngol 136: 852-859.
- Barnes ML, Vaidyanathan S, Williamson PA, Lipworth BJ (2010) The minimal clinically important difference in allergic rhinitis. Clin Exp Allergy 40: 242-250.
- Chowdhury NI, Mace JC, Bodner TE, Alt JA, Deconde AS, et al. (2017) Investigating the minimal clinically important difference for SNOT-22 symptom domains in surgically managed chronic rhinosinusitis. Int Forum Allergy Rhinol 7: 1149-1155.
- Schulz KF, Altman DG, Moher D (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med 7: e1000251.
- Hopkins C, Hettige R, Soni-Jaiswal A, Lakhani R, Carrie S, et al. (2018) CHronicRhinosinusitis Outcome MEasures (CHROME), developing a core outcome set for trials of interventions in chronic rhinosinusitis. Rhinology 56: 22-32.
- Van Rijswijk JB, Boeke EL, Keizer JM, Mulder PG, Blom HM, et al. (2003) Intranasal capsaicin reduces nasal hyperreactivity in idiopathic rhinitis: a double-blind randomized application regimen study. Allergy 58: 754-761.
- Van Gerven L, Alpizar YA, Wouters MM, Hox V, Hauben E, et al. (2014) Capsaicin treatment reduces nasal hyperreactivity and transient receptor potential cation channel subfamily V, receptor 1 (TRPV1) overexpression in patients with idiopathic rhinitis. J Allergy Clin Immunol 133: 1332-1339.
- Gevorgyan A, Segboer C, Gorissen R, van Drunen CM, Fokkens W (2015) Capsaicinfor non?allergic rhinitis. Cochrane Database of Systematic Reviews Pg no. 1-44.
- Georgitis JW, Banov C, Boggs PB, Dockhorn R, Grossman J, et al. (1994) Ipratropium bromide nasal spray in non-allergic rhinitis: efficacy, nasal cytological response and patient evaluation on quality of life. Clin Exp Allergy 24: 1049-1055.
- Marshak T, Yun WK, Hazout C, Sacks R, Harvey RJ (2016) A systematic review of the evidence base for vidianneurectomy in managing rhinitis. J Laryngol Otol 130: S7-S28.
- Segboer CL, Terreehorst I, Gevorgyan A, Hellings PW, van Drunen CM, et al. (2018) Quality of life is significantly impaired in nonallergic rhinitis patients. Allergy 73: 1094-1100.
- Druce HM, Ramsey DL, Karnati S, Carr AN (2018) Topical nasal decongestant oxymetazoline (0.05%) provides relief of nasal symptoms for 12 hours. Rhinology 56: 343-350.
- World Medical Association (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310: 2191-2194.
Citation: Avdeeva KS, Reitsma S, Fokkens WJ (2020) The Effect of Kinetic Oscillation Stimulation on Symptoms of Non-allergic Rhinitis: A Per-protocol Analysis of a Randomized Controlled Trial. J Otolaryng Head Neck Surg 6: 42
Copyright: © 2020 Klementina S Avdeeva, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

