The Impact of Pathological Changes in the Intestinal Microbiocenosis on the Appearance or Intensification of Negative Behavior in Children with ASD
*Corresponding Author(s):
Alexandra Alexandrovna MaximovaMiraculum Medical Center, Tbilisi, Georgia
Tel:+995 595283313,
Email:aleksandra-krasn@mail.ru ; am@maximov.com
Abstract
A causal relationship was found to exist between the many manifestations of negative behavior and the pathogenic microflora predominating in the intestines of children with ASD. Elimination of identified bacterial and fungal infections led to a decrease or disappearance of negative behavior, and, in a relapse, to their return. Negative behavior was associated with an imbalance of neurotransmitters, the effect of metabolites of pathogenic bacteria as neurotoxins, the occurrence of visceral and abdominal pain against the background of direct and indirect effects on the vagus nerve and sympathetic trunk in children with ASD. Parents and clinicians are advised to pay attention to possible problems in the gastrointestinal tract before resorting to psychotropic drugs when dealing with negative behavior in children diagnosed with ASD.
Keywords
ASD; Autism; Bacterial infection; Children; Diet; Fungal infection; Hysterical laughter; Microbiome; Negative behavior; Pathogenic flora; Sleep disorders
Introduction
The first publications that examined the relationship between disturbances in the structure of the intestinal microbiota and the functioning of the human brain appeared several years ago [1]. Each year, the interest in this topic grows, and, today, the number of publications and studies is in the hundreds [2-6]. It has been causally established that Parkinson's and Alzheimer's diseases can directly correlate to the state of gut microbiota. This does not mean that the root cause of the disease is precisely lesions in the gastrointestinal tract, but it does mean that pathological processes in the gastrointestinal tract aggravate and accelerate the course of these serious diseases [7].
The relationship between the gastrointestinal tract and autism has not been generally recognized by official medicine, but here, too, there have been advances in recent years. A number of studies have been published showing that negative behavior in children with autism may be associated with an imbalance in the gut microbiome. Various scientists and doctors have suggested different types of diets and anti-inflammatory protocols as a tool to stabilize the state of the microbiome [8]. America's most renowned and cutting-edge medical centers for autism remain within the DSM-5 (Diagnostic and Statistical Manual of Mental Disorders, 5th Edition), which traditionally classifies autism as a mental illness [9].
At the same time, some begin to attribute the problems of the gastrointestinal tract of children with ASD to the concomitant diseases of autism that require priority attention. For example, the Autism Spectrum Center at Boston Children's Hospital, on its home page, speaks of giving priority attention to the gastroenterological problems of children with autism [10]. The equally famous Johnson Center for Child Health and Development focuses the attention of parents and professionals on the widespread use of "dietary intervention and therapeutic nutrition" in their practice [11]. All this may be seen as advancement and a kind of forced compromise between official doctrine and actual clinical practice.
In most countries of the world understanding the importance of the gastrointestinal tract; when working with children with ASD does not reach clinical practice depriving many children of their chance for possible developmental progress. Miraculum’s practice in Georgia confirms this thesis and supports the belief that in many countries the situation is not much better. The overwhelming majority of clinicians continue to attribute children's stomach pains and other parental complaints to autism. Because of this, they neglect laboratory diagnostics to determine the state of their patients’ microbiome. All parents from the sample considered in this article (41 children) noted during a parental survey that their doctors did not pay due attention to the health of their children. Most explanations from specialists and their answers to parental questions, as a rule, boiled down to the diagnosis of "autism" as an inexplicable and incurable disease.
To change this situation, it is fundamentally important to constantly inform both parents and specialists; especially pediatricians, gastroenterologists, as well as psychiatrists and behavioral analysts (who work with children); to the fact that negative behavior is often caused by pathological changes in the intestinal microflora. By correcting these changes, in most cases, the negative behavior disappears, and the effectiveness of behavioral therapy and the cognitive development of a child rapidly increase.
In this study, we reflect our experience in managing children with ASD as patients with gastrointestinal problems. The relationship between pathological processes in the small and large intestines and manifestations of negative behavior in children with ASD has been shown. Potential treatment strategies are suggested.
Materials and Methods
Purpose of the study
To investigate the dependence of negative behavior in children with ASD on violations of the intestinal microbiocenosis
Research objectives
1. Investigate the relationship of intestinal microbiocenosis disorders to manifestations of negative behavior (anxiety, aggression/self-aggression, uncontrollable tantrums, hysterical laughter), sleep disturbances and emotional liability in children with ASD.
2. Suggest options for diagnosing intestinal microbiocenosis disorders.
3. Suggest potential therapeutic strategies and anti-inflammatory nutritional protocols:
• Combat the overgrowth of specific pathogenic bacterial and fungal infections,
• Correct intestinal microbiocenosis disorders.
4. Show a causal relationship and investigate the possible correlation between the successful correction of intestinal microbiocenosis disorders and the decrease or disappearance of the negative behavior.
Research materials
The relationship between the microbiome and negative behavior was conducted at the Center for Integrative Medicine, Miraculum (Tbilisi, Georgia; www.facebook.com/autism.ge), during a period of 14 months as part of a general study of the dependence of negative behavior in children with ASD on various pathological conditions. During this period, 71 patients with ASD, ages 3 to 12 years, were involved. Diagnoses: childhood autism (F.84) and atypical autism (F.84.1).
For this article, the considered sample was limited only to patients with pathological changes in the structure of the intestinal microflora. This is 58% of the entire sample or 41 children. All children were severely autistic and could not be tested. Accordingly, behavior was assessed with a parental questionnaire, “Modified Checklist for Autism in Toddlers, Revised, with Follow-Up (M-CHAT-R/F)" [12], as well as A. I. Zakharov’s test that assesses the level of anxiety in a child [13].
Research methods
1. Study of the history of the disease and the child's life;
2. Assessment of physical development;
3. Examination by the following specialists: gastroenterologist, neurologist, clinical psychologist, and pediatrician.
4. Laboratory diagnostics:
4.1. Coprogram and bacteriological culture of feces with sensitivity to bacteriophages and antibiotics (Research Institute of Microbiology, Virology, and Immunology named after G. Eliava; Tbilisi, Georgia);
4.2. Measurement of fecal calprotectin level (Mrcheveli Laboratory; Tbilisi, Georgia);
4.3. Measurement of zonulin level in feces (Helix Laboratory Services; Vladikavkaz, Russia);
4.4. Assessment of the microecological status of a person by the method of chromatography-mass spectrometry according to G.A. Osipov (Laboratory of Microbial Chromatography; Moscow, Russia) [14].
Research Results
Laboratory studies made it possible to register the following pathogenic bacterial and fungal infections, the presence of which in the microbiome indicates pathological changes in the intestine (Table 1):
|
NN |
Pathogenic bacterial and fungal infections |
% of Total |
Children with Pathology |
|
|
Growth of pathogenic bacteria and fungi in the colon |
||||
|
1 |
Hemolytic Escherichia coli |
63% |
26 |
|
|
2 |
Helicobacter pylori |
34% |
14 |
|
|
3 |
Candida albicans |
24% |
10 |
|
|
4 |
Hemolytic enterococcus |
22% |
9 |
|
|
5 |
Pseudomonas aeruginosa |
20% |
8 |
|
|
Growth of pathogenic bacteria and fungi in the small intestine |
||||
|
6 |
Micromycetes |
71% |
29 |
|
|
7 |
Streptococcus mutans |
54% |
22 |
|
|
8 |
Clostridium perfringens |
51% |
21 |
|
|
9 |
Candida albicans |
10% |
4 |
|
|
Confirmation of a silent inflammatory process |
||||
|
10 |
Increased levels of fecal calprotectin |
63% |
26 |
|
|
Total children in the sample for intestinal microbiotal disorders |
100% |
41 |
||
|
Total children in the sample for all pathological changes |
71 |
|||
Table 1: The presence of pathogenic bacteria and fungi in the intestine of children with ASD.
In the study of intestinal microbiome, for a number of historical reasons, one analysis is mainly used in modern world practice. This involves the microflora of only the large or only the small intestine. This significantly reduces the effectiveness of any therapeutic strategy that is developed, based on findings in this study.
The main research method for the colon in the United States and other Western countries is 16S rRNA sequencing, which, according to many experts, provides sufficient information on the health of both the large and small intestines. However, based on clinical practice described here, this method fails to provide a clear picture of the small intestine’s microbiome. There are no other widely used laboratory diagnostic tools for the small intestine. Currently, the state of the small intestine can be seen only with the help of mass spectrometry to analyze the metabolites of intestinal bacteria found in a patient’s blood and urine. In Western clinical laboratory diagnostics, this is a completely new direction. The first studies are beginning to appear, but it is too early to talk about practical tests [15].
In Russia, the situation is reversed. When analyzing the microbiome, they rely primarily on the study of the small intestine. In this country, the analysis of the spectrum of organic acids of blood by the method of gas chromatography with mass spectrometry (GC/MS) according to G. A. Osipov has been practiced since 2009 [14]. Several other laboratories in other countries of the world have been using the method in this field for microbial analysis and diagnostic even before 2009. But outside of Russia, this method has not become widespread in clinical practice. Inside Russia, it is also often believed that the analysis of the small intestine will be sufficient to track all the processes occurring in the upper and lower intestines.
We do not consider it correct to use only mono-analyzes. Based on the results of 16S rRNA sequencing, the clinician can only correct a condition in the large intestine, without considering possible problems occurring above in the small intestine. For example, a syndrome of intensive bacterial growth in the small intestine would be excluded. On the other hand, if the clinician relies on GC/ MS studies, then only conditions in the small intestine are corrected with the use of antibiotic therapy and various probiotics/prebiotics. In the end, this can aggravate conditions in the large intestine due to the growth of conditionally pathogenic flora, which received additional nutrition. In turn, this leads to deterioration in the general condition of the gastrointestinal tract, with a subsequent increase in the negative behavior of children with ASD.
In view of the above, an integrated approach to diagnose the state of the gut microbiome was used. During implementation of the project in Georgia, there was no opportunity to perform 16S rRNA sequencing of the microbiome to study the large intestine; therefore, we used an analysis of feces for dysbiosis and a coprogram for a rough assessment. For the small intestine, the GC / MS by G. A. Osipov are the only available and relatively effective method for studying this organ and is constantly used in our practice [14]. Accordingly, all therapeutic strategies were developed considering the complex picture of the microflora in all parts of the intestines.
The laboratory tests available to us identified 7 bacterial and 2 fungal infections in children with ASD, in the colon and/or small intestine. The study of the colon microbiome based on bacterial cultures and PCR diagnostics of feces can show only a small number of the types of bacteria and fungi: in the range from 10 to 30, as in this study. With 16S rRNA sequencing, two orders of magnitude more pathogens can be identified, about 1,700 bacteria and about 1,000 species of fungi. Such a detailed analysis of the microbiome is fundamentally important for selecting targeted pharmacological therapy and maintaining normal flora. For example, a prebiotic - inulin in one child can bring good results and, in another child, can be counterproductive because pathogenic flora grows on it. The same applies to other drugs. Both antibiotics and bacteriophages should be selected for sensitivity based on detailed analyzes.
It is hoped that soon this obvious thesis may be proved in practice. A deeper, more comprehensive and detailed study of the microbiome of the large and small intestine will allow for a targeted selection of drug therapy and effective correction of normal flora, including the creation and use of new bacteriophages against specific types of pathogenic flora. This is a promising pharmacological direction.
Finally, stool analysis for fecal calprotectin and zonulin, by themselves, had no practical diagnostic value since it did not explain the nature of inflammation and did not offer ways to combat it. It was useful, however, from the point of view of evidence-based confirmation and fixation of the process of sluggish inflammation arising from changes in the microbiome and the overgrowth of pathogenic flora. Therefore, for the clinician, an elevated level of calprotectin and/or zonulin may be a biomarker of inflammation and a reasonable place to start a laboratory search for its cause.
Statistical processing of research data
The method of nonparametric processing, namely Spearman's rank correlation coefficient, was used as statistical data processing. To assess the strength of correlations between variables, the Chaddock scale was used to interpret the value of the coefficient. The study revealed direct correlations between the following indicators:
- • Correlation between the level of calprotectin and autistic symptoms. (Determined by the M-CHAT-P / F and A. I. Zakharov tests). Graph 1 shows before treatment, Graph 2 after treatment;
- • Correlation between the level of zonulin and autistic symptoms. Graph 3 shows before treatment, Graph 4 after treatment.
All patients had their fecal calprotectin and fecal zonulin levels measured before and after treatment. Measurements revealed that with the growth of pathogenic flora in the large intestine, the level of calprotectin increased; with the growth of pathogenic flora in the small intestine, the level of zonulin increased. When changes in the microbiocenosis of both upper and lower intestines were noted, levels of both indicators increased. Depending on which part of the intestine the disorders grew fastest and strongest, calprotectin and zonulin indicators also grew faster there.
Treatment tactics, based on diagnosed disorders of the gastrointestinal tract, included: correcting problems caused by the type of pathogenic flora, overcoming enzymatic insufficiency, combating endotoxemia (for more details on treatment protocols, see below).
To confirm our hypothesis about the influence of the state of the gastrointestinal tract on negative behavior and the level of anxiety in children with autism spectrum disorder, we conducted testing on patients before and after treatment. Testing included the "Screening Tool for Functional Assessment" ("Modified Checklist for Autism in Toddlers, Revised, with Follow-Up (M-CHAT-R / F)") [12], as well as the A.I. Zakharov test to assess the level of anxiety in the child [13].
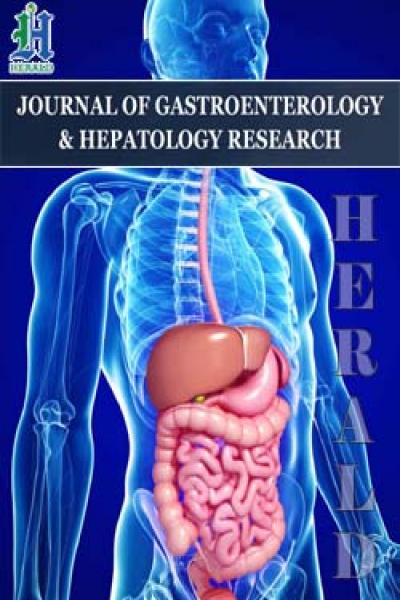
 Spearman correlation coefficient between calprotectin levels:
Spearman correlation coefficient between calprotectin levels:
- • Indicators for M-CHAT-R \ F test: 0.874064 (strong dependence);
- • Indicators for A.I. Zakharov test for level of anxiety: 0.988577 (very strong dependence).
Graph 1: Influence in level of fecal calprotectin on the number of autistic symptoms according to parental questionnaire M-CHAT-P / F and level of anxiety according to A.I. Zakharov.
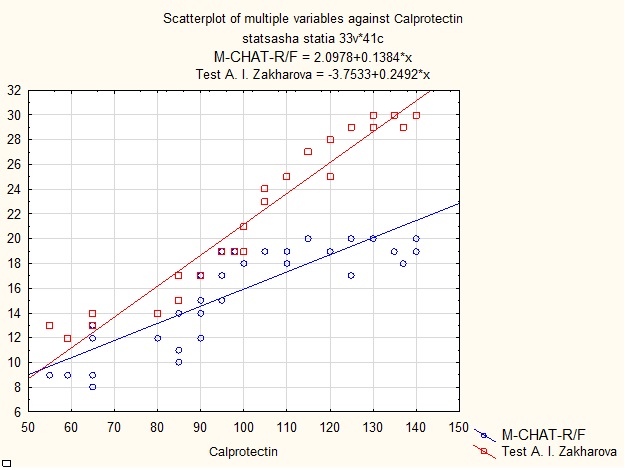
 Spearman correlation coefficient between post-treatment calprotectin levels:
Spearman correlation coefficient between post-treatment calprotectin levels:
- • Indicators for M-CHAT-R \ F test: 0.922176 (very strong dependence);
- • Indicators for A.I. Zakharov test for level of anxiety: 0.996724 (very strong dependence).
Graph 2: Influence of normalization of fecal calprotectin level on number of autistic symptoms according to the M-CHAT-R \ F parental questionnaire and level of anxiety according to A.I. Zakharov test.
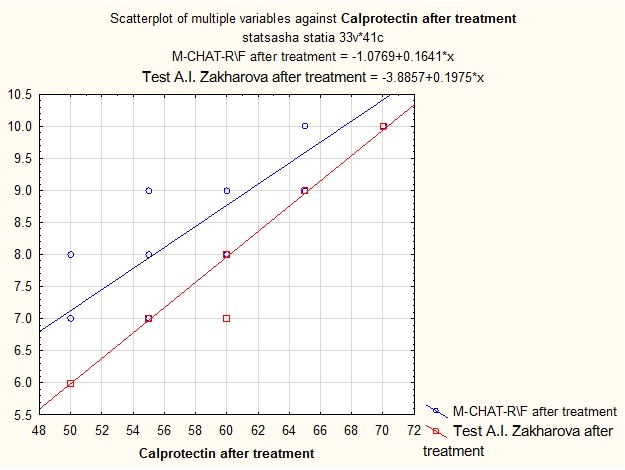
 Spearman correlation coefficient between zonulin levels:
Spearman correlation coefficient between zonulin levels:
- • Indicators for M-CHAT-R \ F test: 0.470662 (average dependence);
- • Indicators for A.I. Zakharov test for level of anxiety: 0.484298 (average dependence).
Graph 3: Influence of zonulin level on number of autistic symptoms according to parental questionnaire M-CHAT-P /F and level of anxiety according to A.I. Zakharov.
The data in graphs 1 and 2 confirm a direct correlation between the state of the gastrointestinal tract and negative behavior/level of anxiety. Colon health is particularly strongly correlated with negative behavior and anxiety levels. Perhaps this is due to the occurrence of abdominal visceral pain, as well as severe endotoxemia in problems of the large intestine.
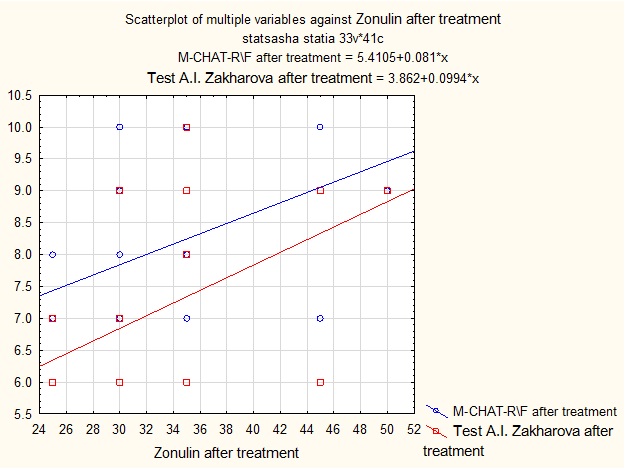 Correlation coefficient according to Spearman between the levels of zonulin after treatment:
Correlation coefficient according to Spearman between the levels of zonulin after treatment:
- • Indicators according to M-CHAT-R \ F test: 0.307596 (weak dependence);
- • Indicators for A.I. Zakharov test for level of anxiety: 0.293469 (weak dependence).
Graph 4: Influence of normalization in the level of zonulin on number of autistic symptoms according to parental questionnaire M-CHAT-R \ F and level of anxiety according to A.I. Zakharov.
After treatment, repeated testing and measurement of fecal calprotectin and zonulin levels were implemented. The results of these are presented in Graphs 3 and 4 and indicate that an improvement in the gastrointestinal tract led to a decrease in negative behavior and anxiety levels. At the same time, stabilization of conditions in the large intestine is significantly more correlated with a decrease in negative behavior and anxiety levels than is stabilization of conditions in the small intestine.
Features of bacterial and fungal infections and associated manifestations of negative behavior
Hemolytic enterococcus, Hemolytic escherichiacoli, Pseudomonasaeruginosa in the colon (correspondingly 26 children or 63%, 9 or 22%, and 8 or 20% of the respective samples).
These pathological changes led primarily to sleep disturbance and low concentrated attention. Occasionally, the growth of the above-named pathological flora led to aggression.
For these pathological changes, antibiotic therapy based on sensitivity was used, but, as a rule, these were antibiotics of the cephalosporin series. Standard therapy was also used to maintain normal intestinal flora — probiotics (usually Hylak Forte and Ultrabiotique) and prebiotics (psyllium). Enzymatic preparations of the Creon type were used, if necessary. The treatment lasted 14-28 days, after which an anti-inflammatory nutritional protocol was prescribed (protein minimization, exclusion of fast carbohydrates, sugars, and starches).
Behavior stabilized approximately 3 weeks after beginning treatment. All patients were advised to follow an anti-inflammatory nutritional protocol for a certain period after treatment to avoid a recurrence of pathological changes.
Growth of Candida albicans in the colon and small intestine (correspondingly, 10 children or 24% and 4 children or 10% of the respective samples).
According to a survey of parents, the growth of this fungus in the intestines was expressed in bouts of sudden hysterical laughter, both during the day and at night. These lasted from one to several minutes. Hysterical laughter often ended in outbursts of aggression or self-aggression. Sleep was disturbed; there was excessive anxiety during sleep with early awakenings and / or frequent episodic waking in the middle of the night, accompanied by crying and / or severe aggression.
Standard anti-candida therapy (Fluconazole and Enterosgel), antihistamines (Aerius or Fenistil), and probiotic (Ultrabiotique) was used. Intensive therapy took about 14 days. Then, to maintain a stable state, an anti-candidiasis nutritional protocol was used, with the exclusion of sweets (including sweet fruits) and fast carbohydrates (various kinds of bakery and pasta, baked goods, sugary carbonated drinks) in combination with the intake of oregano essential oil.
During the first week of treatment, all parents noted a significant deterioration in the condition of their children. There was growing weakness or, to the contrary, intensified aggression, and sleep disturbances increased sharply. This was seen as due to the increasing endotoxemia of the child's body due to the death of pathogenic flora. In the second week of treatment, parents recorded gradual improvements. After about a month, all negative manifestations of behavior (aggression, anxiety) and sleep disturbances disappeared.
Helicobacter pylori infection (14 children or 34% of the sample).
This type of bacterial infection has attracted the attention of scientists and doctors, and recent studies have been devoted to it [16,17]. In this project, the main complaints of parents with children who had such an infection was sleep disturbances, hyperactivity, or a child's “shutdown." The children were treated with antibiotic therapy, an anti-inflammatory nutritional protocol, diet, and a course of pro / prebiotics based on the results of microbiome analyzes. The course of treatment was 1 month. After treatment, parents noted a significant improvement in concentration, “involvement” by the child, and sleep stabilization. Helinorm was used for rotation.
Micromycetesin the small intestine (29 children or 71% of the sample).
Unlike Candida albicans, the growth of this fungal flora did not cause hysterical laughter. It led instead to outbursts of aggression and self-aggression. The parents of 5 children, based on recommendations under this study, had EEG studies performed, and excitation foci were recorded. Sleep disturbances were not always observed.
Treatment involved anti-candidiasis drugs (including Voriconazole) as well as therapy to maintain normal intestinal flora, taking probiotics (usually Hylak Forte and Ultrabiotique), and a prebiotic (psyllium). If necessary, Creon was used as an enzymatic preparation. Treatment lasted14 to 28 days; followed by an anti-inflammatory nutritional protocol (protein minimization; exclusion of fast carbohydrates, sugars, and starch). Oregano essential oil was used in rotation. Behavior stabilized after about a month from the start of treatment. All patients were advised to follow an anti-inflammatory nutritional protocol for a certain period of time after treatment to avoid a recurrence of pathological conditions.
Streptococcus mutans in the small intestine (22 children or 54% of the sample).
With this bacterial infection, parents noted a high level of anxiety and periodic outbreaks of aggression and self-aggression in children. Sleep disturbances were not always recorded.
Phage therapy (Streptococcal bacteriophage) was used to combat bacteria, which was applied per os and per rectum. Treatment with bacteriophages was carried out for 21 days with the simultaneous use of probiotics and prebiotics, and, if necessary, enzyme preparations. The protocol also included enhanced detoxification with a silica sorbent. An anti-inflammatory nutritional protocol was introduced from the first day of treatment.
Parents noted an improvement in the child's condition and a decrease in negative behavior as early as 7 days after treatment began.
Clostridium perfringens in the small intestine (21 children or 51% of the sample).
The overgrowth of this bacterium led to sleep disturbances, high levels of anxiety, and emotional lability. The latter was generally associated mainly with regular abdominal pain. Parents also noted intermittent periods of sudden anxiety, describing these manifestations as "anxiety arising for no reason." This anxiety was expressed as an obsession with something, in stereotypic movements and with fear of everything around them.
The treatment used was antibiotic therapy and normal flora support. Of all the pathological changes we considered, the treatment with Clostridium perfringens turned out to be the longest and most difficult. Doses of antibiotics were used 5-6 times lower than the recommended dosage, but the duration of treatment was extended to a month. An anti-inflammatory nutritional protocol was introduced at the same time. Without this, it is not possible to maintain restoration of intestinal walls.
Parents noted an improvement 1.5 months after starting treatment, but, unlike other bacterial and fungal infections, this improvement was very unstable and reversible. At the slightest disturbance in the diet, both abdominal pain and sleep disturbances returned.
Level of fecal calprotectin
An increase in the level of fecal calprotectin was recorded in all children with pathological changes in the colon. Moreover, when the pathology occurred only in the small intestine, calprotectin values were insignificant or did not register at all. With stabilization of the gastrointestinal tract resulting from treatment, the fecal calprotectin indices returned to normal. This indicator by itself does not carry practical diagnostic value since it does not indicate the reason for its increase. It does point to the need for additional analyzes, first of which is a bacteriological culture of the colon and analysis of feces by the GS / MS method according to Osipov. In other words, fecal calprotectin is an important marker pointing to ongoing sluggish inflammation of the colon, albeit without information about its causes.
Antimicrobial function of propolis
As an additional therapeutic recommendation, it is recommended that all children take a water-soluble form of propolis, which has an antimicrobial function, after the intensive phase of treatment [18]. In this study, propolis intake had a positive effect on the stability of the intestinal microbiome and reduced the recurrence or exacerbation of gastrointestinal diseases. In the parental survey, parents noted a decrease in the frequency of acute respiratory diseases in children, which are attributed, at least partially, to the action of propolis. As a side note, research is currently underway on the beneficial effect of propolis in Parkinson's disease and rheumatoid arthritis [19].
Triggering negative behavior
In general, statistics from the research evidence suggests that the predominance of pathogenic microflora in the intestines led to manifestations of negative behavior in children with ASD. However, this is an observation, not an explanation. The research and evidence-based description of the mechanisms for triggering negative behavior due to pathogenic changes in the microbiome is a separate and independent topic of scientific work. This article is limited to listing the possible options that might launch mechanisms that can function both independently and in conjunction with each other:
- Direct influence of the metabolites of pathogenic bacteria as neurotoxins;
- Indirect effect of the metabolites of pathogenic bacteria on the autonomic nervous system and, specifically, on the vagus nerve and sympathetic trunk;
- Emergence of visceral pain of unknown origin due to neurotoxins on the autonomic nervous system;
- Visceral pain arising from a change in pressure in the stomach and intestines during the contraction and relaxation in muscles of these organs due to pathological changes in the microbiocenosis of the intestines and stomach.
- Visceral pain arising from ischemia of intestinal and stomach tissues due to the formation of acidic metabolic products from pathogenic bacteria;
- Chronic visceral pain resulting from central sensitization and impaired autonomic innervation of internal organs. This may be due to an imbalance of neuropeptides and substance P in children with ASD and the direct effect of neurotoxins of pathogenic bacteria on NMDA receptors;
- Abdominal pains indifferent locations associated with IBS (irritable bowel syndrome) due to disturbed intestinal microbiocenosis and the proliferation of pathogenic bacteria that produce metabolites for excessive synthesis of serotonin;
- Direct effect of serotonin imbalance on the brainstem and limbic system due to disturbed intestinal microbiocenosis;
- Direct influence of excess glutamate on the nervous system from the presence of many producers of this neurotransmitter in the intestines.
At the same time, the current lack of a final answer to the question about the etiology of dysfunction of the autonomic (and central) nervous system in violation of intestinal microbiocenosis is not a reason to deny a child and his parents’ medical care. Pediatricians and psychiatrists working with children with ASD should pay special attention to the manifestations of negative behavior in young patients and refer them to a gastroenterologist and other doctors in clinical practice. In practice, as noted in this study, the correction of microbiocenosis can, in principle, remove the issue of negative behavior.
Interaction of pathogenic microbiota with other pathologies
In most cases, microbiota disorders combined and interacted with other pathological conditions in children. Accordingly, negative behaviors were recorded when the intestinal microbiome was violated in isolation and when they were linked to other pathological conditions (growth of pathogenic flora in the nasopharynx and pharynx, low blood iron levels, increased ASO levels, cortisol imbalance), as described in another article by the current author [20,21].
Stabilization of behavior always begins with stabilization of the gastrointestinal tract. The intestines area platform for many physiological processes in the body that ensure correct functioning of the immune system and the central and autonomic nervous systems, as well as the hypothalamic-pituitary adrenal axis. Therefore, in practice, stabilization of the gastrointestinal tract is always the first step. If manifestations of negative behavior persist, pathological changes that need correcting are determined and a sequence of interrelated treatment protocols is outlined.
At the same time, since autism is the consequence of intricate comorbid pathology, it is often difficult or impossible to immediately determine the main (underlying) pathological factor. There is no doubt that the choice of a specific anti-inflammatory nutritional protocol is based primarily on the specific pathogenic structure of the gut microbiome. However, the final decision (especially in severe cases) should be made considering the hormonal background, the work of all neurometabolic systems, and all pathological factors of the patient identified during the process of diagnosing comorbid pathology.
Based on practice, it is often impossible to use a mono-diet to correct a patient's condition. It is extremely important to monitor in a timely manner the pathological disorders that were previously hidden and then manifested during treatment and concurrently adjust the prescribed nutritional protocol (diet). However, the issue of the interaction of the gastrointestinal tract and other basic systems of a child's body during integrated treatment of a complex comorbid pathology is a topic for another independent article.
The comorbidity of autism does not allow treating only its most pronounced manifestations, which, as a rule, are associated precisely with disorders of the gastrointestinal tract and the nervous system. It is necessary to consistently examine all the main systems and organs of the patient to identify hidden or sluggish pathological processes. This dictates the imperative of an interdisciplinary medical approach to the treatment of autism and the search for interrelated mechanisms that trigger it.
Conclusion
During this study, 9 bacterial or fungal infections were identified, leading to an excessive proliferation of pathogenic intestinal microflora. In the colon, these were Hemolytic Escherichia coli, Helicobacter pylori, Candida albicans, Hemolytic enterococcus, and Pseudomonas aeruginosa. In the small intestine, there were Micromycetes, Streptococcus mutans, Clostridium perfringens, and Candida albicans.
Pathological changes in the intestines of children with ASD in all cases, without exception, led to such negative behavior as aggression/self-aggression, uncontrolled tantrums, increased anxiety and sleep disturbances. In two cases, specific manifestations were added to the general manifestations:
(1) The proliferation of Candida albicans led to outbursts of hysterical laughter followed by bouts of aggression, and
(2) when infected with Helicobacter pylori, parents also noted the appearance of hyperactivity, decreased concentration, and a child’s being "turned off.”
(3) A high correlation was found between
(1) The appearance or intensification of negative behavior and other aforementioned disorders and
(2) a high level of calprotectin in the intestine, indicating the development of a sluggish inflammatory process in the large and/or small intestine. At a norm of 50μg/g, the level of calprotectin ranged from 90 to 145μg/g. In turn, with the growth of pathogenic flora in the small intestine, high levels of zonulin, from 85-110 ng/ml, were detected.
(5) Correction of these changes in the intestinal microflora and the cessation of the overgrowth of pathogenic bacteria and fungi led to a decrease or disappearance of negative behavior.
(6) The following mechanisms for triggering negative behavior could be related:
- • To an imbalance of neurotransmitters,
- • To the influence of the metabolites of pathogenic bacteria as neurotoxins, or
- • To the occurrence of visceral and abdominal pain against a background of direct and indirect effects on the vagus nerve and the sympathetic trunk in children with ASD.
With any trigger mechanism, the negative behavior of children decreased or disappeared as the growth of pathogenic microflora stopped and or a positive correction of intestinal microbiocenosis occurred. This was noted by the parents of all children without exception. Due to the selected therapeutic strategies, sleep stabilized, anxiety, tantrums, bouts of aggression/self-aggression, and hysterical laughter disappeared, concentrated attention, and "involvement" of the child in the cognitive process improved.
These observations causally associated through clinical practice, allows the following conclusions and recommendations:
- • Parents and healthcare professionals should not attribute all negative behaviors to autism. It should be remembered that these manifestations may be the result of pathological changes in a child's gastrointestinal tract, which need to be diagnosed and corrected. Accordingly, it is necessary to consult with a gastroenterologist and other specialists.
- • Parents and healthcare professionals should not attribute all negative behaviors to autism. It should be remembered that these manifestations may be the result of pathological changes in a child's gastrointestinal tract, which need to be diagnosed and corrected. Accordingly, it is necessary to consult with a gastroenterologist and other specialists.
- • These recommendations apply directly to psychologists, behavioral analysts, speech pathologists, tutors, and other professionals who work with children diagnosed with autism spectrum disorder. If there is a suspicion that medical reasons might be causing the negative behavior, children should be sent for a specialized consultation, and the corrective and cognitive load on children should not be expanded. Moreover, with the disappearance of negative behavior, the effectiveness of behavioral and corrective therapy increases many times over. For children who have passed or are undergoing our medical protocols, this is confirmed by the experience of the Resource Class (for schoolchildren 12-13 years old) and the Children's Resource Group (for children 3-6 years old) at the Miraculum Center.
- • Fits of hysterical laughter can be a symptom of a proliferating Candida albicans. It can, therefore, be an additional diagnostic sign for both parents and professionals.
- • The gastrointestinal tract is a platform for many physiological processes in the body that ensures correct functioning of the immune system and the central and autonomic nervous systems, as well as the hypothalamic-pituitary adrenal axis. Therefore, stabilization of the main systems of a child's body should begin with the intestines. Concurrently, keep in mind that disorders of the gastrointestinal tract can be linked to pathological changes in other bodily systems. This dictates the need for an interdisciplinary medical approach when diagnosing pathological conditions in children with ASD.
- • Finally, the relationship between intestinal microbiocenosis and negative behavior must be considered when prescribing drug therapy for children with ASD. These children are often prescribed antidepressants, hypnotics, and / or antipsychotics for high levels of anxiety, agitation, and / or sleep disturbances. Clinicians, however, should first pay attention to possible pathological processes that are the root cause of negative behavior. Only after pathological conditions are confidently excluded from the list of causes for negative behavior, can the doctor consider the possibility of connecting drugs from the psychotropic class to therapy.
(7) Patient Consent Statements: All guardians of patients signed an agreement to participate in this study.
References
- Children's autism: research and practice (2008) In Ed: Kasatkin VN, Center for Psychological, Medical and Social Support for Children and Adolescents ISBN: 978-5-9900666-3-2.
- Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, et al. (2015) Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord 30:350-358.
- Wood H (2015) Gut reactions—can changes in the intestinal microbiome provide new insights into Parkinson disease? Nat Rev Neurol11: 66.
- GenerosoJS, Giridharan VV, Lee J, Macedo D, Barichello T (2020) The role of the microbiota-gut-brain axis in neuropsychiatric disorders. Braz J Psychiatry.
- Van Ameringen M, Turna J, Patterson B, Pipe A, Mao RQ, et al. (2019) The gut microbiome in psychiatry: A primer for clinicians. Depress Anxiety 36: 1004-1025.
- Ngo ST, Restuadi R, McCrae AF, Van Eijk RP, Garton F, et al. (2020) Progression and survival of patients with motor neuron disease relative to their fecal microbiota. Amyotroph Lateral Scler Frontotemporal Degener 21: 549-562.
- Riccio P, Rossano R (2019) Undigested Food and Gut Microbiota May Cooperate in the Pathogenesis of Neuroinflammatory Diseases: A Matter of Barriers and a Proposal on the Origin of Organ Specificity. Nutrients 11: 2714.
- Gogou M , Kolios G (2020) Is there place for nutrition in the treatment of children with autism spectrum disorder? Psychiatriki 31: 57-69.
- https://www.psychiatry.org/psychiatrists/practice/dsm
- http://www.childrenshospital.org/centers-and-services/programs/a-_-e/autism-spectrum-center-program
- http://www.johnson-center.org/healthcare
- Robins DL, Fein D, Barton M (2009) Modified Checklist for Autism in Toddlers, Revised, with Follow-Up (M-CHAT-R/F).
- Rafikovna MA (2015) A.I. test Zakharova to assess the level of anxiety of the child.
- Strukova EG, Efremov AA, Gontova AA, Osipov GA, Sarmatova NI (2009) Determination of the microecological status and diagnosis of infections of the human body using the method of chromatography-mass spectrometry. J Sib Fed Univ Chem 4: 351-358.
- Paprotny L, Celejewska A, Frajberg M, Wianowska D (2019) Development and validation of GC-MS/MS method useful in diagnosing intestinal dysbiosis. J Chromatogr B Analyt Technol Biomed Life Sci 1130-1131: 121822.
- Tucker RM, Augustin AD, Hayee BH, Bjarnason I, Taylor D, et al. (2020) Role of Helicobactersin Neuropsychiatric Disease: A Systematic Review in Idiopathic Parkinsonism. J Clin Med 9: 2159.
- Dobbs SM, Charlett A, Dobbs RJ, Weller C, Iguodala O, et al. (2013) Antimicrobial surveillance in idiopathic parkinsonism: indication-specific improvement in hypokinesia following Helicobacter pylori eradication and non-specific effect of antimicrobials for other indications in worsening rigidity. Helicobacter 18: 187-196.
- Petruzzi L, Rosaria Corbo M, Campaniello D, Speranza B, Sinigaglia M, et al. (2020) Antifungal and Antibacterial Effect of Propolis: A Comparative Hit for Food-Borne Pseudomonas, Enterobacteriaceae and Fungi. Foods 9: 559.
- Ayikobua ET, Kasolo J, Kasozi KI, Eze ED, Safiriyu A, et al. (2020) Synergistic action of propolis with levodopa in the management of Parkinsonism in Drosophila melanogaster. J Complement Integr Med 17.
- Maximova AA (2020) Dependence of Negative Behavior on Somatic Pathological Processes in ASD Children // Universum: medicine and pharmacology. Int Sci J
- Ibrahim MS, Balhaddad AA, Garcia IM, Hefni E, Collares FM, et al. (2020) Tooth sealing formulation with bacteria-killing surface and on-demand ion release/recharge inhibits early childhood caries key pathogens. J Biomed Mater Res B Appl Biomater 108: 3217-3227.
Citation: Maximova AA (2021) The Impact of Pathological Changes in the Intestinal Microbiocenosis on the Appearance or Intensification of Negative Behavior in Children with ASD. J Gastroenterol Hepatology Res 6: 032.
Copyright: © 2021 Alexandra Alexandrovna Maximova, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.