
The Effects of Ginsenoside Rg2 on Trastuzumab-Induced Cardiotoxicity
*Corresponding Author(s):
Xiaoyong QiGraduate School, Hebei Medical University, Shi Jiazhuang, Hebei Province, China
Tel:+86 031186095739,
Email:47126458@qq.com
Abstract
Objectives: Trastuzumab (TZM) shows significant effects in early-stage HER2-positive breast cancer, but it is also followed by severe cardiotoxicity. Ginsenoside Rg2 has been demonstrated usefully against myocardial injury and apoptosis. However, whether ginsenoside Rg2 can protect cardiomyocytes with TZM-induced toxicity is not clear. The study is to investigate its possible mechanism.Methods:30 wistar male rats were divided in 3 groups (control group, TZM group and TZM+Rg2 group), cardiac ultrasound was performed before and after the experiment; cardiomyocytes (H9C2) was divided in 3 groups (control group, TZM group and TZM+Rg2 group), Annexin V test and Western blot were performed in the experiment.
Results: Ginsenoside Rg2 inhibited the deterioration of cardiac function in rats, ginsenoside Rg2 inhibited cardiomyocyte apoptosis, increased the expression of Caspase-3,Caspase-9 and BAX in cell experiment. Conclusions: Ginsenoside Rg2 could protect cardiomyocytes in TZM-induced toxicity and it might be via inhibiting cell apoptosis.
Keywords
Breast cancer; Cardiotoxicity; Cell apoptosis; Ginsenoside Rg2; Trastuzumab
Introduction
Trastuzumab (TZM) is a humanized monoclonal antibody that targets on Human Epidermal growth factor receptor 2 (HER2) and shows significant effects in early-stage breast cancer [1]. I the majority of patients with HER2-positive breast cancer, TZM has a significantly increasing in the overall survival [2].. However, TZM has a following cardiac side effects, including QTprolongation, bradycardia, hypertension, thromboembolic disease, congestive heart failure and ischemic heart disease, despite its beneficial effects [3,4].
Ginseng (Panax ginseng) is a traditional Chinese herbal medicine that has been widely used for thousands of years in East Asia, including China, Korea and Bhutan [5]. Ginsenoside is extracted from ginseng and plays the most important role among its components, including anti-inflammatory, antioxidant, anticancer, immunomodulatory and anti-stress effects [6-8]. Ginsenoside Rg2 is one compound of ginsenoside and has been proved multiple roles, including improving neurological performance, enhancing memory and exhibiting protective effects against hydrogen peroxide-induced injury and apoptosis in human cardiomyocytes [9-12].
This study aimed to investigate whether ginsenoside Rg2 could protect HCMs against TZM-induced toxicity in vitro and cardiac function in animal experiment.
Materials And Methods
Animal experiments
The experiment was approved by the Animal Care and Use Committee of Hebei Medical University; they were in compliancewiththeNationalInstitutesofHealthGuideforCareandUseofLaboratoryAnimals. 30 male wistar rats (160±10g) were randomly divided into 3 groups (n=10 per group):control group, TZM group and TZM+Rg2 group. TZM was given by intraperitoneal injection with a dose of 12mg/kg, then at a dose of 6mg/kg per day for 6 consecutive days, the ginsenoside Rg2 was given by intraperitoneal injection with a dose of 15mg/kg, the subsequent experiments were conducted after 24 hours, the control group was injected with the same dose of normal saline. Cardiac ultrasound was performed in the last day and Left Ventricular End-diastolic Diameter (LVEDD), Left Ventricular End-systolic Diameter (LVESD), Left Ventricular Ejection Fraction (LVEF) and Left Ventricular Fractional Shortening (LVFS) were recorded.
Cell experiments
Human primary HCMs (Applied Biological Materials, Inc.) were cultured in DMEM (Sigma-Aldrich; Merck KGaA) containing 10% FBS (Thermo Fisher Scientific, Inc.), maintained at 37°C and 5% CO2. They were divided in 3 groups as the same as the rats.TZM group was processed by TZM with a dose of 10μg/mL for 24h,TZM+Rg2 group was preprocessed by Rg2[purity>98% (by high-performance liquid chromatography analysis); MedChemExpress) with a dose of 0. 025mmol/ L for 24h, then the subsequent experiment was performed, the control was processed by the same dose of DMSO. Annex in V TEST and Western blotting were performed after TZM administration in 24h.
Cell proliferation assay: HCMs (5x103 cells/well) were seeded in a 96-well plate in triplicate. The cells were subsequently treated with Rg2 and TZM, maintained for 24h at 37°C and 5% CO2, MTT solution (5mg/ml) was added into every plate and then cultured in 3h at 37°C. Remove the culture carefully and add DMSO in every plate. Using ELISA plate reader (Bio-Rad Laboratories, Inc.) to check absorption of 490nm wave.
Apoptosis assay: HCMs (2x105 cells/well) were seeded in a 6-well plate and then treated with Rg2 and TZM. Using Annexin V staining kit (BD Biosciences) to check the cell apoptosis. The cells were centrfugated for 5minutes by 500-1000r/min, abandoned the culture, washed by FBS once, centrfugated for 5minutes by 500-1000r/min again. Using 100ul marking solution to heavy-hanging cells, incubating without light for 10-15minutes. Add 5μl Annexin V and 10μl propidium Iodide, placed in a dark room for 15 minutes, using a FACSCanto II flow cytometer (BD Biosciences) to check the cell apoptosis.
Western blotting: HCMs were rinsed and lysed using RIPAlysis buffer (EMD Millipore) containing a protease and phosphatase inhibitor. Subsequently, the cell lysates were vortexedon ice five times within 20 min and centrifuged for 10 minat 10,000 x g at 4°C. The protein concentration was determined using a bicinchoninic acid protein quantification kit (PromegaCorporation). Protein aliquots (30 µg) were subjected toSDS-PAGE on a 10% gel and subsequently transferred on to PVDF membranes (EMD Millipore). The membranes were blocked using 5% non-fat milk for 1 h at room temperature. The membranes were then incubated with the following primary antibodies: Anti-phosphorylated (p)-AKT (cat. no. ab38449),anti-p-mTOR (cat. no. ab84400), anti-autophagy protein 5(ATG5, cat. no. ab109490), anti-beclin 1 (cat. no. ab210498),anti-microtubule associated protein 1 light chain 3α (LC3,cat. no. ab62721) overnight at 4°C. All primary antibodies wereused at a 1:200 dilution and were purchased from Abcam. Following washing with PBS, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Abcam) for 1 h at room temperature. An ECL reagent kit (SantaCruz Biotechnology, Inc.) was used to visualize the immu-noreactive bands according to the manufacturer's protocol. Protein band intensities were quantified using Image J software (v1.8.0.112; National Institutes of Health). β-actin (1:200 dilution; cat. no. ab8227; Abcam) acted as the internal control.
Statistical analyses
All data are presented as mean ± standard deviation. One-way analysis of variance was used to assess statistical differences; pair wise analysis was performed by posthoc Tukey tests. All statistical analyses were performed using SPSS Statistics, version 19.0 (IBM, Armonk, NY, USA).Differences with P < 0.05 were considered statistically significant.
Results
The protective effect of ginsenoside Rg2 on cardiac function in rats
Before administration there was no difference among 3 groups in LVEDD, LVESD, LVEF and LVFS (table 1); there was an obvious increasing of LVEDD and LVESD and an obvious decreasing of LVEF and LVFS in TZM group, it was statistically significant compared with control group (P < 0.05); in TZM+Rg2 group, the changes were not obvious and there was no statistical difference between it and control group (P>0.05 Figures 1-4).
|
|
Control group |
TZM group |
TZM+Rg2 group |
|
LVEDD(mm) |
6.32±0.54 |
6.34±0.40 |
6.25±0.43 |
|
LVESD(mm) |
3.36±0.62 |
3.31±0.81 |
3.26±0.59 |
|
LVEF (%) |
78.05±5.81 |
80.72±8.10 |
81.77±6.46 |
|
LVFS (%) |
49.25±7.39 |
51.60±9.19 |
52.7±8.17 |
Table 1: Echocardiographic index of left ventricle in three groups of rats.
There was no statistical difference compared with control group, P>0.05. 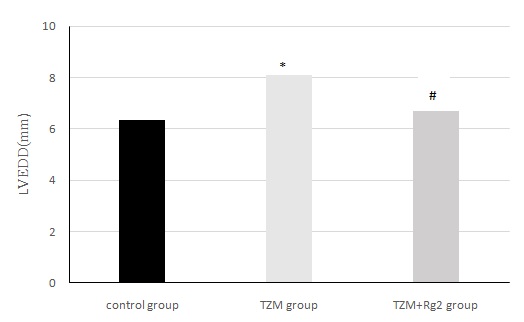
Figure1: Changes of cardiac LVEDD in three groups of rats after medication administration.
*Compared with control group(P < 0.05),#:Compared with control group(P>0.05)
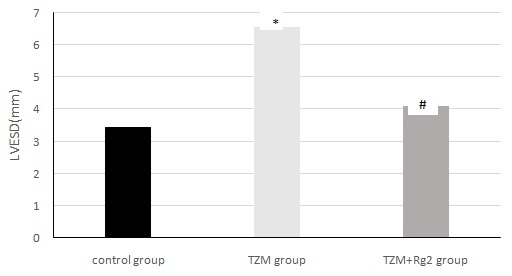 Figure 2: Changes of cardiac LVESD in three groups of rats after medication administration.
Figure 2: Changes of cardiac LVESD in three groups of rats after medication administration.
*Compared with control group (P < 0.05),#:Compared with control group(P>0.05)
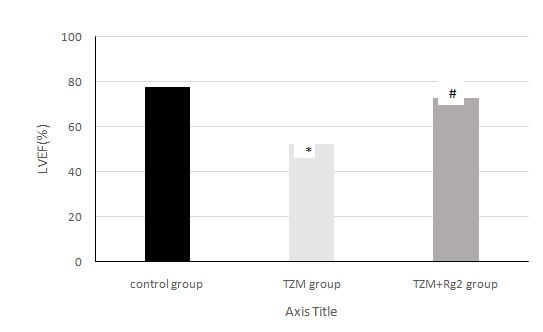 Figure 3: Changes of cardiac LVEF in three groups of rats after medication administration.
Figure 3: Changes of cardiac LVEF in three groups of rats after medication administration.
*Compared with control group (P < 0.05),#:Compared with control group(P>0.05)
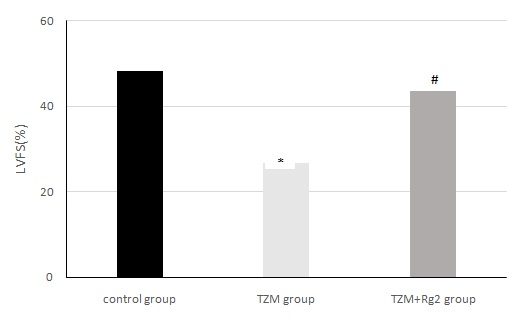 Figure 4: Changes of cardiac LVFS in three groups of rats after medication administration.
Figure 4: Changes of cardiac LVFS in three groups of rats after medication administration.
*Compared with control group (P < 0.05),#:Compared with control group(P>0.05)
The protective effects of ginsenoside Rg2 in TZM-induced cardiotoxicity of HCMs
In the colone test, we checked the cell proliferation, investigated that TZM could inhibit the proliferation and pretreatment with ginsenoside Rg2 improved the proliferation (Figure 5). In Annex in V test, we checked the cell apoptosis, TZM obviously promoted the apoptosis, pretreatment with ginsenoside Rg2 prevented the process (Figure 6).In western blot test, we checked the expressions of Caspase-3, Caspase-9 and BAX in the cells, there was a significant up-regulation in TZM group compared with control group, pretreatment with ginsenoside Rg2 could inhibit the up-regulation (Figure 7).
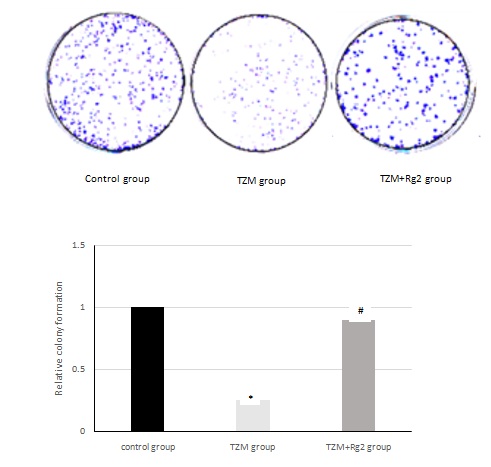 Figure 5: Effects of ginsenoside Rg2 on cell proliferation.
Figure 5: Effects of ginsenoside Rg2 on cell proliferation.
*Compared with control group(P < 0.05),#:Compared with control group(P>0.05).
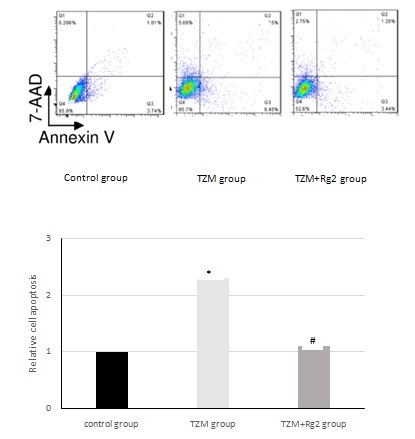 Figure 6: Effects of ginsenoside Rg2 on cell apoptosis.
Figure 6: Effects of ginsenoside Rg2 on cell apoptosis.
*Compared with control group (P<0.05), #:Compared with control group (P>0.05).
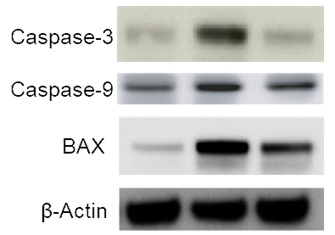 Figure 7: Effects of ginsenoside Rg2 on apoptosis-related proteins.
Figure 7: Effects of ginsenoside Rg2 on apoptosis-related proteins.
Discussion
In this study, we found that TZM induced toxicity via animal experiment and in vitro model of HCMs. The affection of HCMs included decreasing cell proliferation, increasing cell apoptosis and up-regulating the expressions of Caspase-3, Caspase-9 and BAX. However, the TZM-induced cardiotoxicity and Caspase-3, Caspase-9 and BAX up-regulation were reversed by ginsenoside Rg2. In animal experiment, TZM induced obvious cardiac disfunction showen as heart dilatation and LVEF decreasing, but pretreatment with ginsenoside Rg2 prevent the proceeding.
Ginseng has an important medical value and various compound prepatations have been used to treat cardiac disease. Ginsenoside Rg2 is a constituent of panaxatriol, previous studies have showen that ginsenoside Rg2 can resistant hypoxia injuries, reduce cell apoptosis and protect myocardial cells [13,14]. Recently, the other effects of ginsenoside Rg2 were reported more and more. Ginsenoside Rg2 can repair the UV-induced DNA damage, possibly via the pathway of regulating the P53 protein [15,16]. A reported study showed that ginsenoside Rg2 exerted protective effects against hydrogen peroxide-induced injury and apoptosis in HCMs [17]. Furthermore, ginsenoside Rg2 could increase autophagy and activated the p53/AMPK signaling pathway in MCF-7 breast cancer cells and decrease lipopolysac charide-induced Bax, caspase-3 and -9 expressions and exerted an antiapoptotic effect in neurons [18,19]. Furthermore, Wang et al., [20] investigated that ginsenoside Rg2 could inhibit the proliferation of breast cancer cells in vitro.
Collectively, in the study, we found that TZM could cause cell apoptosis, heart enlargement and cardiac disfunction, up-regulate the expressions of Caspase-3, Caspase-9 and BAX. It revealed that TZM induced cardiomyocyte injuries might be by cell apoptosis. Pretreatment with ginsenoside Rg2 inhibited the deterioration of cardiac function, down-regulate the expressions of Caspase-3, Caspase-9 and BAX, prompted that ginsenoside Rg2 could protect cardiomyocyte possibly via inhibiting cell apoptosis. Ginsenoside Rg2 may serve as a potential clinical agent to prevent TZM-related cardiotoxicity and may provide significant beneficial effects for patients with breast cancer. However, the data presented in this study requires further in vivo validation prior to the clinical application of ginsenoside Rg2.
Declaration of Conflicting Interest
The authors declare that there is no conflict of interest.
Funding
No funding was received.
References
- Gershon N, Berchenko Y, Hall PS, Goldstein DA (2019) Cost effectiveness and affordability of trastuzumab in sub-Saharan Africa for early stage. Cost Eff Resour Alloc 17: 5.
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, et al. (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365: 1273-1283.
- Mazzotta M, Krasniqi E, Barchiesi G, Pizzuti L, Tomao F, et al. (2019) Long-term safety and real-world effectiveness of trastuzumab in breast cancer. J Clin Med 8: 254.
- Sato A, Yoshihisa A, Miyata-Tatsumi M, Oikawa M, Kobayashi A, et al. (2019) Valvular heart disease as a possible predictor of trastuzumab-induced cardiotoxicity in patients with breast cancer. Mol Clin Oncol 10: 37-42.
- Xu QM, Jia D, Gao HW, Zhang MM, He WJ, et al. (2013) In vitro and in vivo protective effects of gingenosides on acute renal injury induced by cantharidin.J Functional Foods 5: 2012-2018.
- Christensen LP (2009) Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. J Adv Food Nutr Res 55: 1-99.
- Fu W, Sui D, Yu X, Gou D, Zhou Y, et al. (2015) Protective effects of ginsenoside Rg2 against H2O2-induced injury and apoptosis in H9c2 cells. Int J Clin Exp Med 8: 19938-19947.
- Gou D, Pei X, Wang J, Wang Y, Hu C, et al. (2020) Antiarrhythmic effects of ginsenoside Rg2 on calcium chloride-induced arrhythmic without oral toxicity. Journal of Ginseng Research 44: 717-724.
- Zhang G, Liu A, Zhou Y, San X, Jin T, et al. (2008) Panax ginseng ginsenoside-Rg2 protects memory impairment via anti-apoptosis in a rat model with vascular dementia. J Ethnopharmacol 115: 441-448.
- Fan Y, Wang N, Rocchi A, Zhang W, Vassar R, et al. (2017) Identification of natural products with neuronal and metabolic benefits through autophagy induction. Autophagy 13: 41-56.
- Chung Y, Jeong S, Choi HS, Ro S, Lee JS, et al. (2018) Upregulation of autophagy by Ginsenoside Rg2 in MCF-7 cells. Anim Cells Syst (Seoul) 22: 382-389.
- Fu W, Sui D, Yu X, Gou D, Zhou Y, et al. (2015) Protective effects of ginsenoside Rg2 against H2O2-induced injury and apoptosis in H9c2 cells. Int J Clin Exp Med 8: 19938-19947.
- Sun Y, Liu Y, Chen K (2016) Roles and mechanisms of ginsenoside in cardiovacular diseases: Progress and Perspectives. Science China (Life Sciences) 59: 292-298.
- Cui J, Wang J, Zheng M, Gou D, Liu C, et al. (2017) Ginsenoside Rg2 Protects PC12 cells against β-amyloid25-35 -induced apoptosis via the phosphoinositide 3-kinase/Akt pathway. Chemico-Biological Interactions 9: 152-161.
- Chung Y, Jeong S, Choi HS, Ro S, Lee JS, et al. (2018) UPregulation of AutoPhagy by Ginsenoside Rg2 in MCF-7 Cells. Animal cells and systems 22: 382-389.
- Chung YH, Jeong SA, Choi HS, Ro S, Lee JS, et al. (2018) Protective effects of ginsenoside Rg2 and astaxanthin mixture against UVB-induced DNA damage. Animal cells and systems 22: 400-406.
- Fu W, Sui D, Yu X, Gou D, Zhou Y, et al. (2015) Protective effects of ginsenoside Rg2 against H2O2-induced injury and apoptosis in H9c2 cells. Int J Clin Exp Med 8: 19938-19947.
- Chung Y, Jeong S, Choi HS, Ro S, Lee JS, et al. (2018) Upregulation of autophagy by Ginsenoside Rg2 in MCF-7 cells.Anim Cells Syst (Seoul) 22: 382-389.
- Chung YH, Jeong SA, Choi HS, Ro S, Lee JS, et al. (2018) Protective effects of ginsenoside Rg2 and astaxanthin mixtureagainst UVB-induced DNA damage. Anim Cells Syst (Seoul) 22: 400-406.
- Wang CZ, Aung HH, Zhang B, Sun S, Li XL, et al. (2008) Chemopreventive effects of heat-processed Panax quinquefolius root on human breast cancer cells. Anticancer Res 28: 2545-2551.
Citation: Liu G, Qi X (2021) The Effects of Ginsenoside Rg2 on Trastuzumab-Induced Cardiotoxicity. J Altern Complement Integr Med 7: 179.
Copyright: © 2021 Guang Liu, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

