
The links between inflammatory parameters in acute and chronic inflammatory responses in terms of the surgical stress response and Systemic Lupus Erythematosus
*Corresponding Author(s):
Barbara LisowskaAnaesthesiology Department, Carolina Medical Centre, Warsaw, Poland
Email:blisowska19@gmail.com
Abstract
Inflammation is a biological response of the immune system that can be induced by a variety of factors. Two types of inflammatory response exist, namely, the acute and chronic responses. Acute inflammation is a physiological response to trauma, while chronic inflammation is a pathological state which has an important role in the onset and progression of many diseases, including autoimmune diseases. The presence of chronic inflammation may impact the severity of an acute inflammatory response.
The cells and mediators of the innate and acquired responses are involved in both acute and chronic inflammation. Cytokines are inflammatory modulators, the activation of which is mandatory for any inflammatory response. Surgery and anesthesia induce acute inflammatory responses primarily by activating the cytokine production pathway.
The dysregulation of cytokine activation plays a crucial role in chronic inflammation and also underlies the pathogenesis of Systemic Lupus Erythematosus (SLE), an autoimmune disease with a multifactorial etiology that includes many immunological changes that cause chronic inflammation and affect both innate and adaptive responses.
In this review, we discuss the complex mechanisms of inflammation and we focus on the correlations and roles of inflammatory mediators in both acute and chronic inflammation with examples of stress responses to surgery and systemic lupus erythematosus.
Keywords
Acute; Chronic Inflammation; Stress Surgery; Systemic Lupus Erythematosus.
Introduction
Inflammation is a biological response of the immune system that can be caused by different factors, including trauma, pathogens, and toxic compounds. There are two types of inflammatory responses: acute inflammation, which is a physiological and protective reaction to injury, and chronic inflammation, which plays an important role in the pathogenesis of many diseases, including autoimmune diseases [1].
Both acute and chronic inflammation involve the cells and mediators of the innate and acquired responses. Cytokines, as well-known inflammatory modulators, combine these responses through cross-communication in a complex information distribution network [2].
Systemic Lupus Erythematosus (SLE) is an autoimmune disease with a complex and heterogenous etiology. Many genetic and environmental factors cause the characteristic hormonal and immunological dysregulations in innate and adaptive responses. No single mechanism is known to explain the complexity of SLE’s pathogenesis. Chronic inflammation, with central roles played by neutrophils, interferon-alpha, transcription factors, and signaling dysfunction, is fundamental to the development and progression of SLE. Dysregulation of apoptosis, defects in the removal of circulating immune complexes and their deposition in tissues, the accumulation of B and T helper lymphocytes, and an impairment in the function of suppressive T lymphocytes also play pivotal roles in the development of this disease [1,2]. The diagnostic criteria for SLE are presented in (Table 1).
|
DEVELOPMENT AND VALIDATION OF SLE CLASSIFICATION CRITERIA |
|||
|
ENTRY CRITERION Antinuclear antibodies ANA at a titre of ≥1:80 on Hep-2 cells or an equivalent positive test. |
|||
|
If absent, do not classify as SLE; if present, apply additive criteria. |
|||
|
Addditive criteria |
|||
|
Clinical domains and criteria |
Weight |
Immunology domains and criteria |
Weight |
|
Constitutional |
Antiphospholipid antibodies |
||
|
Fever |
2 |
Anti-cardiolipin or anti-β2GP1 antibodies or lupus anticoagulant |
2 |
|
Hematologic |
Complement proteins |
||
|
Leukopenia |
3 |
Low C3 or C4 Low C3 and C4 |
3 4 |
|
Thrombocytopenia |
4 |
SLE-specific antibodies |
|
|
Autoimmune hemolysis |
4 |
Anti-dsDNA or anti-Smith antibody |
6 |
|
Neuropsychiatric |
|
||
|
Delirium |
2 |
||
|
Psychosis |
3 |
||
|
Seizure |
5 |
||
|
Mucocutaneous |
|||
|
Non-scarring alopecia |
2 |
||
|
Oral ulcers |
2 |
||
|
Subacute cutaneous |
4 |
||
|
Acute cutaneous lupus |
6 |
||
|
Serosal |
|||
|
Pleural or pericardial effusion |
5 |
||
|
Acute pericarditis |
6 |
||
|
Musculoskeletal |
|
||
|
Joint involvement |
6 |
||
|
Renal |
|
||
|
Proteinuria of >0.5 g/24h |
4 |
||
|
Renal biopsy Class II or V LN |
8 |
||
|
Renal biopsy Class III or IV LN |
10 |
||
|
CLASSIFY AS SLE WITH A SCORE OF ≥10 |
|||
|
Aringer M. et al., European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus [65] |
|||
Table 1: SLE Classification Criteria
After surgical trauma, the inflammatory response, through the activation of the immune and neuroendocrine systems, works to alleviate the injury and return to a state of previous homeostasis. Moreover, a reduction in protein synthesis has been observed, and the metabolic changes seem to be proportional to the extent of the surgery [3]. The intensity of a patient’s response to surgery stress depends on many factors, including the extent and approach of the surgery, the general condition and nutritional status of the patient, the presence of chronic diseases and infections, and the use of medications (Chronic and Perioperative) [4,5].
The immune system’s response to surgical trauma can be described from a local or a systemic perspective. The early inflammatory response triggers local changes in the injured tissue with capillary dilatation and an accumulation of neutrophils and monocytes. Leukocytes gather at the site of the injury and stimulate the release of pro-inflammatory cytokines. Cytokines, which participate in the development of the acute inflammatory response, act via the activation of the innate response cells, blood proteins, and other inflammatory mediators.
Then, in an acquired immunological response, the Antigen-Presenting Cells (APCs), including Dendritic Cells (DCs), macrophages, and B cells, retrieve antigens from the peripheral tissues and present them to T cells. Acquired immunity is characterized by its specificity, and it includes both the T- and B-cell populations of the lymphocytes. The role of B lymphocytes is not limited to humoral responses. The cellular immune response is carried out primarily by various T-lymphocyte subpopulations [6].
The next step comprises a compensatory response that triggers a cascade of reactions. The released anti-inflammatory compounds are mobilized to suppress the inflammation and restore homeostasis. The process of inflammatory response reduction is characterized by a decrease in leukocyte accumulation at the site of the injury, initiation of apoptosis, and a drop in the synthesis and release of the pro-inflammatory cytokines. Additionally, there is a decrease in lymphocyte proliferation and a release of anti-inflammatory cytokines such as transforming growth factor (TGF-β), IL-10, soluble TNF receptor, and IL-1 receptor antagonist (IL-1Ra) [6]. The inflammatory response to surgical tissue damage is outlined in (Figure 1).
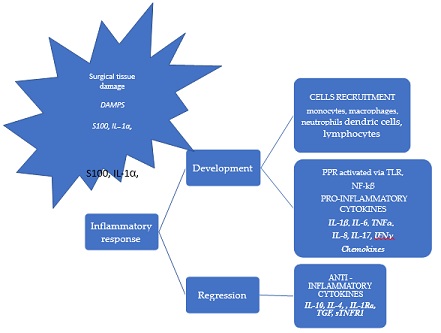 Figure 1: Inflammatory response to surgical tissue damage.
Figure 1: Inflammatory response to surgical tissue damage.
Local and systemic inflammatory responses to surgical trauma. The cytokine network is an example of intercellular signaling and communication based on the multidirectional activity of cytokines linked by signal-dependent pathways. The cells of the innate immune system,such as monocytes, macrophages, and neutrophils, are involved in the local immune response, DAMPs, S100 and IL-1α are released by damaged cells. Cytokines and chemokines play key roles in the induction and maintenance of innate and systemic immunity, including both cellular and humoral responses. The cytokine balance between highlighted in red text, and the anti-inflammatory cytokines are highlighted in black text. TNF-α tumor necrosis factor; IFN-γ, interferon γ IL-1Ra, interleukin -1 receptor antagonist; TGF, transforming growth factor; sTNFR1, soluble tumor necrosis factor receptor 1.
Chronic inflammation may modify the severity of an acute inflammation response by creating an imbalance between the pro- and anti-inflammatory responses. A predominance of anti-inflammatory factors was observed in patients with Rheumatoid Arthritis (RA) after orthopedic surgery (Postoperative Fluid Drainage). Moreover, the acute inflammatory response (Local and Systemic) was positively correlated with the age of the patient and the duration of the RA [7].
Chronic inflammation can also provoke an increased risk of postoperative infection and delays in wound healing.
Long-term, drawn-out chronic inflammation followed by multiorgan immunopathological changes is frequently mediated by vasculitis. Systemic Lupus Erythematosus (SLE) is characterized by chronic systematic inflammation, which causes the multiorgan damage outlined in (Figure 2) [8,9]. Acute and chronic inflammation pathomechanisms and outcomes are presented in (Table 2).
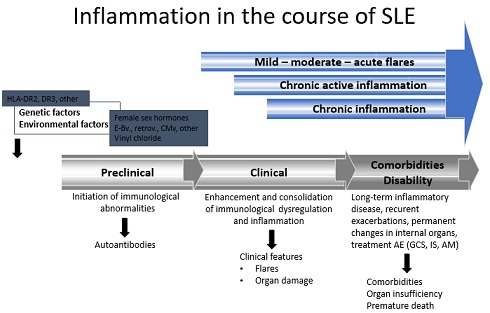 Figure 2: Inflammation in the course of SLE
Figure 2: Inflammation in the course of SLE
|
Acute inflammation |
Chronic inflammation |
|
Causative factor |
|
|
Pathogens, injured tissues |
Persistent acute inflammation due to non-degradable pathogens and autoimmune reactions |
|
Primary mediators |
|
|
Vasoactive amines, eicosanoids |
IFN-α, other cytokines, growth factors, reactive oxygen species, and hydrolytic enzymes |
|
Pathomechanism |
|
|
Capillary dilatation
Neutrophils and monocytes accumulation
Pro-inflammatory cytokines release
Activation of cells of the innate response, blood proteins, and other inflammatory mediators
APCs present antigens to T cells |
Neutrophil influx Increased IFN-α levels and transcription factors Signaling dysfunction Dysregulation of apoptosis Defects in the removal of circulating ICs Increased deposition of ICs in tissues Accumulation of lymphocytes B and Th Autoantibody hypersecretion Decreased function of suppressive T cells |
|
Outcomes |
|
|
Resolution, abscess formation, chronic inflammation |
Tissue destruction, fibrosis |
|
Suppression of inflammation and restoration of homeostasis Decreased accumulation of leukocytes Initiation of apoptosis Decreased synthesis and release of pro-inflammatory cytokines Decreased lymphocyte proliferation Increased anti-inflammatory cytokines (TGF-β, IL-10, sTNF-R, and IL-1Ra) |
Multiorgan damage due to chronic persistent inflammation and side effects of treatment Pre-term mortality |
|
APCs, antigen-presenting cells; ICs, immunological complexes. |
|
Table 2: Acute and Chronic Inflammation: Pathomechanisms and Outcomes
Therefore, patients with SLE, as well as patients with RA, require particularly careful preoperative assessment and determination of individual anesthetic management, depending on the degree of the multiorgan disorders caused by SLE [10]. Patients with SLE typically manifest renal insufficiency as a form of lupus nephritis, as well as cardiovascular disturbances with symptoms of pericarditis, myocarditis, arthrosclerosis, and myocardial ischemia. Respiratory problems may involve pleuritis, pleural effusion, alveolar hemorrhage, and interstitial lung disease [11]. The possibility of difficult intubation also exists because of subglottic stenosis and/or laryngeal oedema. Moreover, hematological disturbances associated with SLE, such as anemia, thrombocytopenia, and lupus anticoagulant-hypoprothrombinemia syndrome, can increase the risk of bleeding complications perioperatively [11]. The other key points are dysfunctions of the central and peripheral nervous systems and a higher risk of postoperative pancreatitis, protein-losing enteropathy, and malabsorption [12].
Management strategies for SLE include long-term treatment with many immunosuppressants, including corticosteroids, which are associated with a high risk of postoperative hypothalamic–pituitary–adrenal axis insufficiency, infections, glucose imbalance, and wound-healing complications [12].
In summary, SLE patients are particularly challenging for anesthesiologists and they require individualized management strategies to ensure safe anesthesia.
In this review, we discuss the multiple components and mechanisms of inflammatory responses, with a special focus on several of the key pro-inflammatory cytokines and inflammatory mediators of chronic and acute inflammatory responses involved in SLE and the stress response to surgery.
Main Mechanisms of Inflammatory Responses in Sle and the Stress Response to Surgery
Cytokines are major modulators of acute and chronic inflammatory responses. Under conditions of pathology, an imbalanced dysregulation of cytokines may result in systemic inflammatory responses or immunosuppression, leading to autoimmune diseases.
Cytokines are a diverse group of molecules (glycoproteins, peptides, and small proteins). They act via specific receptors and are involved in many processes in both the innate and acquired immune responses. A characteristic feature of their action is their interaction via auto-, para-, and endocrine pathways, with synergistic or antagonistic effects.
All cytokines exhibit pleiotropy (a single cytokine has multiple biological activities) and redundancy (shared biological activity) to a greater or lesser extent. These features of cytokines can be explained by the binding of cytokines to common receptor subunits with the activity of the corresponding intracellular signaling pathways [13, 14].
Cytokines participate in growth, differentiation, action, and apoptosis in populations of cells from the lymphocytic and hemopoietic systems, which means that they are involved in inflammation processes as stimulants and suppressors of the inflammatory response. The complexity of connections and reactions, including the functional interactions between the cells involved in the immune response, is best described as a cytokine network [5].
Cytokines act by binding to specific receptors, which most commonly trigger the NF-κB (nuclear factor κB), MAPK (Mitogen-Activated Protein Kinase), JAK (Janus kinases), and STAT (Signal Transducers and Activators of Transcription) inflammatory signaling pathways. Subsequently, active STAT proteins are transferred into the nucleus of the cell, where they act as transcription factors regulating the expression levels of various genes via the activation of transcription factors NFκB and AP-1 (activator protein 1) [15-17].
There is evidence that cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin 1 IL-1, interleukin 6 (IL-6), interleukin 3 (IL-3), interleukin 22 (IL-22), interleukin 10 (IL-10), and interferon alpha (INF-a) play significant roles in the pathogenesis of SLE; for this reason, they can be used as biomarkers for monitoring the severity and activity of this disease [18-20].
The first pro-inflammatory cytokines released by macrophages and monocytes are TNF-α and IL-1. Then, they initiate the synthesis and increased secretion of other pro-inflammatory cytokines, including IL-6, IL-8, and numerous endogenous inflammatory mediators, such as growth factors, components of the complement cascade, and adhesion molecules [18].
The cytokine IL-6 is a major mediator in acute and chronic inflammation. Il-6 levels are significantly elevated in the early acute response, especially in patients with impaired immune systems. Chronic inflammation may also cause an increased risk of postoperative infection and complications in wound healing, which may be correlated with preoperatively confirmed high levels of pro-inflammatory cytokines [7].
It should be emphasized that other pro-inflammatory cytokines also take part in the development and course of the inflammatory response. For example, it appears that dysregulation of the IL-10/IL12 balance is involved in the attenuation of the cellular response seen in SLE [9].
Many previous studies have confirmed a link between the surgical approach, the risk of postoperative complications, and increases in the plasma levels of cytokines (IL-6, TNF-α, and IL-1β) [21,22]. The overproduction of these cytokines is often associated with postoperative complications, making cytokines appropriate prognostic biomarkers [21,23,24].
The contributions of monocytes and B and T lymphocytes to the dysregulation of cytokine synthesis and signaling manifest as an overproduction of IL-10 and IL-6, which has also been confirmed in SLE patients [25]. Moreover, this predominance of IL-6 and IL-10 is correlated with SLE progression [20,26].
SLE activity is also associated with an increase in Th17 lymphocyte subpopulations, which leads to increases in IL-17A, IL17F, and IL-22 levels. The imbalance between the subpopulations of Th17 and T-regulatory cells is known as an abnormal characteristic of SLE. The subpopulation of regulatory T lymphocytes (Tregs) primarily prevents an excessive immune response and prevents the development of autoimmune disorders. There is a significant reduction in the subpopulation of regulatory T cells and their function in SLE. An increased level of IL17 is observed in lupus nephritis. Moreover, the overexpression of Th17 has been confirmed in the skin, lungs, and kidneys of patients with SLE [27].
Using animal studies, Ogura et al. confirmed the role of IL-17 in the development of the acute and chronic inflammatory responses. This process takes place through stimulation of inflammatory cytokines and chemokines and an increased proliferation of neutrophils. The immune cells are then transferred into inflamed tissues. The authors also described the significant role of the mutual relationship between lL-17 and IL-6; the expression of IL-6 is increased after IL-17A stimulation, and then IL-6 evokes the differentiation of Th17 that may promote the release of IL-17 by Th17 [15].
Moreover, Min et al., revealed that IL-17, as a pro-inflammatory cytokine, can also play a significant role in the immune stress response. IL-17A stimulates the production of the chemokines MCP1, CCL3 (MIP-1α), CCL7 (MCP3), and CCL20 (MIP-3a), and, through this, it supports the translocation of macrophages and lymphocytes into damaged tissues [28].
Zhang et al., observed different postoperative serum levels of IL-1b, IL-17, and TNF-α in four groups of patients rated from excellent (HHS>90) to poor (HSS<70) according to their Harris Hip Scores (HHS). The authors confirmed lower postoperative serum levels of evaluated cytokines in the patients with higher HHS. According to the obtained results, they considered these cytokines to be potential prognostic indicators of better patient recovery after surgery [29]. Moreover, Millar et al. confirmed the role of IL-17A, along with others such as IL-1, IL-6, TNF-α, and chemokines, in early tendinopathy. According to their research, the authors proposed IL17A as a potential inflammatory regulator in tendon remodeling [30].
Interleukin-17A, as a member of the IL-17 family of cytokines, is a pro-inflammatory mediator and is increasingly involved in a variety of immune and inflammatory disorders. The expression of IL-17A has been described in several cell types, including Th17 cells. The multiple activation of IL-17A can cause tissue destruction and cell degeneration due to inflammation by inducing the synthesis of cytokines, including IL-1, IL-6, TNF-α, and chemokines, by the cells of the immune system [31].
Granulocyte–Macrophage Colony-Stimulating Factor (GM-CSF) is the link between the chronic and acute inflammatory responses. GM-CSF is derived from the Colony-Stimulating Factor (CSF) superfamily. GM-CSF is released by lymphocytes and innate lymphoid cells such as macrophages, monocytes, mast cells, vascular endothelial cells, and fibroblasts [32]. The results of Torre et al. confirmed the important role of GM-CSF in the regulation of polymorphonuclear and mononuclear progenitors in wound healing. For example, in SIRS patients, a significant reduction in serum GM-CSF levels was found [33].
It is worth emphasizing the role of GM-CSF in the context of the chronic inflammatory process. GM-CSF plays significant but diverse roles in some autoimmune diseases. For example, the effect of GM-CSF is related to the progression of Rheumatoid Arthritis (RA) and Multiple Sclerosis (MS). In contrast, a protective role of GM-CSF is found in relation to other pathologies, such as SLE, Miastenia Gravis (MG), Hashimoto's Thyroiditis (HT), and Inflammatory Bowel Disease (IBD). In SLE, its protective role appears to be associated with the amelioration of neutrophil apoptosis [34]. Therefore, it is worth considering the therapeutic potential of GM-CSF in the context of treating autoimmune diseases.
The dysregulation of innate immunity plays a critical role in the pathogenesis of SLE. Type 1 interferons play a significant role in this process, particularly IFN-α [1]. This leads to the activation and proliferation of lymphocytes, dendritic cells, and natural killer NK cells, which disrupts autoimmune tolerance. During infection, the presence of genetic material of bacteria and/or viruses increases the production of IFN-α/β, which, in turn, activate B cells to produce antinuclear antibodies [35]. Normally, when the apoptotic process is not impaired, immune pathway activation as a result of exposure to autoantigens is prevented. In contrast, in SLE, the disruption of apoptosis leads to the increased production of autoantybodies. As a consequence, the production of IFN-α increases and immune processes intensify such that T-helper cells are activated, antigens are presented by dendritic cells, and the following cytokines are secreted: IL-1, -2, -4, -6, and -8 [1].
Additionally, in patients with SLE, IFN-γ intensifies the transformation of monocytes in dendritic cells, which recognize antigens, increase the production of IFN-γ, and modulate other immune processes [9].
Interferons (IFNs) are known as pleiotropic cytokines, and they coordinate several biological functions such as antiviral, antitumor, and immunomodulatory responses, in which they are primarily represented by IFN-α and IFN-β. The interferon-gamma (IFN-γ) signaling pathway takes part in the regulation of inflammation, apoptosis, and the cell cycle. Interferon-gamma is released from stimulated Lymphocytes (Th1), Natural Killer (NK) cells, Natural Killer T Cells (NKT), B cells, and APCs [36]. In particular, the role of interferon-gamma is very important because of the involvement of IFN-γ in macrophage translocation at wound sites. Moreover, IFN-γ acts as a pro-inflammatory agent through the stimulation of cytotoxic T cells and the activation of the complement system [31].
Pattern recognition receptors (PRPs) such as Toll-Like Receptors (TLRs) play another important role in the disruption of innate immunity in SLE. TLR-3, TLR-7, TLR-8, and TLR-9 play important roles in the pathogenesis of SLE due to their ability to bind dsDNA and ssRNA. TLR-9 can activate dendritic cells and consequently increase IFN-α production. TLR7 regulates the production of IL-1 and IL-23 and promotes the proliferation of Th17 [37,38].
Toll-like receptors are involved in both types of immune response. They are found on monocytes, macrophages, neutrophils, dendritics, and T and B lymphocytes.
TLRs recognize the Danger-Associated Molecular Patterns (DAMPs) released by damaged cells and work by activating the intracellular NF-κB signaling pathway [39, 40].
Another group of cytokines associated with SLE and the acute inflammatory response are chemokines, which are a group of small proteins with chemotactic properties that recruit cells to the site of inflammation. Chemokines are released by various immune cells, primarily monocytes, macrophages, and endothelial and stromal cells, in response to inflammation. Furthermore, it should be noted that many other activated cells are capable of releasing chemokines. Leukocytes activated by pro-inflammatory cytokines are a source of chemokines and should be considered a hard link between chemokines and cytokines. Changes in leukocyte levels may potentially reflect the regulation of chemokines. Chemokines are classified into four subfamilies based on their chemical structure, which is related to the location of the first two cysteines and the amino acid residues between them at the N-terminal end of the polypeptide [41]. It is worth emphasizing the double nomenclature of chemokines, which describes them as belonging to a subfamily and corresponds to their influence on cells as described by researchers after their discovery. An example is CCL1, which is known as a Monocyte Chemoattractant-1 (MCP1) protein [42]. Through the activation of signaling pathways, chemokines are involved in all protective and destructive immune and inflammatory responses. They are also involved in the pathogenesis of many human diseases, such as autoimmune diseases and neogenesis, where inflammation is an essential part of the cancer microenvironment. Chemokines act through receptors that are expressed on the cell surface as 7-transmembrane proteins that are structurally related to superfamily G-Protein-Coupled Receptors (GPCRs) [43,44].
The results presented by Krzystek et al. revealed the important role of chemokines in surgical stress. The authors showed temporary changes in the chemokines responsible for leukocyte migration and concluded that the upregulation of chemokines is related to the type of surgical technique used and that it may be useful in assessing the risk of postoperative complications [45].
Chemokines, along with other cytokines, are known to be part of immunoregulatory networks that also play significant roles in the pathogenesis of SLE. Chemokines may influence the course of SLE and are associated with the early development of several complications, particularly lupus nephritis [46]. Chemokines are able to recruit monocytes, neutrophils, and lymphocytes into the inflammatory region and activate leukocyte subsets through their receptors. The expression of some chemokines, including MCP-1/CCL2 and IL-8/CXCL8, in the glomerular compartment during active SLE nephritis confirms their involvement in renal injury [44]. A human study presented by Zeng et al. showed the relationship between serum CXCL1 chemokine concentration and disease activity in a patient with SLE. The promotional role of CXCL1 in the pathogenesis of SLE was also confirmed by revealing the relationship between a higher chemokine concentration and disease activity [47]. Moreover, CXCL1 has also been described as a Growth-Related Oncogene α (GRO-α) that acts through CXCR2 receptors to promote leukocyte accumulation during inflammation. CXCL1 belongs to the CXC chemokine family, and its participation in many processes, such as inflammation, angiogenesis, and tumor progression, has been confirmed by evidence from many studies. Therefore, GRO-α expression may be a potential therapeutic target and prognostic marker in autoimmune and neoplastic diseases [48-51].
Another example of a chemokine involved in the pathogenesis of SLE is CXCL13, which is recognized as a B-cell-contributed response chemokine and a B-Lymphocyte Chemoattractant (BLC) due to its strong chemotactic effect on B cells. CXCL13 acts through CXCR5 receptors.
The serum concentration of CXCL13 is significantly higher in SLE patients, and this high level is also positively associated with SLE Disease Activity Index (SLEDAI) score. In addition, animal and human studies have shown that CXCL13 and CXCR5 are overexpressed in the renal cortex of patients with lupus nephritis [41,52].
The S100 A8 and A9 (S100A8/A9) proteins are known as alarmins belonging to the S100 family of calcium-binding proteins. Their expression has been confirmed in many cells, including neutrophils, monocytes, thrombocytes, and dendritic cells. Kinetic changes in the concentrations of S100A8/A9 in many diseases with chronic inflammatory etiology, such as SLE, joint diseases, diabetes mellitus, and cardiovascular diseases, may indicate a potential role of these proteins in diagnosis, prognosis, and therapy [53].
Alarmins are endogenous DAMP molecules that play different roles under normal conditions, and are released when cells are damaged as a result of cell necrosis or the activation of secretory pathways. Alarmins act through several receptors, including RAGE (a pattern recognition receptor), TLR4, and Formyl Peptide Receptors (FPRs) belonging to the category of G-protein-coupled receptors. Alarmins, along with all DAMPs, are an integral part of the inflammatory response, promoting the synthesis of pro-inflammatory mediators [40,54].
The results of several studies have confirmed that both acute psychological and polytraumatic stress act to increase the levels of the pro-inflammatory alarmins S100A8/A9 and sRAGE (soluble receptor) in serum [54-57]. Moreover, the results of these studies illustrate the importance of these parameters and their potential use as early predictive markers of increased risk of post-traumatic complications as a result of organ dysfunction.
The results presented by Wei Qijiao et al. indicated the involvement of S100A8 dysregulation in the pathogenesis of SLE. The scientists examined 13 genes, and an upregulation was confirmed (for S100A8) in only in one case. The glomerular expression of S100A8 was significantly elevated in renal biopsies from SLE patients compared to those from a control group of nephrectomy cancer patients [58].
The cellular immune response is mediated by various T-cell subpopulations (helper, cytotoxic, and regulatory T cells) that are capable of recognizing specific antigens. The activation of B and T cells after antigen presentation by Antigen-Presenting Cells (APCs), most often Dendritic Cells (DCs), induces an acquired immune response. CD4+ Helper T (Th) cells are functionally divided into different subsets—Th1, Th2, and Th17 cells—according to the cytokines they secrete and their immune roles.
Thus, lymphocytes release the following cytokines: Th1 cells release IFN-γ, TNF-α, and IL-2 and Th2 cells release IL-4, IL-5, IL-9, IL-13, and IL-25 but not IFN-γ, and Th17 cells release IL-17. Moreover, Th2 participates in the stimulation of B cells to produce antibodies belonging to the humoral immune response [9,39,59].
The dysregulation of acquired immunity plays a key role in the disruption of tolerance-dependent T-lymphocyte dysfunction. This leads to the dysregulation of the signaling pathway and, consequently, to abnormal cytokine secretion. Moreover, it ensures the activation of B cells [1]. A characteristic feature of SLE is the chronic inflammatory process, with the release of autoantibodies by overactive B lymphocytes that play significant roles in SLE pathogenesis though participation in the synthesis of pro-inflammatory cytokines (such as IL-1) and activities such as those of antigen-presenting cells [60].
In the pathogenesis of SLE, an imbalance in the proportions between Th1 and Th2 cells is observed. These cells are subtypes of CD 4+ T cells, and they are divided into the Th1 and Th2 subpopulations according to the secretion of different types of cytokines. Th1 produces TNF-α, IL-2, and IFNs, which are involved in the inflammatory process of autoimmunity. The subpopulation of Th2 cells produces IL-4, IL-6, and IL-10, the key roles of which are the proliferation and activation of B cells and the production of IgG. In SLE, the balance between Th1 and Th2 is impaired, and the growth of a subpopulation of Th2 cells leads to the activation of B cells and the production of autoantibodies is observed [1].
Surgery also induces various changes in the Th1/Th2 ratio. It may be associated with an attenuation of the postoperative cell-mediated response [61].
Decker et al., reported the effect of surgical stress on the Th1/Th2 balance, noting the downregulation of Th1 and upregulation of Th2 humoral-mediated immunity. Moreover, the authors confirmed that the intensity of this shift is linked to the surgical technique (open versus laparoscopic) [62]. In turn, Lee at al. assessed changes in the Th1/Th2 ratio in cancer patients undergoing surgery. They found that a shift towards an increased Th1 response without a rise in IL-6 levels improved the prognosis for hepatocellular carcinoma in patients after transarterial chemoembolization [63].
Research conducted by Lee assessed the effects of Dexmedetomidine (an α agonist). The obtained results showed that intraoperatively administered dexmedetomidine was associated with an increase in the ratio of IFN-gamma/IL-4 and IL-17/IL-10, depending on the dosage regimen. The results seemed to suggest a response to the Th1/Th2 and T17/Treg balance shifting towards Th1 and Th17 [64,65].
Thus, the presented research results indicate that factors related to surgical stress, in addition to the drugs used during anesthesia, contribute to the modulation of the inflammatory response. For this reason, in patients with impaired immune systems, careful consideration should be given when selecting the type of anesthesia with regard to changes in the immune system. The mechanisms of the surgical stress response are presented in (Figure 1).
Concluding Remarks and Outlook for the Future
We are fully aware that the examples presented herein represent a small part of the molecular link between acute and chronic inflammation. Serious immune system disorders induced by surgery in patients with chronic inflammation may theoretically modulate both types of inflammatory responses in unexpected directions, leading to potentially high risk of complications. These problems may be dependent on the context of the multifactorial response of the body and influenced by multiorgan failure due to chronic inflammation in SLE patients.
In contrast to patients with properly functioning immune systems, the choice of therapy related to the type of surgery and anesthesia in immunocompromised patients has a decisive influence on the results of the treatment and the further course of the chronic disease. The idea of administering modified anesthesia to patients with impaired immune systems could create new therapeutic benefits, as well as challenges.
It should be predicted that an increase in empirical knowledge about the molecular mechanisms of the acute and chronic inflammatory responses will facilitate improved decision making in personalized therapy, taking into account the functioning of the immune system.
Highlights
- The acute inflammatory response due to surgical trauma is initiated through immune cell activity via various cytokine and chemokine interactions.
- Systemic lupus erythematosus is an autoimmune disease with chronic inflammation in pathogenesis, with both impaired innate and adaptive responses.
- The presence of chronic inflammation modifies the acute inflammatory response.
- The common components of and interactions between acute and chronic inflammation are critical for understanding the molecular mechanisms of the inflammatory response pathways in the surgical immune responses of SLE patients.
Author`s Contribution
BL, conceptualization and writing—original draft preparation; AS, writing—original draft preparation; ADG, editing and assistance with literature search and references; DK, editing and assistance with literature search and references; MO, critical revisions to the manuscript for important intellectual content, and review and editing.
All authors have approved the final version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
Not applicable
Conflicts Of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Pan L, Lu M, Wang JH, Xu M, Yang SR (2020) Immunological pathogenesis and treatment of systemic lupus erythematosus.World J Pediat 16: 19-30.
- FanouriakisA, Tziolos N, Bertsias G, Boumpas DT (2021) Update οn the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis 80: 14-25.
- Finnerty C, Mabvuure NT, Ali A, Kozar RA, Herndon DN (2013) The Surgically Induced Stress Response. J. Parenter. Enteral. Nutr 37: 21-29.
- Chen L, Deng, Cui HH, Fang J, Zuo Z, et al. (2018) Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9: 7204-7218.
- Lisowska B, Szymanska M, Nowacka E, Olszewska M (2013) Anesthesiology and the cytokine network. Postepy Hig Med Dosw 67: 761-769.
- Maslinski W, Kontny E (2015) Podstawy immunologii dla reumatologó Warszawa: Narodowy Instytut Geriatrii.
- Lisowska B, Maslinski W, Maldyk P, Zabek J, Baranowska E (2008) The role of cytokines in inflammatory response after total knee arthroplasty in patients with rheumatoid arthritis. Rheumatol Int 28: 667-671.
- GottschalkTA, Tsantikos E, Hibbs ML (2015) Pathogenic inflammation and its therapeutic targeting in systemic lupus erythematosus. Front. Immunol 6: 550.
- Mok CC, Lau CS (2003) Pathogenesis of systemic lupus erythematosus. Clin. Pathol 56: 481-490.
- Lisowska B, Rutkowska SL, Maldyk P, Cwiek R (2008) Anaesthesiological problems in patients with rheumatoid arthritis undergoing orthopaedic surgeries. Clin Rheumatol 27: 553-55
- Khokhar RS, Baaj J, Al-Saeed A, Sheraz M (2015) Anesthetic management of patient with systemic lupus erythematosus and antiphospholipid antibodies syndrome for laparoscopic nephrectomy and cholecystectomy. Saudi J Anaesth 9: 91-93.
- Gualtierotti R, Parisi M, Ingegnoli F (2018) Perioperative Management of Patients with Inflammatory Rheumatic Diseases Undergoing Major Orthopaedic Surgery: A Practical Overview. Adv Ther 35: 439-456.
- Pajtasz-Piasecka E (1996) Cytokine redundancy-unity in diversity. Postepy Hig Med Dosw 50: 557-580.
- Nicola NA (1994) Cytokines pleiotropy and redundancy: a view from the receptor. Stem Cells 12: 3-12.
- Ogura H, Murakami M, OkuyamaY, Tsuruoka M, Kitabayashi C, et al. (2008) Interleukin-17 Promotes Autoimmunity by Triggering a Positive-Feedback Loop via Interleukin-6 Induction. Immunity 29: 628-636.
- Watowich SS, Wu H, Socolovsky M, Klingmuller U, Constantinescu SN, et al. (1996) Cytokine receptor signal transduction and the control of hematopoietic cell development. Annu Rev Cell Dev Biol 12: 91-128.
- Robb L (2007) Cytokine Receptors and Hematopoietic Differentiation. Oncogene 26: 6715-6723.
- Renner K, Hermann FJ, Schmidbauer K, Talke Y, Rodriguez Gomez M, et al. (2015) IL-3 contributes to development of lupus nephritis in MRL/ lpr mice. Kidney Int 80: 1088-1098.
- Hu L, Hu J, Chen L, Zhang Y, Wang Q, et al. (2021) Interleukin-22 From Type 3 Innate Lymphoid Cells Aggravates Lupus Nephritis by Promoting Macrophage Infiltration in Lupus-Prone Mice. Front Immunol 26: 584414.
- Pita MS, Citores MJ, Castejon R, Bango YM, Ureta TP, et al. (2009) Monocytes and T lymphocytes contribute to a predominance of interleukin 6 and interleukin 10 in systemic lupus erythematosus. Cytometry B Clin Cytom 76: 261-270.
- Baigrie RJ, Lamont PM, Kwiatkowski D, Dallman MJ, Morris PJ (1992) Systemic cytokine response after major surgery. Br J Surg 79: 757-60.
- Oka Y, Murata A, Nishijima J, Yasuda T, Hiraoka N, et al. (1992) Circulating interleukin 6 as a useful marker for predicting postoperative complications. Cytokine 4: 298-
- Okholm C, Goetze JP, Svendsen LB, Achiam MP (2014) Inflammatory response in laparoscopic vs. open surgery for gastric cancer. Scand J Gastroenterol 49: 1027-1034.
- Serpa NA, Campos PP, Hemmes SN, Bos LD, Bluth T, et al. (2017) Kinetics of plasma biomarkers of inflammation and lung injury in surgical patients with or without postoperative pulmonary complications. Eur J Anaesthesiol 34: 229-238.
- Cash H, Relle M, Menke J, Brochhausen C, Jones SA (2010) Interleukin 6 (IL-6) deficiency delays lupus nephritis in MRL-Faslpr mice: the IL-6 pathway as a new therapeutic target in treatment of autoimmune kidney disease in systemic lupus erythematosus. J Rheumatol 37: 60-70.
- Llorente L, Patin RY, Wijdenes J, Varela AJ, Maillot MC, et al. (1993) Spontaneous production of interleukin-10 by B lymphocytes and monocytes in systemic lupus erythematosus. Eur Cytokine Netw 4: 421-427.
- Yang J, Chu Y, Yang X, Gao D, Zhu L, et (2009) Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum 60: 1472-1483.
- Shin MS, Leea N, Kanga I (2011) Effector T cell subsets in systemic lupus erythematosus: update focusing on Th17 cells. Curr Opin Rheumatol 23: 444-448.
- Zhang H, Tai H, Ma Y, Li A, Dang Z, et al. (2019) Postoperative Serum Levels of Interleukin-1b (IL-1b), Interleukin-17 (IL-17), and Tumor Necrosis Factor-a (TNF-a) in Patients Following Hip Replacement Surgery for Traumatic Fractured Femoral Neck: A Retrospective Study. Med Sci Monit 25: 6120-6127.
- Millar NL, Akbar M, Campbell AL, Reilly JH, Kerr SC, et al. (2016) IL-17A mediates inflammatory and tissue remodelling events in early human tendinopathy. Sci. Rep 6: 27149.
- Ohl K, Tenbrock K (2011) Inflammatory Cytokines in Systemic Lupus Erythematosus. J. Biomed Biotechnol 11: 432595.
- Mu X, Liu K, Li H, Wang FS (2021) Granulocyte-macrophage colony-stimulating factor: an immunotarget for sepsis and COVID-19. Cell Mol Immunol 18: 2057-2058.
- Torre D, Tambini R, Manfredi M, Chiaranda M, Campi P, et al. (2003) Circulating levels of granulocyte macrophage colony-stimulating factor in patients with the systemic inflammatory response syndrome. J Infect 47: 296-299.
- Lotfi N, Thome R, Rezaei N, Zhang GX, Rezaei A, et al. (2019) Roles of GM-CSF in the Pathogenesis of Autoimmune Diseases: An Update. Front Immunol 10: 1265.
- Taniguchi T, Takaoka A (2002) The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr Opin Immunol 14: 111-116.
- Castro F, Cardoso AP, Gonçalves RM, Serre K, Oliveira MJ (2018) Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front Immunol 9: 847.
- Kontaki E, Boumpas DT (2010) Innate immunity in systemic lupus erythematosus: sensing endogenous nucleic acids. J Autoimmun 35: 206-211.
- Pisetsky DS, Ullal AJ (2010) The blood nucleome in the pathogenesis of SLE. Autoimmun Rev 10: 35-
- Dabrowska A, Slotwinski R (2014) The immune response to surgery and infection. Centr Eur J Immunol 39: 532-537.
- Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81: 1-5.
- Liao X, Pirapakaran T, Luo XM (2016) Chemokines and Chemokine Receptors in the Development of Lupus Nephritis. Mediators of Inflammation 16: 6012715.
- Taha HA, Abdallah NH, Salem MS, Azza H, Hamouda A, et al. (2017) Urinary and tissue monocyte chemoattractant protein1 (MCP1) in lupus nephritis patients. The Egyptian Rheumatologist 39: 145-150.
- Fard GS, Shahir M, Taheri M, Salimi A (2021) A review on the role of chemokines in the pathogenesis of systemic lupus erythematosus Cytokine 146: 155640.
- Hughes C, Nibbs R (2018) A guide to chemokines and their receptors. FEBS Journal 285: 2944-
- Krzystek-Korpacka M, Zawadzki M, Lewandowska P, Szufnarowski K, Bednarz-Misa I, et al. (2019) Distinct Chemokine Dynamics in Early Postoperative Period after Open and Robotic Colorectal Surgery. J Clin Med 8: 879.
- Rovin BH (2008) The chemokine network in systemic lupus erythematous nephritis. Front Biosci 13: 904-922.
- Zeng Y, Lin Q, Yu L, Wang X, Lin Y, et al. (2021) Chemokine CXCL1 as a potential marker ofdisease activity in systemic lupus erythematosus. BMC Immunology 22: 82.
- Bechara C, Chai H, Lin PH, Yao Q, Chen C (2007)) Growth related oncogene-alpha (GRO-alpha): roles in atherosclerosis, angiogenesis and other infammatory conditions. Med Sci Monit 13: 87-90.
- Caunt M, Hu L, Tang T, Brooks PC, Ibrahim S, et al. (2006) Growth-regulated oncogene is pivotal in thrombin-induced angiogenesis. Cancer Res 66: 4125-4132.
- Loukinova E, Dong G, Enamorado-Ayalya I, Thomas GR, Chen Z, et al. (2000) Growth regulated oncogene-alpha expression by murine squamous cell carcinoma promotes tumor growth, metastasis, leukocyte infiltration and angiogenesis by a host CXC receptor-2 dependent mechanism. 19: 3477-3486.
- Guo F, Long L, Wang J, Wang Y, Liu Y, et al. (2019) Insights on CXC chemokine receptor 2 in breast cancer: An emerging target for oncotherapy. Oncol Lett 18: 5699-5708.
- Lee HT, Shiao YM, Wu TH, Chen WS, Hsu YH, et al. (2010) Serum BLC/CXCL13 concentrations and renal expression of CXCL13/CXCR5 in patients with systemic lupus erythematosus and lupus nephritis. J Rheumatol 37(1): 45-52.
- WangS, Song R, Wang Z, Jing Z, Wang S, et al. (2018) S100A8/A9 in Inflammation. Front Immunol 9: 1298.
- Zhuang Y, Wang L, Guo J, Sun D, Wang Y, et al. (2022) Molecular recognition of formylpeptides and diverse agonists by the formylpeptide receptors FPR1 and FPR2. Nat Commun 13: 1054.
- Jonasson L, Larsen HG, Lundberg AK, GullstrandB, Bengtsson AA, et al. (2017) Stress-induced release of the S100A8/A9 alarmin is elevated in coronary artery disease patients with impaired cortisol response. Sci Rep 7:
- Cohen M, Carles M, Brohi K, Calfee C, Rahn P, et al. (2010) Early release of soluble receptor for advanced glycation endproducts after severe trauma in humans. J Trauma 68: 1273-1278.
- Joly P, Marshall JC, Tessier PA, Massé C, Page N, et al. (2017) S100A8/A9 and sRAGE kinetic after polytrauma; an explorative observational study. Scand J Trauma Resusc Emerg Med 25:
- Qijiao W, Zhihan C, Makota P, Qing Y, Fei G, et al. (2022) Glomerular Expression of S100A8 in Lupus Nephritis: An Integrated Bioinformatics Analysis. Front Immunol 13: 843576.
- Shin MS, Lee N, Kang I (2011) Effector T cell subsets in systemic lupus erythematosus: update focusing on Th17 cells. Curr Opin Rheumatol 23: 444-448.
- Chan OT, Madaio MP, Shlomchik MJ (1999) The central and multiple roles of B cells in lupus pathogenesis. Immunol Rev 169: 107-121.
- Cardinale F, Chinellato I, Caimmi S, Peroni DG, Franceschini F, et al. (2011) Perioperative Period: Immunological Modifications.Int J Immunopatho Pharmaco 24: 3-12.
- Decker D, Schöndorf, M, Bidlingmaier F, Hirner A, von Ruecker AA (1996) Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up-regulation of antibody-mediated immunity commensurate to the trauma. Surgery 119: 316-325.
- Lee HL, Jang JW, Lee SW, Yoo SH, Kwon JH, et al. (2019) Inflammatory cytokines and change of Th1/Th2 balance as prognostic indicators for hepatocellular carcinoma in patients treated with transarterial chemoembolization. Sci Rep 9:
- Lee J, Han H, Choi W, Yoo S, Baek S, et al. (2018) Immunomodulatory effects of intraoperative dexmedetomidine on T helper 1, T helper 2, T helper 17 and regulatory T cells cytokine levels and their balance: a prospective, randomised, double-blind, dose-response clinical study. BMC Anesthesiology 18: 164.
- Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, et al. (2019) European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol 71:1400-1412.
Citation: Lisowska B, Saletra A, Golab AD, Kosson D, Olesinska M (2023) The links between inflammatory parameters in acute and chronic inflammatory responses in terms of the surgical stress response and Systemic Lupus Erythematosus. J Clin Immunol Immunother 9: 076.
Copyright: © 2023 Barbara Lisowska, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

