
The Mechanism of Antihepatocellular Carcinoma and Toxicity Analysis of Huachanin
*Corresponding Author(s):
Zhetian XueShanghai Starriver Bilingual School, China
Tel:+86 133 91329280,
Email:xuebravo@sina.com
Abstract
Huachanin is a chemical borrowed from Traditional Chinese Medication practices that is commonly utilized in the treatment of cancer. Its mixture attribute serves in alignment with the typical multiple-target effects of Traditional Chinese Medicine. A majority of researches on Huachanin, however, examine its influences only on single pathways. This review integrates several major mechanisms by which Huachanin acquires hepatoma cancer suppressing functions, namely, the down regulation of Aurora Kinase, induction of apoptosis, and the inhibition of cell autophagy. The review then analyses Huachanin’s cause on nontumor tissue malfunction while suppressing tumor growth, aiming to provide insight into balancing the toxicity and efficacy in Huachanin treatments to maximize its medical potential.
Keywords
Huachanin; Mechanism of Antihepatocellular Carcinoma; Toad; Toxicity Analysis
Introduction
Toad is the common name of the anuran and toad family. Toad’s size is large, and its skin is rough. The back of the toad is covered with large and small bumps, which are sebaceous glands. The large pair of glands are the postauricular ones, located on the side of the head above the tympanic membrane. The white venom secreted by these glands is the raw material for making toad puffs.
Toadstool is a precious Chinese herbal medicine. According to Chinese Medicine, a Shanghai Science and Technology Press trial textbook for higher medical schools, toads are fishy, warm, and poisonous. It belongs to the heart meridian and has the effects of detoxification, pain relief and orifice opening. The Chinese Folk Chinese Medicine Research and Development Association’s unique recipe for cancer records that toad skin is fishy, cool and slightly poisonous, with the functions of detoxification, diuresis, decongestion and treatment of various types of swelling and cancer. It is mainly used for sores, swellings, and children’s noma, and is commonly used for tumors in modern days. Based on The Complete Book of Chinese Herbs of Far Eastern Publishing House, toad clover can treat cancer, leukemia, lymphoma, etc.
Chemical Composition Of Toadstool
The chemical composition of a toadstool is complex. The chemical components isolated from toadstool mainly include toadstool alkaloids, indole alkaloids, cyclic amides, small-molecule cyclic peptides, sterols, and other compounds. And toadstool alkaloids can be divided into two categories: toadstool ligands and toadstool toxins. Toadstool toxins are derivatives of toadstool ligands, substituted by arginine bicarbonate and sulfate at the 3-position [1]. They are both the active and toxic components of toadstools.
Bufadienolides are a class of steroids with α-pyrone ring (six-membered unsaturated lactone ring) attached to C-17 position, cis or trans-concentration of A/B ring, trans-concentration of B/C ring, and cis-concentration of C/D, and C-3 polyhydroxy substitution. The components of this class mostly have various pharmacological activities, such as cardiotonic, local anesthetic, anti-shock, antiviral, etc. Especially, their antitumor activity is very significant, and the IC50 of tumor cell inhibition rate is 1~1,00 nmol/L.
Ma studied the chemical composition of chloroform extracts of toadstool [2]. According to Ma’s research, three main toad steroid components were isolated: cinobufagin (1), lipid (resibufagenin, 2) and bufalin (3). Five steroids were identified by nuclear magnetic resonance hydrogen spectroscopy (1H-NMR) and carbon spectroscopy (13C-NMR), as shown in figure 1.
Bufotalin (bufotalin, 4), marinobufagin (5), cinobufotalin (6), and 12β-hydroxy-bufalin (12β-hydroxyl-bufalin, 7) 5,7β-dihydroxy-bufalin (5,7β-dihydroxyl- resibufageni n, 8) 5,7β-dihydroxyl- resibufageni n, 8, 5,7β-dihydroxy-toad venom ligand and Chinese toad venom group are the leading representative substances of toad steroids, which are the main active and toxic components of toadstools.
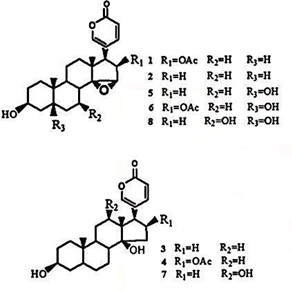 Figure 1: The chemical composition of compounds 1-8.
Figure 1: The chemical composition of compounds 1-8.
As recorded in the Materia Medica Huiyan, toadstool is a medicine for healing chancre, eliminating swelling and relieving healing poison. Modern pharmacological studies have shown that toadstool has potent analgesic, cardiotonic, anti-infective and anti-tumor effects. It is widely used to treat various malignant tumors, chronic hepatitis B, acute and chronic respiratory tract infections, heart disease, etc., and local anesthesia and surface anesthesia [3].
Mechanism Of Action Of Huachanin On Inhibiting Hepatocellular Carcinoma
Down-regulation of Aurora Kinase Expression and Promotion of Cell Cycle Arrest in Hepatocellular Carcinoma
Aurora kinases are serine/threonine kinases with a highly conserved catalytic domain, containing an autophosphorylation site [4]. The Aurora kinase family includes Aurora A, Aurora B and Aurora C isoforms. which are deeply involved in regulating cell mitosis and cell cycle. Cell division is an extremely complex physiological process. Cells complete the replication of chromosomes and distribute the replicated genetic material equally between the two daughter cells to complete mitosis. Aurora A, a member of the Aurora kinase family, is one of the critical mitotic protein kinases that monitor the proper functioning of the cell cycle, ensuring the fidelity of cell division and genetic stability. Its abnormal expression will lead to cell cycle disruption and ultimately aneuploid cell formation. Aurora A is genetically amplified or highly expressed in various human tumors (Figure 2).
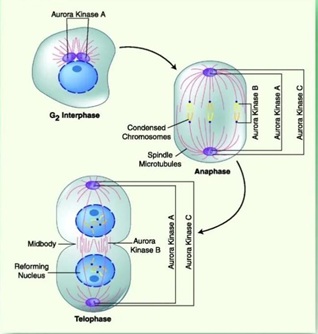 Figure 2: Aurora Kinase inhibition on mitotic division.
Figure 2: Aurora Kinase inhibition on mitotic division.
Given that overexpression of Aurora A has been demonstrated in a variety of cancers [5], its small molecule kinase inhibitors can inhibit the proliferation, migration and invasion of cancer cells. In recent years, this has become a research hotspot for novel anti-tumor targeting drugs in the cell cycle field.
The expression levels of AURKA and AURKB mRNA showed a significant negative correlation with the survival of hepatocellular carcinoma patients. For example, the higher the expression of AURKA and AURKB is, the shorter the survival of hepatocellular carcinoma patients can be [6]. Wang found that the expression of AURKA, AURKB, TPX2, SMC2, TOP2A and CDK1 was significantly reduced after 12 and 24 h of the action of Chinese toadstool ligands on human hepatoma HepG2 cells.
AURKA, AURKB, TPX2, SMC2, TOP2A and CDK1 protein expression were significantly reduced after the ligands were applied to human hepatocellular carcinoma HepG2 cells for 12 and 24 h (P < 0.01). The expressions of AURKA, AURKB, TPX2, SMC2, TOP2A and CDK1, were significantly reduced after 12 h of Toadstool effect on human hepatocellular carcinoma HepG2 cells.
AURKA, AURKB, TPX2, SMC2 and CDK1 protein expression were significantly reduced after 12 h of Toadstool's action on human hepatoma HepG2 cells (P < 0.05, 0.01). TOP2A, AURKA, AURKB, TPX2, SMC2 and CDK1 protein expression were significantly reduced after 24 h of action (P < 0.01). Therefore, the expression of AURKB, which promotes cytoplasmic segregation in late mitosis, was also down-regulated, resulting in impaired chromosome assembly and centrosome formation. It causes the failure of sister chromatids and cytoplasm to segregate normally and the failure of cells to pass through the G2/M phase. This leads to cell cycle arrest in the G2/M phase, which inhibits the proliferation of hepatocellular carcinoma cells.
Induction of Apoptosis
Although the detailed mechanism of the apoptotic process is not fully understood, it is well established that caspases, i.e., cysteine proteases, play a crucial role in the execution of apoptosis [7]. The process of apoptosis is a cascade reaction, in which caspases are activated and undergo apoptotic proteases. They are present in the cytoplasm and are highly homologous, and structurally similar. All Caspase family proteins are found in the cytoplasm, are highly homologous, structurally identical, and contain a cysteine activation site that specifically breaks peptide bonds after aspartic acid residues. Fourteen Caspases have been identified and are involved in regulating various biological processes, closely related to apoptosis, in addition to cell growth, differentiation, proliferation, and motility.
Caspase family proteins can be classified into three major groups based on their structure and function, (1) apoptotic initiators, (2) apoptotic executors and (3) inflammatory mediators, mainly including Caspase-2,8,9,10,3,6,7. They perform efficient cleavage of intracellular proteins in a cascade amplification manner, ultimately leading to apoptosis. The apoptosis initiation factors, Caspase 2, 8, 9, 10, are involved in the initiation of apoptosis and apoptosis execution factors. Caspases 3, 6, and 7 are involved in apoptosis execution. Inflammation-mediating factors mainly include Caspase-1, 4, 5, 11, 12, 13, and they mediate the inflammatory response and play a secondary role in the death receptor-mediated apoptotic pathway.
Wang showed that huachanin has good inhibitory activity on the proliferation of HepG2 cells in hepatocellular carcinoma cells, and its effect is related to the induction of apoptosis in tumor cells [8]. Huachanin induced apoptosis of HepG2 cells through the mitochondrial signaling pathway. First, by upregulating the expression of Bax protein and downregulating the expression of Bcl-2 protein, huachanin decreases the mitochondrial membrane potential and releases cytochrome C into the cytoplasm. Second, in the presence of ATP, cytochrome C forms a complex with Apaf-1. Third, the formed complex activates capspase-9 zymogen, activating caspase-3 and cleaved poly ADP. Caspase 3, which cleaves poly ADP-Ribose Polymerase (PARP) to cleaved-RARP, leads to DNA fragmentation and apoptosis.
To detect the effect of huachanin on the expression of caspase-3 and caspase-9 in HepG2 cells, western blotting was performed on caspase-3 and caspase-9, and after 0.04ug/mL of huachanin was applied to HepG2 cells for 24 hours, the levels of caspase-3 and caspase-9 decreased. Caspase-3 and Caspase-9 proteins decreased after 0.04ug/mL was used to HepG2 cells for 24 hours, suggesting that the induction of apoptosis in HepG2 cells in vitro is related to the regulation of Caspase-3 and Caspase-9 protein expression (Figure 3).
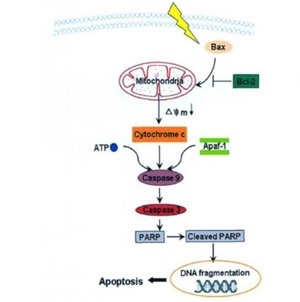 Figure 3: The mitochondria-mediated apoptosis pathway induced by cinobufacini.
Figure 3: The mitochondria-mediated apoptosis pathway induced by cinobufacini.
Inhibition of cellular autophagy
Autophagy is a highly conserved cell biological behavior in eukaryotic cells [9], which is the process of autophagy and digestion of cells. During this process, cells wrap intracellular cytoplasm and small organelles through a bilayer membrane structure and transport them to lysosomes for metabolism. Cells provide nutrients and energy, maintain the cell's metabolic homeostasis, and relieve pressure.
Autophagy can be divided into basal state autophagy and induced state autophagy. Autophagy in the basal state has a low metabolic rate and maintains the homeostasis of the intracellular environment, mainly by removing proteins and damaging organelles that tend to aggregate [10]. Under the induction of unfavorable microenvironments, such as hypoxia and nutrient deprivation, cells maintain cell survival by activating and up-regulating the level of autophagy to degrade intracellular protein aggregates, oxidized lipids, damaged organelles and other materials [11].
The tumor microenvironment mainly comprises cancer cells, non-malignant epithelial cells, fibroblasts, and blood. It consists of tubules, immune cells and an extracellular matrix, which interact and play an important role in tumorigenesis, proliferation and metastasis. As an important mechanism for tumor cell survival, autophagy can play a regulatory role between tumor cells and tumor microenvironment. It has been found that hepatic tumor stem cells CD133+ cells can maintain cell survival by activating autophagy in a hypoxic and nutrient-deficient environment [12]. Tumor cells generate autophagic catabolism to maintain tumor cell survival and relieve the stress of the external environment on tumor cells.
Autophagy was first discovered in yeast in response to starvation, where autophagy maintains amino acid levels and upregulates mitochondrial function to support the survival of yeast cells under starvation conditions [13].
In addition, activation of the Ras oncogene induces the growth of tumors, and these tumor cells enhance survival in stressful environments by upregulating autophagy levels [14].
Tumor dormancy is one of the biological properties of malignant tumor cells. On the one hand, it allows tumors to escape from surgery and radiotherapy, leading to tumor recurrence after the reactivation of dormant cells to proliferate. On the other hand, it maintains cellular activity when tumors metastasize [15]. Autophagy enables dormant tumor cells to resist apoptosis and has an important protective effect on their survival.
Therefore, autophagy has different roles in different stages of tumorigenesis and development. It also plays different roles in treating tumors, and the correct use of autophagy can effectively improve the therapeutic effect and delay tumor recurrence [16].
Han observed from electron microscopy experiments and protein blotting assay that huachanin could inhibit humans [17]. Han’s results showed that the autophagosome formation of human SMMC-7721 hepatocellular carcinoma cells was inhibited, and the expression level of autophagy marker proteins Beclin 1, LC3-II expression level, which confirmed that Toadstool could inhibit autophagy in human hepatocellular carcinoma cells. Human hepatocellular carcinoma cell lines SMMC-7721 and Bel-7402 are established as the subjects, and a tumor-bearing mouse model is built to study the inhibitory effects of toadoxilin on autophagy of human hepatocellular carcinoma cells in vitro and in vivo. The role and status of autophagy in the induction of human liver cancer cell death are clarified.
Toxicity analysis of Toadstool
Toadstool has intense pharmacological activity, wide clinical application and remarkable efficacy, especially in anti-tumor, cardiac strength, anesthesia and pain relief. In clinical practice, toadstool is used in various Chinese herbal prescriptions, including Toadstool Ling, Chinese Toadstool Essence, Six Gods Pill, Musk Heart Protective Pill, Plum Blossom Dotted Tongue Pill, Toadstool Injection, etc. For example, Musk Heart Protective Pill comprises seven Chinese herbs, including musk, Suhe Xiang, toadstool and cinnamon. It is used to treat chest tightness, angina pectoris and myocardial infarction caused by myocardial ischemia. In addition, toadstool is a very toxic drug, and improper use of the drug can cause serious toxic side effects.
Toadstool toxin and toadstool ligands mainly act on the heart vagus nerve center or terminals. They can act directly on the heart muscle, causing slow heart rate, arrhythmia and other slow arrhythmia symptoms. They can also cause atrioventricular conduction block, leading to cardiac arrest in systole [18-20].
Indole alkyl compounds have hallucinogenic effects. Catechol compounds can cause violent contraction of microscopic blood vessels in various organs and tissues, leading to tissue ischemia and hypoxia [21]. Symptoms of acute toadstool poisoning in humans are vomiting, shortness of breath, muscle cramps, convulsions and cardiac arrhythmias. Although the toxicity of toadstool has been recognized for a long time, the current research mainly focuses on analysing the chemical composition and pharmacological effects of toadstool. In contrast, the mechanism of toxic action of toadstool is still unclear [22].
The results of acute toxicity experiments show that the LD50 of various components of toadstool in mice are 41.0 mg/kg (intravenous injection), 96.6 mg/kg (subcutaneous injection), and 36.24 mg/kg (intraperitoneal injection). The lethal doses of various components of Toadstool are 41.0 mg/kg (intravenous injection), 96.6 mg/kg (subcutaneous injection), 36.24 mg/kg (intraperitoneal injection) for Toadstool. Among them, 2.2 mg/kg (intraperitoneal injection) are for Toadstool, 4.38 mg/kg (intraperitoneal injection) are for Toadstool, and 4.25 mg/kg (rapid intravenous injection), 15 mg/kg (slow intravenous injection), 14 mg/kg (intraperitoneal injection), 124.5 mg/kg (intraperitoneal injection) are for Toadstool.
The LD50 for toadstool in dogs is about 0.36 mg/kg (intravenous) and the oral LD50 is about 0.98 mg/kg. Acute poisoning in mice by intravenous or intraperitoneal injection of toadstool is characterized by shortness of breath, muscle spasm, cardiac arrhythmia, and finally paralysis and death, for which atropine has a certain detoxifying effect, but epinephrine does not. The toxicity of toadstool is greatly reduced after boiling [23].
Ma [24] argued that the Chinese herbal medicine Niuhuang could effectively alleviate the side effects, such as cardiac arrhythmia caused by toadflax and could be used as an antidote for toadflax poisoning.
The results of Zhong’s acute and subchronic toxicity tests showed the toxicity of Toadflax injection [25]. The target organs were mainly the liver and kidney, which showed enlargement of the liver and atrophy of the kidney, with hepatocyte swelling, degeneration, hyperplasia and renal tubular necrosis as the characteristic lesions. At the end of the recovery period, the remaining animals were observed and tested in the same way. No noticeable toxic reactions and pathological changes were observed in the medium and low dose groups. The liver and kidney functions recovered well, indicating that the dose (≤5.0 mg/kg) was not toxic. It was found that no significant toxic reactions and pathological changes were observed in the middle and low dose groups, and the recovery of liver and kidney function was good, indicating that the damaging lesions caused by the drug at this dose (≤5.0 mg/kg) were reversible and did not produce delayed toxic reactions. In contrast, the higher dose (≥10.0 mg/kg) showed good recovery. 0 mg/kg had poor recovery effects. The results also indicate that the acute toxicity of toadstool injection is high. The long-term use of large doses can lead to liver and kidney damage, so the clinical application should pay attention to the dose and treatment course.
To further analyze the material basis of toadstool cardiotoxicity, Jiang et al. used HPLC [26]. A total of nine toad steroid components, including lipotoxic ligand and nisatoxin, were measured in toadstool perfused hearts. Comparing the content of each component to the total steroids before and after perfusion, the affinity of each toadstool component to the heart may be different: an increase in the content after perfusion indicates a strong affinity for the heart; a decrease in the content suggests a weak affinity. The variation in the ratio of each compound may be the material basis for the in vitro cardiotoxicity of a toadstool in guinea pigs, but further studies are needed to elucidate the specific effects of each compound.
Conclusion
The toadstool has been widely used in traditional Chinese medicine, and the current studies on anticancer are mainly focused on chemical composition and mechanism of action. However, the chemical composition of toadstool is complex, the chemical composition of various experimental tests is not consistent, and there are few reports on the mechanism of action of each component on tumors. Research on the toxicology of toadstool, both an effective and toxic component, is few. It is necessary to thoroughly study the threshold of its effectiveness and toxicity dose, and develop low-toxicity, effective and quality-controlled toadstool products to promote its safe and reasonable application in clinical practice.
References
- Xiyan W, Min H, Zhimin W (2010) Research progress on the chemical composition of toadstools. Chinese Journal of Experimental Formulation 16: 207-220.
- Snapchi M, Baojing Z, Xie D (2009) Study on the steroidal components of toadstools in Chinese medicine. Advances in Modern Biomedicine 9: 3519-3522.
- Ruilin G, Yixiang S (2006) Clinical application of toadstool. Chinese clinical rehabilitation 30: 142-144.
- Tang A, Gao K, Chu L, Zhang R, Yang J, et al. (2017) Aurora kinases: Novel therapy targets in cancers. Oncotarget 8: 23937-23954.
- He SH (2011) Study on the mechanism of oncogene AURKA in tumor development. Beijing Peking Union Medical College, China.
- Jiabao W, Zhongwei X, Zhimei W, Erqing D, Ruicheng X (2017) Study on the mechanism of down-regulation of aurora kinase expression and promotion of cell cycle block in hepatocellular carcinoma by the active ingredients of Toadstool. Chinese herbal medicine 48: 3796-3801.
- Guo SC, Zeng D (2000) Caspase family and apoptosis. Chinese Journal of Cell Biology 6: 163-169.
- Wang DL (2010) Screening, identification and mechanism of action of the effective components of Huachanin against hepatocellular carcinoma. Shandong University, China.
- Lozy F, Karantza V (2012) Autophagy and cancer cell metabolism. Semin Cell Dev Biol 23: 395-401.
- Eskelinen EL (2011) The dual role of autophagy in cancer. Curr Opin Pharmacol 11: 294-300.
- Chen N, Wadsworth VK (2009) Role and regulation of autophagy in cancer. Biochim Biophys Acta 1793: 1516-1523.
- Song YJ, Zhang SS, Guo XL, Sun K, Han ZP, et al. (2013) Autophagy contributes to the survival of CD133+ HCC stem cells in the hypoxia and nutrient-deprived tumor microenvironment. Cancer Lett 339: 70-81.
- Suzuki SW, Onodera J, Ohsumi Y (2011) Starvation-induced cell death in autophagy-deficient yeast mutants results from mitochondrial dysfunction. PLoS One 6: 17412.
- Yang S, Wang X, Contino G (2011) Pancreatic cancers require autophagy for tumor growth. Genes Dev 25: 717-729.
- Gewirtz DA (2009) Autophagy, senescence and tumor dormancy in cancer therapy. Autophagy 5: 1232-1234.
- Shanwei S, Yi L (2015) Autophagy and its relationship with tumor proliferation, invasion, and treatment. West China Journal of Stomatology 33: 98-103.
- Mengfei H (2020) The role and mechanism of autophagy in bufalin-induced apoptosis and aerobic glycolysis in human hepatoma cells. Chinese People's Liberation Army Naval Medical College, China.
- Xiaoping L, Zheng Z, Ping H (2011) A metabolomic study on the acute toxicity of toad venom. Journal of Chemistry in Colleges and Universities 32: 38-43.
- Kennedy DJ, Vetteth S, Periyasamy SM (2006) Pathogenesis of experimental uremic cardiomyopathy. High Blood Pressure 47: 488-495.
- Bick RJ, Poindexter BJ, Sweney RR (2002) The effect of traditional Chinese medicine "Chen Su" on the instantaneous heat of isolated cardiomyocytes. Cardiotoxicity due to more than just blockade of Na, K-ATPase. Life Science 72: 699-709.
- Armstrong SC, Shivell LC, Ganote CE (2001) Simian hemorrhage and osmotic fragility are associated factors of irreversible damage in isolated rabbit cardiomyocytes after preconditioning. J Mol Cell Cardiol 33: 149-160.
- Xie JT, Wang H, Attele AS, Yuan CS (2000) Effects of resibufogenin from toad venom on isolated Purkinje fibers. Am J Chin Med 28: 187-196.
- Chinese Materia Medica Editorial Committee of the State Administration of Traditional Chinese Medicine (1999) Chinese Materia Medica. Shanghai. Shanghai Science and Technology Press 1999: 8362-8363.
- Ma H, Zhou J, Jiang J, Duan J, Xu H, et al. (2012) The novel antidote Bezoar Bovis prevents the cardiotoxicity of Toad (Bufo bufo gargarizans Canto) Venom in mice. Exp Toxicol Pathol 64: 417-423.
- Zhong SHH, Hu TJ, Hao LH, Zhao H, Deng YH, et al. (2013) Acute and subchronic toxicity study of toadstool injection. Chinese Journal of Veterinary Medicine 49: 68-71.
- Jiejun J, Jing Z, Hongyue M, Fenqiang Y, Jingran D, et al. (2022) Study on the toxic effects and material basis of Toadstool on guinea pig isolated heart. Chinese Experimental Formulary Miscellaneous, China.
Citation: Xue Z (2022) The Mechanism of Antihepatocellular Carcinoma and Toxicity Analysis of Huachanin. J Altern Complement Integr Med 8: 284.
Copyright: © 2022 Zhetian Xue, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

