
The Effectiveness of Autologous Platelet-Rich Plasma (PRP) in the Therapy of Infertile Men with Non-Abstractive Azoospermia
*Corresponding Author(s):
Zakwan KhraitBritish Syrian Ivf And Fetal Medicine Centre, Al-Rasheed Hospital, Damascus, Syria, Syrian Arab Republic
Tel:+971 555242644,
Email:zakwankhrait@hotmail.com
Abstract
Objectives: To evaluate the effectiveness of autologous PRP in the therapy of infertile men with nonobstructive Azoospermia.
Methods: Seventy-one patients received ½ ml PRP in each testicle which was prepared by centrifuging patient’s own blood. FNA parameters and FSH levels of the patients were measured before and after the procedure. Testosterone level was measured before the procedure. All the required data for the study was collected retrospectively from the hospital records.
Results: A couple of NOA cases developed normal spermatogenesis. Post-procedure FSH was higher than preprocedural FSH (MD 1.737, p .560). Patients with spermatocytes in the initial FNA report showed a lower percentage of azoospermia than their counterparts (11.4% vs 44.4%).
Conclusion: Numerous studies have proven PRP to have a statistically significant level of regenerative potential in different branches of medicine. PRP therapy has been successful for the treatment of some causes of female infertility. The treatment potential of PRP for male infertility may not be underestimated.
INTRODUCTION
Platelet Rich Plasma (PRP), with its rich growth factor composition, has been already proven beneficial in regenerative therapy [4]. Some of the specific beneficial effects of PRP are accelerated angiogenesis and anabolism, inflammation control, cell migration, differentiation and proliferation were identified by a few previous studies [5-7]. As a result, PRP is nowadays used in various clinical scenarios that required improved tissue regeneration in maxillofacial surgery, neurobiology, orthopedics, sports medicine and ophthalmology [3,8,9]. Potential benefits of PRP have also been studied in the field of gynecology; a study revealed PRP can increase endometrial thickness and improve the pregnancy outcome with thin endometrium [9]. According to Colombo GVL, PRP has the potential to reduce the implantation failures by increasing the expression of adhesion molecules, ovarian rejuvenation and folliculogenesis reactivation in peri-menopausal women [2,10]. PRP can improve the structural and functional impairment of the testis.
Male factor causes approximately 30% to 50% of infertile couples. Non-Obstructive Azoospermia (NOA) is a failure of spermatogenesis within the testis [11]. In the past, males with Non-Obstructive Azoospermia (NOA) had no therapeutic options outside of assisted reproductive techniques to conceive a biological child. These patients were bound to rely on options of donor sperm or adoption [12]. Then several sources had suggested about hormonal therapies and stem cell therapy for NOA [11,13]. Till date, no human study was done to identify potential benefits of PRP in NOA treatment. Given all the aforementioned positive outcomes of PRP therapy in various medical fields, little is known regarding the application of PRP in the testicular injection. In our current study, we aim to know the potential benefit of autologous PRP therapy of infertile men with Non-Obstructive Azoospermia (NOA), if so we try to select the criteria of PRP in (NOA) patients.
MATERIAL AND METHODS
Study design: Retrospective chart-review study
Platelet-Rich Plasma (PRP) is prepared from fresh whole blood which is collected from a peripheral vein, stored in Acid Citrate Dextrose solution A (ACD-A) anticoagulant and processed to increase platelets by separating various components of blood, hence the injected fluid prepared from autologous blood by centrifugation using Dr. PRP kits and equipment (USA). Consent form was given and signed.
Under sedation men had been undergone PRP intra-testicular injection with ½ ml in each testicle using fine needle, the injections were repeated to those who have more than one failed TESE previously with no mature sperm where found.
Male factor causes approximately 30% to 50% of infertile couples. The Azoospermia diagnosis of referral patients or outpatients have been established on the basis of, at least, two semen analysis evaluation done at separated occasions, if no spermatozoa are observed in the wet preparation, we followed the World Health Organization (WHO) recommendations as to perform an examination of the centrifuged sample (300 X g or greater for 15 minutes ), followed by detailed history taking including general health, libido, sexual health, past fertility, sexual activity and previous exposure to surgery, drugs, mumps infection, irradiation and physical examination. Physical examination includes genital and local examination for detection of signs of Klinefelter syndrome, testicular atrophy, absence of vas and investigations includes FSH and Testosterone while some other hormones which may influence the spermatogenesis procedure e.g., Estradiol and Prolactin not included in this study but focused on FSH and Testosterone level. 2-4 months following PRP, the TESA (Testicular Sperm Aspiration) was performed after confirmed semen analysis azoospermia was performed shortly prior to second TESA.
STATISTICAL ANALYSIS
RESULTS
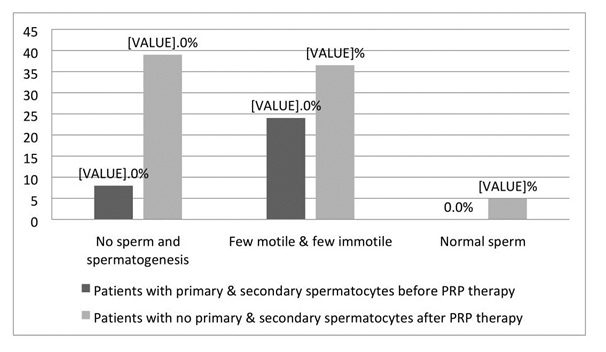 Figure 1: The following chart is showing the percentages of ‘no sperm and no spermatogenesis’, ‘few motile & few immotile’ and ‘normal sperm’ in final FNA reports for patients with primary & secondary spermatocytes prior to PRP therapy (n = 50) and patients with no primary & secondary spermatocytes prior to PRP therapy (n = 41).
Figure 1: The following chart is showing the percentages of ‘no sperm and no spermatogenesis’, ‘few motile & few immotile’ and ‘normal sperm’ in final FNA reports for patients with primary & secondary spermatocytes prior to PRP therapy (n = 50) and patients with no primary & secondary spermatocytes prior to PRP therapy (n = 41).|
Parameters |
N (%)/mean ± SD |
|
Age in years |
37.60 ± 6.66 |
|
Initial FNA/ SA parameters |
|
|
Primary & secondary spermatocytes |
50 (54.9) |
|
No sperm and no spermatogenesis |
22 (24.2) |
|
Few motile and few immotile sperms |
13 (14.3) |
|
Oligospermia |
3 (3.3) |
|
Other |
3 (3.3) |
|
Initial FSH |
22.89 ± 13.12 |
|
Testosterone level (ng/dL) |
571.08 ± 844.72 |
|
Protocol |
|
|
No treatment |
61 (67.0) |
|
Letrazole |
30 (33.0) |
|
Final FNA report |
|
|
Primary & secondary spermatocytes |
17 (18.7) |
|
No sperm and no spermatogenesis |
20 (22.0) |
|
Few motile & few immotile sperms |
27 (29.7) |
|
Oligospermia |
2 (2.2) |
|
Normal sperm |
2 (2.2) |
|
Missing |
23 (25.3) |
|
Final FSH |
24.56 ± 23.43 |
|
|
Mean |
SD |
95%CI |
t |
p-value |
|
Initial FSH - final FSH (in all cases, n=71) |
-1.737 |
±24.280 |
4.185-7.660 |
-0.586 |
0.560 |
|
Initial FSH - final FSH (in patients with primary & secondary spermatocytes before PRP therapy, n=35) |
2.288 |
±15.825 |
7.899-3.323 |
0.831 |
0.412 |
DISCUSSION
We have observed FSH level to increase after PRP therapy in the current study. The present study also showed the cases who had primary and secondary spermatogenesis prior to PRP therapy had a lower percentage (11.4%) of azoospermia compared to their counterparts (44.4%). PRP therapy has proliferative effects on endometrium and ultimately increase implantation rate and reduce female fertility [9]. As per the male infertile we have observed improvement in terms of hormonal level and spermatogenesis in the current study. In the current study, we did not observe any deteriorating effect of PRP therapy.
CONCLUSION
REFERENCES
- Wang HL, Avila G (2007) Platelet Rich Plasma: Myth or Reality? Eur J Dent 1: 192-194.
- Pantos K, Nitsos N, Kokkali G, Vaxevanoglou T, Markomichali C, et al. (2016) Ovarian rejuvenation and folliculogenesis reactivation in peri-menopausal women after autologous platelet-rich plasma treatment. P-401.
- Anitua E, de la Fuente M, Ferrando M, Quintana F, Larreategui Z, et al. (2016) Biological effects of plasma rich in growth factors (PRGF) on human endometrial fibroblasts. Eur J Obstet Gynecol Reprod Biol 206: 125-130.
- Sekerci CA, Tanidir Y, Sener TE, Sener G, Cevik O, et al. (2017) Effects of platelet-rich plasma against experimental ischemia/reperfusion injury in rat testis. J Pediatr Urol 13: 317.
- Lenz J, Celander D, Crowther RL, Patarca R, Perkins DW, et al. (1984) Determination of the leukaemogenicity of a murine retrovirus by sequences within the long terminal repeat. Nature 308: 467-470.
- Pietrzak WS, Eppley BL (2005) Platelet rich plasma: biology and new technology. J Craniofac Surg 16: 1043-1054.
- Borrione P, Gianfrancesco AD, Pereira MT, Pigozzi F (2010) Platelet-rich plasma in muscle healing. Am J Phys Med Rehabil 89: 854-861.
- Alio JL, Arnalich-Montiel F, Rodriguez AE (2012) The role of "eye platelet rich plasma" (E-PRP) for wound healing in ophthalmology. Curr Pharm Biotechnol 13: 1257-1265.
- Chang Y, Li J, Chen Y, Wei L, Yang X, et al. (2015) Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med 8: 1286-1290.
- Colombo GVL, Fanton V, Sosa D, Criado Scholz E, Lotti J, et al. (2017). Use of platelet rich plasma in human infertility. J Biol Regul Homeost Agents 31: 179-182.
- Kumar R (2013) Medical management of non-obstructive azoospermia. Clinics (Sao Paulo) 68: 75-79.
- Vij SC, Sabanegh E Jr, Agarwal A (2018) Biological therapy for non-obstructive azoospermia. Expert Opin Biol Ther 18: 19-23.
- Hendriks S, Dancet EA, Meissner A, van der Veen F, Mochtar MH, et al. (2014) Perspectives of infertile men on future stem cell treatments for nonobstructive azoospermia. Reprod Biomed Online 28: 650-657.
- Valeriy Z, Olena K, Olena K (2014) Platelet-rich plasma induces morphofunctional restoration of mice testes following doxorubomycine hydrochloride exposure. J Exp Clin Med 31: 183-187.
- Matz EL, Pearlman AM, Terlecki RP (2018) Safety and feasibility of platelet rich fibrin matrix injections for treatment of common urologic conditions. Investig Clin Urol 59: 61-65.
- Bir SC, Esaki J, Marui A, Yamahara K, Tsubota H, et al. (2009) Angiogenic properties of sustained release platelet-rich plasma: Characterization in-vitro and in the ischemic hind limb of the mouse. J Vasc Surg 50: 870-879.
- Battinelli EM, Markens BA, Italiano JE Jr (2011) Release of angiogenesis regulatory proteins from platelet alpha granules: Modulation of physiologic and pathologic angiogenesis. Blood 118: 1359-1369.
- Sanchez-Gonzalez DJ, Méndez-Bolaina E, Trejo-Bahena NI (2014) Platelet-rich plasma peptides: Key for regeneration. Int J Pept 532519: 10.
Citation: Al-Nasser R, Khrait Z, Jamali S (2018) The Effectiveness of Autologous Platelet-Rich Plasma (PRP) in the Therapy of Infertile Men with Non-Abstractive Azoospermia. J Reprod Med Gynecol Obstet 3: 011.
Copyright: © 2018 Rami Al-Nasser, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

