The Most Relevant Extraintestinal Manifestations of Inflammatory Bowel Disease: A Multidisciplinary Approach
*Corresponding Author(s):
Carlos Alexandre Antunes De BritoDepartment Of Internal Medicine, Center Of Medical Science Of Federal University Of Pernambuco, Avenue Professor Moraes Rego, S/n, Recife, Pernambuco, Brazil; Division Of Immunology, Autoimune Research Institute, Recife, Brazil, Division Of Gastroenterology, Hospital Das Clinicas, Federal University Of Pernambuco, Recife, Brazil
Tel:+55 81 21268534,
Email:cbritoc@gmail.com
Keywords
Crohn's disease; Extraintestinal manifestations; Inflammatory bowel disease; Ulcerative colitis
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are types of inflammatory bowel disease (IBD) whose etiopathogenesis involves the interplay between immunological, genetic, microbiotic, and environmental factors and whose clinical manifestations can be chronic, recurrent, or of different intensities, varying from mild forms to severe cases associated with complications [1,2].
In addition to the involvement of the intestine, IBD may present with a broad spectrum of systemic manifestations and affects approximately a third of patients. It is frequently associated with significant morbidity and has a variable clinical course, sometimes occurring independently of bowel disease activity and requiring a therapeutic approach different from that adopted for the intestine [2,3,4].
A better understanding of the immunopathogenesis of IBD and the identification of therapeutic targets have led to the expansion of the therapeutic arsenal, thereby changing the natural history of bowel disease; however, the number of clinical trials remains limited for many of these extraintestinal manifestations (EIMs). The frequency and importance of these manifestations demand attention by gastroenterologists and a multidisciplinary approach for adequate diagnosis and case management.
In this review, we discuss the most frequent and relevant EIMs of IBD by highlighting the epidemiological, clinical, and diagnostic aspects and the main available therapeutic options
Extraintestinal Manifestations
There is a broad spectrum of EIMs, with some occurring as a consequence of bowel inflammation, surgical procedures, and side effects of drugs (e.g., arthralgia, osteopenia, osteoporosis, nephrolithiasis, cholelithiasis, glaucoma, cataracts, psoriasis) and others sharing some etiopathogenic mechanisms with IBD, such as genetic factors and cellular and humoral immune response (e.g., spondyloarthritis, pyoderma gangrenosum, uveitis, primary sclerosing cholangitis [PSC], pneumopathy, cardiac and neurological involvement) (Table 1). Moreover, some of these manifestations may precede the onset of bowel disease, occur independently of disease activity, or differ in the response to the drugs used to treat the intestinal disease [5,6].
|
Joints |
|
Peripheral arthropathy Axial arthropathy Osteoporosis |
|
Mucocutaneous |
|
Pyoderma gangrenosum Erythema nodosum Other: aphthous stomatitis, psoriasis, Sweet’s syndrome, hidradenitis suppurativa |
|
Ophthalmologic |
|
Episcleritis |
|
Hepatobiliary |
|
Primary sclerosing cholangitis Cholelithiasis |
|
Renal |
|
Nephrolithiasis |
|
Vascular |
|
Thromboembolic manifestations (deep vein thrombosis, pulmonary thromboembolism, portal vein thrombosis) |
Table 1: Main extraintestinal manifestations of inflammatory bowel disease in clinical practice.
EIMs are reported with frequencies ranging between 7% and 52% of patients with IBD. They are more frequent among CD patients, women, individuals with ileocolonic disease in CD, and those with pancolitis in UC [3,4,7-15].
The manifestations often precede the diagnosis of the inflammatory disease by months to years [3,12,13]. In a Swiss cohort of 1249 patients with IBD, 25.8% were diagnosed with the manifestations, on average, 5 months (range, 0–25 months) before the diagnosis of bowel disease [13]; however, a higher frequency and longer interval were reported in another study conducted in Greece, in which 38.6% of patient shad the EIMs between 1 and 10 years (mean, 3 years) before IBD [12].
Approximately 25% of patients with EIMs have two or more manifestations, with the association between joint and skin manifestations being more frequent [13,15]. In an analysis of 362 patients with EIMs, Vavricka S et al reported more than one EIM in 37% of cases, with two manifestations occurring in 26.5% of cases, three manifestations in 4.9%, four manifestations in 2.5%, and up to five manifestations in 2.7% throughout the course of the disease [11].
Because of the high frequency of some of these manifestations and the associated morbidity, there is a need for a specific approach that allows their identification and early diagnosis and adequate choice of treatment. In addition to making gastroenterologists aware of the importance of this topic, it is necessary to create protocols and flowcharts for the main EIMs of IBD, with the participation of other specialties and implementation of a multidisciplinary follow-up.
Joint Manifestations
The involvement of joints in IBD is frequent, accounting for 60–70% of all EIMs. Arthritis is reported in approximately 15% of patients with IBD, varying between 6% and 33% and reaching 50% when complaints of non inflammatory joint pain are included [3,4,7-16]. As described for the phenotype of EIMs, joint involvement is more frequent in CD, in women, in ileocolonic disease in CD, and in pancolitis in UC [4,16].
Despite the high frequency of joint manifestations, gastroenterologists often do not include in their clinical practice a screening for the detection of joint complaints or have difficulty in characterizing the clinical pattern of the disease, which delays diagnosis or the required treatment [17,18]. In a study including 269 patients with complaints of joint pain who were evaluated by rheumatologists, 50.5% met the criteria for spondyloarthritis, among whom 53% had peripheral clinical features, 20.6% had axial disease, and 26.4% had a combination of both [18].
Delays in diagnosis were reduced after the creation of a clinical unit for an integrated approach by gastroenterology and rheumatology specialists. The multidisciplinary approach improved the management of the rheumatological diseases in patients with IBD and allowed a more comprehensive care.
Besides the need for specific approaches to symptom control, in conditions such as axial spondyloarthritisit is important not to miss the window of opportunity to introduce disease-modifying drugs and to control the joint disease early and thus avoid permanent joint damage.
Clinical questionnaires have been developed with the aim of being administered by gastroenterologists to improve suspicion and detection of spondyloarthritis [19-21]. Queiro R et, al. assessed two questionnaires administered by gastroenterologists to patients with IBD to detect spondyloarthritis; with the use of simple questions, the questionnaires showed a sensitivity of 87.5% and 82.2% and a specificity of 89.8% and 87.4% for the detection of cases of peripheral and axial spondyloarthritis, respectively. The authors suggested that the high specificity of the questionnaires, together with their simplicity, made them adequate tools for the detection of spondyloarthritis in gastroenterology consultations [21] (Table 2).
|
Questionnaire for peripheral arthritis |
Questionnaire for axial arthritis |
|
(i) Do you have joint pain? (ii) When you wake up in the morning, do you notice stiffness in your joints that lasts for 30 minutes or more? (iii) Do you have or have you had a swollen joint? |
(i) Do you have back pain? (ii) When waking up in the morning, do you notice stiffness in your back that lasts for 30 minutes or more? (iii) Do you have or have you had back pain that wakes you up or interrupts your sleep? |
Table 2: Screening questionnaires for the detection of spondyloarthritis in inflammatory bowel disease.
An affirmative answer to two questions showed a sensitivity of 87.5% and a specificity of 89.8% for the detection of cases of peripheral spondyloarthritis and a sensitivity of 82.2% and a specificity of 87.4% for the detection of axial spondyloarthritis [21].
Classification of spondyloarthritis in IBD
Spondyloarthritis in IBD is classified into axial and peripheral arthritis. The peripheral form is divided into type I and type II, while the axial form includes isolated sacroiliitis and ankylosing spondylitis.
Peripheral arthritis accounts for more than 60% of cases of inflammatory arthropathies in IBD. Meanwhile, sacroiliitis has been described as the most frequent axial disease, but the findings have been conflicting in some studies [3,4,9,12,14]. The differences in prevalence may be related to the lack of homogeneity in patient characteristics among the studies and the use of different criteria for the classification of spondyloarthritis.
The classification of peripheral spondyloarthritis was proposed in 1998 and is used in gastroenterology guidelines, although it has not been validated [2,16].
Type I peripheral spondyloarthritis have a pauciarticular pattern and may precede bowel disease or appear at its onset. It mostly affects the large joints, knees and ankles in particular, and is strongly associated with active intestinal disease and other extra-articular manifestations (such as erythema nodosum [EN] and uveitis). Type II peripheral spondyloarthritis has a polyarticular pattern and affects the small joints, especially the metacarpophalangeal, distal inter phalangeal and wrist joints. It exhibits a symmetrical pattern and may progress to chronic arthritis, leading to erosion and deformities. It occurs independently of IBD activity and is not associated with extra-articular manifestations except uveitis [16,22,23] (Table 3).
Inflammatory axial joint involvement has been reported in 2–7%, with presentations of sacroiliitis and inflammatory back pain and with peripheral manifestations such as enthesitis, dactylitis, and asymmetrical monoarthritis or oligoarthritis predominantly affecting the lower limbs [3,4,9,12,14,16].
|
Characteristics |
Type I (pauciarticular) |
Type II (polyarticular) |
|
Number of joints |
<5 |
≥5 |
|
Symmetry |
Asymmetrical |
Symmetrical |
|
Duration |
6–10 weeks |
Months to years |
|
Most affected joints |
Large joints |
Small joints |
|
Predominant site |
Lower limbs |
Upper limbs |
|
Association with IBD activity |
Yes |
No |
|
Association with other EIMs |
Frequent |
Rare, except for uveitis |
|
Structural joint damage |
No |
Yes |
|
Association with HLA |
B27, B35, and DRβ1*0103 |
B44 |
Table 3: Clinical patterns and differences between types of peripheral spondyloarthritis in inflammatory bowel disease.
EIMs: Extraintestinal Manifestations; HLA: Human Leucocyte Antigen; IBD, Inflammatory Bowel Disease. Adapted from Vavricka et al [13].
Axial spondyloarthritis is divided into two subgroups: ankylosing spondylitis, in which radiographic sacroiliitis is defined according to the modified New York (mNY) criteria, and non-radiographic spondyloarthritis (nr-axSpA), which occurs at an earlier stage and in which patients are considered to have axial spondyloarthritis even before relevant structural damage to the sacroiliac joints occurs, thus allowing adequate early treatment [24-26] (Figure 1).
Treatment of spondyloarthritis in IBD
Adequate therapeutic management requires the knowledge of the recommendations/consensus for the treatment of axial and peripheral spondyloarthropathies, choosing the best drug for the two diseases.
In recent years, the main goal of spondyloarthritis treatment has been clinical musculoskeletal remission (arthritis, enthesitis, dactylitis, and axial disease) [27-29]. First-line therapies include the use of non steroidal anti-inflammatory drugs (NSAIDs), sulfasalazine, intra-articular injections, and non pharmacological interventions such as education about the disease, exercise, physical therapy, rehabilitation, and patients joining associations and self-help groups. Second-line therapies include conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), such as methotrexate, and biological disease-modifying antirheumatic drugs (bDMARDs). Additional therapies for special situations may include the continuous use of analgesics and surgery (Figure 1).
Despite the high frequency of joint manifestations in IBD, the guidelines of the specialty address the topic only superficially, with little emphasis on the need to actively monitor these manifestations, the relevance of an early differential diagnosis, and differences between the spondyloarthritis and their treatment.
The third European consensus of 2017 discusses and recommends, in a few paragraphs, treating the IBD flare to control the symptoms of type I spondyloarthritis, in addition to the use of sulfasalazine, rest, and physical therapy [2]. The consensus guidelines of the British Society of Gastroenterology of 2019 consider the possibility of using an intra-articular corticosteroid in patients with persistent symptoms and that small group of patients may need sulfasalazine, methotrexate, or anti-tumor necrosis factor (TNF) therapy [30].
With regard to type II spondyloarthritis, the European consensus considers the use of NSAIDs and systemic corticosteroids, while the British Society of Gastroenterology recommends referral to a rheumatologist for the consideration of immunomodulator or biological therapy and highlights the need to rule out secondary joint pain caused by the use of drugs such as azathioprine or by osteonecrosis.
Regarding axial disease, the European consensus considers the use of anti-TNF agents for refractory or NSAID-intolerant patients [2]. On the other hand, the British group in 2019 recommends physical therapy, the use of NSAIDs, and early progression to treatment with anti-TNF agents [30].
Because spondyloarthritis is a frequent EIM of IBD that requires a complex approach, it is necessary to broaden the discussion on the topic, namely through the creation of specific protocols developed with rheumatologists to guide the investigation and treatment of the disease, reduce the associated morbidity and the risk of permanent Sequel that occur in some cases due to delayed diagnosis, and establish an adequate therapeutic approach.
The ideal approach for the treatment of spondyloarthritis in IBD is to choose an effective treatment for both of its types, thus avoiding, whenever possible, the association of multiple drugs and reducing side effects. Some drugs used in rheumatology are not effective and have been associated with worsening of bowel disease, while others are effective in IBD but the doses used are different from those prescribed for the control of intestinal inflammatory activity.
In patients who have intestinal disease in remission but have joint disease that is not under control, the treatment can be directed to the EIM, ensuring the use of the lowest number of drugs that are effective for the two diseases. For example, patients with UC in remission who receive amino salicylates (5-ASA) or thiopurines and require biological therapy to control spondyloarthritis may be switched to monotherapy with an anti-TNF agent while disease remission is objectively monitored and serum drug levels are measured when recommended [30].
Some first- and second-line biologics for the treatment of spondyloarthritis may not be effective in IBD such as secukinumab, an interleukin (IL)-17 inhibitor, or be associated with a risk of bowel disease reactivation, as was described for etanercept, an anti-TNF agent [31-33]. A total of 443 cases of inflammatory disease and 43 cases of flares were reported as adverse events related to the use of etanercept. Not all patients had bowel disease regression after the suspension of etanercept, requiring the introduction of new drugs, including different anti-TNF agents approved for IBD [33].
Moreover, gastroenterologists need to be aware of vedolizumab (VDZ), an anti-integrin used exclusively for IBD and that has been associated with the appearance of new or recurrent joint manifestations [34]. However, other authors reported that although the frequency of these events was higher with VDZ than with anti-TNF therapy, it did not differ from that observed when a placebo was used [35,36]. The efficacy of VDZ in the treatment of EIMs is uncertain; the drug may be beneficial in the treatment of manifestations associated with the activity of the inflammatory disease, as in type I peripheral spondyloarthritis, and may not be effective for other types of spondyloarthritis [35].
In a multicenter study involving patients with previous arthropathy, 40.8% (64/157) had worsening of spondyloarthritis (peripheral, sacroiliitis, or ankylosing spondylitis) after using VDZ. Between the group with worsening disease and the group whose previous joint disease remained stable, there were no differences regarding the concomitant use of corticosteroids, use of combined therapy with thiopurine/methotrexate, and biological therapy. Worsening of the joint manifestation occurred in 59% of cases after 3 months of using VDZ, and the use of therapeutic regimens with shorter intervals (4 weeks) was more frequent in the group with stable joint disease [37].
Ustekinumab, an IL-12/IL-23 inhibitor, was shown to be effective in the control of bowel disease activity in UC and CD; however, only few studies have assessed the efficacy of this drug in the control of extraintestinal joint manifestations. Guillo, et al analyzed the results of three high-quality studies included in a systematic review and found that the drug was beneficial in improving arthralgia and psoriatic arthritis but there was no benefit regarding axial spondyloarthritis [38]. Ustekinumab was not shown to be effective in the treatment of axial or peripheral disease in rheumatology and is not used in the specialty’s guidelines; therefore, patients with active axial spondyloarthritis will have to switch to biological anti-TNF, and patients with peripheral disease may attempt to maintain ustekinumab for intestinal disease and associate sulfasalazine or methotrexate for joint disease.
Other molecules such as tofacitinib, Janus kinase inhibitor are used as UC treatment as well as in other rheumatological diseases such as rheumatoid arthritis and psoriasis arthritis; however this drug is not indicated to treat spondyloarthritis or ankylosant spondylitis.
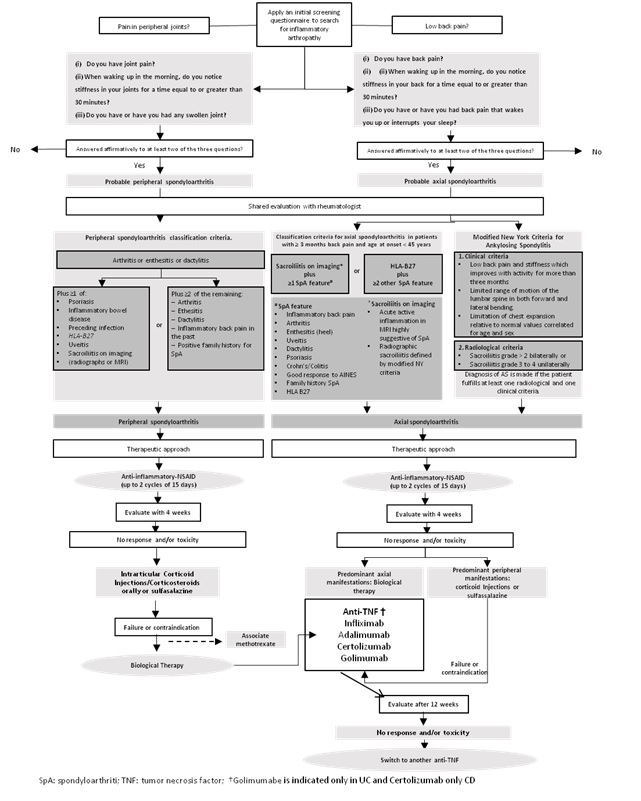 Figure 1: Therapeutic approach to spondyloarthritis in patients with inflammatory bowel disease.
Figure 1: Therapeutic approach to spondyloarthritis in patients with inflammatory bowel disease.
The management of patients with joint manifestations requires the systematization of the diagnostic and therapeutic approach that considers several aspects—such as systematic monitoring and characterization of symptoms, physical examination findings, objective definition of criteria for the different types of spondyloarthritis, intensity of the symptoms, inflammatory activity of the bowel disease, available options of treatment, and efficacy and side effects of the drugs—through the development of algorithms for a staged approach (Figure 1).
Metabolic bone disease.
Different factors contribute to the emergence of metabolic disease in IBD, such as inflammatory activity, intestinal resection, malabsorption, malnutrition, lack of physical activity, smoking and especially users of corticosteroids [1,2,30]. The frequency of osteopenia has been described to occur in about 20-40% of patients and osteoporosis in around 7-15%, being more frequent in patients with CD, among women, with higher frequencies in patients on long-term use of costicosteroids [9,12,30]. The risk of vertebrae and hip fracture is increased in these patients. The diagnosis of osteoporosis and osteopenia is based on a T-score of
Patients who use corticosteroids to treat a disease flare and those who use corticosteroids frequently or for a long time should receive calcium 500–1000 mg/day and vitamin D supplementation [800–1000 IU / day]. If osteoporosis is confirmed or if there is a high risk for this complication when there is a need to start corticosteroids, bisphosphonate may be added to the therapeutic regimen. Complementary non-medication measures should be recommended, such as performing physical activities, reducing smoking and drinking [1,2,30].
Cutaneous Manifestations
Cutaneous manifestations represent the second most common form of extraintestinal involvement in IBD, affecting 20% of patients. Pyoderma gangrenosum (PG) and Erythema nodosum (EN) are the most frequent cutaneous manifestations. Other disorders include oral aphthous ulcer, hidradenitis, psoriasis, and rare dermatologic diseases such as Sweet’s syndrome and bowel-associated dermatosis-arthritis syndrome [3,4,7-10,12,14,39-41]. This article will present the cutaneous manifestations EN and PG.
Erythema nodosum
EN is the most common dermatologic manifestation of IBD, occurring in approximately 3% of patients with UC and 8% of patients with CD. It is more common among female IBD patients [3,4,7-9,12,14,39,42,43]. Lesions typically consist of painful raised, red or violet subcutaneous nodules that are 1–5 cm in diameter. The nodules are most commonly located on the extensor surfaces of the extremities, particularly over the anterior tibial area and occasionally on the arms and trunk [44,45].
Biopsy of these lesions shows focal panniculitis. However, the diagnosis is most often clinical, and biopsy is required only in atypical cases (e.g., patients with no lesions on the legs, persistence beyond 6–8 weeks, or the development of ulceration).
The appearance of EN usually parallels intestinal disease activity. Moreover, the presence of EIMs associated with intestinal disease in suspected intestinal disease remission should prompt physicians to further investigate gastrointestinal disease activity even in the context of asymptomatic patients [44,45].
Treatment
Treatment directed at the underlying IBD usually results in resolution of EN lesions without scar formation. In the case of refractory EN or when in doubt, referral to a dermatologist can help establish a definite diagnosis. In severe cases where lesions can be very painful, a short course of oral corticosteroids (0.5–1 mg/kg/day for 1 or 2 weeks) usually leads to rapid resolution of EN [46]. Anti-TNF agents may serve as rescue therapy [47-51]. Löfberg, et al. demonstrated a decrease of EN prevalence after a 20-week course of adalimumab, from 2.4% (23/945) to 0.4% (4/942) [49]. In a very recently published retrospective analysis of the Swiss IBD cohort study, EN had an anti-TNF response rate of 80% (8/10) [47].
Pyoderma gangrenosum
PG is the second most common dermatologic manifestation of IBD. The PG incidence in individual studies ranged from 0.4% to 5%. PG is more common in UC than in CD [3,4,7-9,12,14,39,52,53].Patients with severe disease and colonic involvement are most likely to develop this complication [54]. In a recent meta-analysis, States V et al showed an association between PG and female sex, EN, and ocular EIM [53].
PG can occur in parallel with IBD activity or take an independent course. PG tends to recur following successful treatment in more than 25% of cases, often in the same place as the initial episode [55]. The lesions initially appear as single or multiple erythematous papules or pustules that are often preceded by trauma to the skin (pathergy).They occur most commonly on the lower legs and peristomal areas in up to 80% and 18% of cases, respectively; however, PG can appear anywhere on the body surface [55]. Subsequent necrosis of the dermis leads to the development of deep ulcerations that contain purulent material that is usually sterile on culture [59].
PG has four different clinical: 1) Ulcerative (classic): begins with a pustule, painful, with erythematous halo, which quickly evolves to an ulcer with a violet border, and regresses leaving a pink atrophic scar. It is associated with IBD, arthritis and malignancies, and treatment consists of systemic immunosuppressive therapy; 2) Pustular: rare form, with multiple sterile, discreet, self-limited pustules that usually regress, without leaving a scar, often associated with fever and arthralgia, IBD and Behçet's disease; 3) Bullous: papules, purples and bluish, superficial, bullous and hemorrhagic lesions, of sudden onset, associated with myeloproliferative disorders; 4) Vegetative: superficial warty lesions, more localized and non-aggressive, with a non-purulent base, with a predilection for the trunk, head and neck [58,59].
The most common presentation of PG is ulcerative (classic) disease. A diagnosis of PG rests upon the recognition of consistent clinical and histologic findings and the exclusion of other inflammatory or ulcerative cutaneous disorders, including malignancy [58,60]. In a previous study, biopsy revealed nonspecific findings consistent with a sterile abscess [61,62].
Treatment
In general, patients are managed with a combination of topical and/or systemic therapies that suppress the inflammatory process and wound care measures that optimize the environment for wound healing (Figure 2). In case intestinal disease activity is present, treating the underlying IBD often results in improvement of PG. Although initial signs of improvement may be evident within days of treatment, weeks to months are often required to achieve complete ulcer healing [62,63].Surgical interventions (excision) should be avoided if possible, as traumas may worsen PG lesions [61].
The severity of PG influences the choice of initial therapy. For patients with mild PG (few or superficial lesions), the local administration of corticosteroids (e.g., clobetasol 0.05% ointment daily, triamcinolone acetonide) or a calcineurin inhibitor (e.g., tacrolimus 0.1% ointment daily) can be sufficient for treatment [64,65].
In contrast, systemic therapy is typically necessary for patients with moderate/severe disease (multiple or deep lesions). Systemic glucocorticoids are the first-line treatments. Glucocorticoids are the most common systemic drugs prescribed since a rapid response is often observed. Treatment is initiated with 0.5–1.5 mg/kg/day of oral prednisone or its equivalent, with a maximum dose of 60 mg of prednisone per day. In very aggressive or painful disease, intravenous pulse glucocorticoids (methylprednisolone 1 mg/kg/day for 3–5 days, followed by prednisone) [56,62,66-68].
Systemic cyclosporine is an alternative first-line treatment for patients who cannot tolerate systemic glucocorticoid therapy. Treatment is initiated at a dose of 4–5 mg/kg, which is subsequently tapered as tolerated. However, cyclosporine can lead to unfavorable side effects, such as renal toxicity and hypertension [62,67].
Immunosuppressive agents such mycophenolate mofetil, methotrexate, and azathioprine have also been utilized to treat PG. However, the onset of action of these drugs is slower than that of systemic glucocorticoids and cyclosporine. These agents are generally considered most beneficial when used as adjunctive or glucocorticoid-sparing agents rather than as monotherapy [52,62,66].
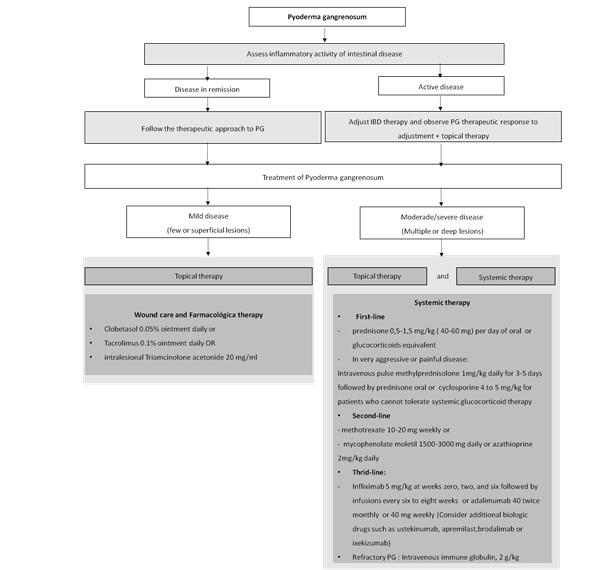 Figure 2: Therapeutic approach to Pyoderma gangrenosum in patients with inflammatory bowel disease.
Figure 2: Therapeutic approach to Pyoderma gangrenosum in patients with inflammatory bowel disease.
Biologics have been increasingly used as an adjunctive treatment for PG lesions that fail to respond to first and second-line systemic therapies. The benefit of anti-TNF therapy in managing mucocutaneous manifestations in IBD seems to be linked with a pathogenic TNF-α-dependent mechanism, common to IBD and PG [47]. Anti-TNF agents have changed PG management in recent years: interventional trials reported clinical improvement rates of 69–100% and complete remission in 21–25% of cases [59].
Infliximab should be considered if a rapid response to corticosteroids cannot be achieved. The drug is administered at a dose of 5 mg/kg infliximab at weeks 0, 2, and 6, followed by infusions every 6–8 weeks [45,47]. Other biologic TNF-α inhibitors may be beneficial in PG, such as adalimumab(40 mg weekly, 40 mg twice monthly, and other regimens),which has been associated with ulcer healing in case reports [66,70,71]. The efficacy of certolizumab pegol (another anti-TNF agent utilized for CD) in PG remains to be determined [47,66].
VDZ is a humanized IgG-1 monoclonal antibody, specifically targeting the a4b7 integrin of migrating lymphocytes, and is an approved induction and maintenance treatment for both UC and CD. A systematic literature review showed that VDZ does not appear to be efficacious for treating cutaneous manifestations but may reduce the occurrence of new EIMs [36].
Ustekinumab has been used successfully to treat PG, demonstrating clearance of recalcitrant lesions in several reports [72]. It has also been documented as a treatment drug for adalimumab-induced PG [73]. Its mechanism of action may be explained by inhibiting the increased expression of IL-23 in PG lesions. According to a systematic literature review, psoriasis, PG, and EN were assessed in seven studies, including 65 patients, and showed high response rates to ustekinumab treatment [38]. In another study, Guenova et al demonstrated that administration of ustekinumab at 45 mg twice per week at weeks 0 and 4 resulted in complete resolution of a chronic wound at 14 weeks, with no evidence of relapse after 6 months [74].
PG tends to develop drug resistance and becomes recalcitrant to available drug therapies. Antagonists to IL-1 seem to be a promising alternative. Furthermore, IL-17 is thought to play an important role in neutrophil migration into PG lesions. Emerging biologics against IL-17 and IL-7R such as ixekizumab and brodalumab are potential therapies, although their efficacy has not yet been reported. Recently, the phosphodiesterase-4 inhibitor apremilast was used successfully to treat PG [75,76].
Intravenous immune globulin (IVIg) is a treatment option for refractory PG. In a study of 49 patients with refractory PG, the most common dosage used was 2 g/kg or higher, which is higher than the dose used for other indications. When IVIG was administered in conjunction with systemic steroids in 87.8% of cases, alone in 7.7% of cases, and with a different immunosuppressive agent in 2.0% of cases, a complete or partial response was noted in 87.8% of patients, and no response in 12.2% of patients [77].
In patients with peristomal PG, closure of the stoma might lead to resolution of the PG lesions. Topical calcineurin inhibitors (pimecrolimus or tacrolimus) are an alternative treatment, but a dermatologist’s advice should be sought. Daily wound care should be performed in collaboration with a wound-care specialist [78]. A recent systematic review of peristomal PG described that most patients had a multimodal approach for treatment, but there is no consensus on the type of intervention. In both univariate and multivariate analyses, the presence of a permanent stoma was a risk factor for developing both PG and peristomal PG in our cohort [79].
Hepatobiliary Manifestations: Primary Sclerosing Cholangitis
PSC is the most important hepatobiliary manifestation of IBD because of its frequency, severity, and complex therapeutic approach. PSC is a chronic cholestatic liver disease of autoimmune etiology in which there is inflammation and fibrosis of the intra- and extrahepatic biliary ducts and that has a progressive course. The therapeutic arsenal currently available has a limited benefit in terms of modifying the natural history of the disease, with liver transplantation often being the only effective and definite treatment. Therefore, it is necessary to monitor patients with PSC rigorously, with the involvement of a multidisciplinary team of gastroenterologists, hepatologists, and endoscopists.
PSC is the most prevalent liver manifestation of IBD, with a frequency among cohorts varying from 0.6% to 10.5%. The disease predominantly affects men aged between 25 and 45 years. PSC is more associated with UC than with CD, and pancolitis is the intestinal pattern most frequently found in patients with UC [3,4,7- 9,12,14].
The risk of biliary tract cancer and colon cancer is high among patients with PSC, and these diseases account for 40-50% of mortality in PSC. Cholangiocarcinoma is the most frequent type of hepatobiliary cancer associated with PSC, with an estimated annual incidence of 0.5–1.5% and a lifetime incidence of 20%. The estimated incidence of gallbladder cancer is 3–14%, and that of hepatocellular carcinoma is 0.3–2.8%. It is estimated that the risk of colon cancer is 2–5 times higher in patients with PSC and IBD than in those with IBD alone [80-82].
Most patients with PSC are asymptomatic at the time of diagnosis, and the elevation of alkaline phosphatase (ALP) levels is an important sign for diagnosis. Symptoms such as fatigue and pruritus may occur in the initial stages, and jaundice, choluria, acholic stools, hepatosplenomegaly, ascites, and encephalopathy may appear as the disease progresses [83]. Episodes of infectious processes in cholangitis caused by bacterial infection secondary to obstruction may also occur.
With regard to laboratory findings, elevation of ALP levels is an evidence of the cholestatic component of PSC. Gamma-glutamyl transferase may also be elevated and confirms the biliary origin of the liver disease. Bilirubin levels are usually normal at the time of diagnosis and tend to increase as the disease progresses. Moreover, there is a slight increase in the levels of aminotransferases [84].
Diagnosis
Ultrasonography (US) may be performed initially when the ALP level is elevated, to rule out extrahepatic cholestasis, including choledocholithiasis and biliary tract cancer. If the US findings are normal, without dilatation of the biliary ducts, the patients should undergo a non-invasive magnetic resonance cholangiography (MRCP), but it may eventually be necessary to perform endoscopic retrograde cholangiopancreatography (ERCP). Although ERCP is an invasive procedure and is associated with the risk of pancreatitis, it may be fundamental in the therapeutic management of obstructions, removal of calculi, and collection of samples for brush cytology or biopsy to investigate cancer processes [83,85,86].
The characteristic findings of ERC are intrahepatic and extrahepatic multifocal stenosis of the biliary duct (“beaded” appearance), mild biliary dilation, diverticular pouches, and “pruned tree” appearance in the chronic stage [86].
Liver biopsy is rarely used to confirm the diagnosis and is indicated in cases of persistently elevated levels of canalicular enzymes despite normal findings on imaging examinations to detect small duct PSC or identify autoimmune hepatitis. The characteristic feature of the liver biopsy in PSC is concentric periductal fibrosis (“onion skinning”), but it is not always observed.
Some guidelines recommend that patients with PSC be tested for IgG4 at least once to detect the specific form of PSC associated with IgG4, which may progress more rapidly [83,84,87].
The 2019 British Society of Gastroenterology guidelines for PSC indicate that liver biochemistry with typical cholestasis and cholangiographic findings, in the absence of other identifiable causes of secondary sclerosing cholangitis, is usually sufficient to diagnose PSC [85].
The differential diagnosis is broad and includes choledocholithiasis, cholangiocarcinoma, IgG4-related cholangitis, autoimmune hepatitis, primary biliary cirrhosis, overlap syndrome (autoimmune cholangiopathy), HIV-associated cholangiopathy, and cystic fibrosis [86,87].
Treatment of PSC
To date, there is no proven effective pharmacological treatment for patients with PSC [83]. The efficacy of the therapeutic drug arsenal for PSC is questionable, with some drugs being used for specific cases, and clinicians in their practice have questions regarding when to use the drug and at what doses (Figure 3).
Ursodeoxycholic acid (UDCA) has been shown in some studies to improve liver biochemistry but not histology or disease progression [85]. In a prospective study, the withdrawal of UDCA after 3 months in 26 patients was shown to cause significant deterioration of liver biochemistry and to worsen pruritus, which indicates that patients benefit from the prolonged use of UDCA [88]. However, higher doses of 28–30 mg/kg/day were associated with worsening of the disease and with more side effects [89]. In another study, the highest doses also increased the risk of colon cancer [90]. Despite the limited scientific evidence of a beneficial effect, some algorithms have been proposed to assess the use of UDCA at doses of 13–15 mg/kg/day for 6 months, and if there is normalization, a reduction in ALP by 40%, a decrease in levels to lower than 1.5 times the upper limit of the normal range, or improvement of pruritus, the drug may then be maintained in the long term [91].
A further reason for the use of UDCA is the reduction of the risk of cancer; however, two meta-analysis studies did not show an effect on the reduction in the incidence of colorectal cancer in PSC patients. In one of the meta-analysis studies, Hansen et al observed a tendency for the reduction of the risk of colorectal cancer in a subgroup of patients who received medium to low doses of UDCA (
The use of UDCA is not recommended by many international guidelines [83,85,87,94]. The American Gastroenterological Association (AGA) guidelines for the treatment of PSC do not make specific recommendations for its use; they only define as consensus that doses higher than 28 mg/kg/day should not be administered [83]. The British Society of Gastroenterology recommends that UDCA should not be used for the routine treatment of newly diagnosed PSC and that patients who already received the drug should avoid doses of 28–30 mg/kg/day. The use of this drug with the aim of reducing the risk of colorectal cancer is also not recommended by this society [85].
Other therapies proposed for PSC include immunosuppressant agents (e.g., azathioprine, cyclosporine, methotrexate, mycophenolate mofetil, tacrolimus), corticosteroids, monoclonal antibodies (e.g., anti-TNF agents, VDZ), fibrates, and antimicrobial drugs (e.g., metronidazole, vancomycin). However, these drugs were not shown to be effective in preventing disease progression and only improved the levels of cholestatic enzymes [95-100]. The use of these medications is not recommended by recent guidelines [83,85,87,94]. Liver transplantation is indicated for advanced liver disease or in the presence of complications [83,85,87,94]. AGA (2015) considers the indication for transplantation when the Model for End-Stage Liver Disease (MELD) scores exceeds 14 or in cases complicated by cholangiocarcinoma [83]. The presence of untreatable pruritus and recurrence of cholangitis are also accepted indications for orthotopic liver transplantation in the UK and should justify the early referral for liver transplantation [85]. There currence of PSC in transplanted livers is observed in 10–40% of cases and is more frequent among men, patients who did not undergo colectomy, and/or those whose colitis remained active after transplantation.
Treatment of symptoms and complications
Pruritus significantly affects quality of life, and pharmacological treatment must be initiated (Figure 3). The use of skin emollients and antihistamines has limited effectiveness. The drug of choice to control pruritus is cholestyramine (anion exchange resin), at a dose of 4–16 g/day. If this fails, rifampicinat a dose of 150–300 mg twice daily, naltrexone (opioid μ-receptor antagonist) at a dose of up to 50 mg/day, sertraline (selective serotonin reuptake inhibitor) at a dose of 75–100 mg/day, or phenobarbital at a dose of 90 mg at bedtime may be used [83,85,94].
The use of UDCA and corticosteroids for their life of symptoms has shown limited results. Patients who experience persistent and progressive pruritus should undergo imaging examinations, and if a dominant narrowing of the biliary duct is detected, an endoscopic approach with dilation or placement of a stent should be adopted to relieve pruritus [1,86,87,94].
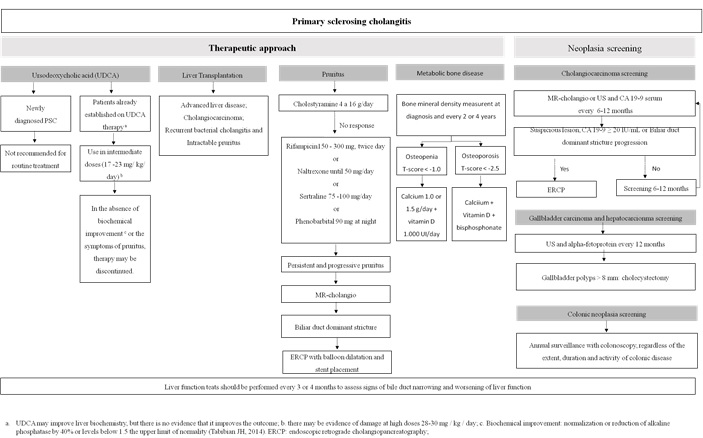 Figure 3: Therapeutic approach and neoplasia screening in patients with primary sclerosing cholangitis and inflammatory bowel disease.
Figure 3: Therapeutic approach and neoplasia screening in patients with primary sclerosing cholangitis and inflammatory bowel disease.
Metabolic bone disease has been associated with chronic cholestatic disease. In addition, patients with long-term IBD or exposure to corticosteroids are more predisposed to osteopenia and osteoporosis [1,83]. The diagnosis of osteoporosis and osteopenia is based on a T-score of
Follow-up and cancer screening
Liver biochemistry tests should be performed every 3 or 4 months to evaluate signs of narrowing of the biliary duct and worsening of liver function [83] (Figure 3).
The European Society of Gastrointestinal Endoscopy (ESGE) and the European Association for the Study of the Liver (EASL) suggest that ERCP with ductal sampling (such as brush cytology or endobiliary biopsy) should be considered in cases of PSC with the following characteristics: (i) clinically relevant symptoms or deterioration (jaundice, cholangitis, pruritus), (ii) rapid increase in the levels of cholestatic enzymes, or (iii) new dominant stenosis or progression of the existing dominant stenosis identified by MRCP in the context of appropriate clinical findings [86]. Worsening of pain in the right upper quadrant of the abdomen, fatigue, and weight loss also require a thorough evaluation. In the presence of dominant stenosis, dilation or placement of a stent should be considered [83,86,87,94].
Screening of cholangiocarcinoma every 6-12 months with MRCP or US and determination of carbohydrate antigen (CA) 19-9 should be considered [82,83,85]. Meanwhile, ERCP should be considered when there are suspicious lesions on imaging, elevated CA 19-9 levels (≥20 IU/mL), or rapid clinical deterioration and stenosis progression [82,94]. The British Society of Gastroenterology does not include CA 19-9 in the screening because it considers that the test has low accuracy [85].
Screening of gallbladder cancer and hepatocellular carcinoma should be performed with US annually. All patients with PSC and gallbladder polyps larger than 8 mm or gallbladder masses of any size should be evaluated for cholecystectomy. In patients with liver cirrhosis, US screening intervals may be reduced to 6 months [82,83].
Patients with UC and concomitant PSC should undergo an annual screening colonoscopy after a diagnosis of PSC, regardless of the activity, extension, and duration of the UC [2,82,83,85-87].
Ophthalmologic Manifestations
Ophthalmologic manifestations are important EIMs because of their frequency and the risk of complications if not adequately treated. The prevalence of ophthalmologic disorders in population studies varies between 1% and 11.1% [3,4,7,9,10,11,12,14,15,101], and ophthalmologic disorders are associated with both UC and CD. Females, patients with colonic involvement in CD, and those with more extensive forms of UC are the groups most at risk [12,101,102].
Genetic susceptibility and immunopathogenic auto antibodies against organ-specific cells shared by the colon and extracolonic organs may be responsible for extraintestinal involvement in IBD [103,104].
The most common ocular manifestations associated with IBD are episcleritis and anterior uveitis, with scleritis and intermediate and posterior uveitis being rarer [12,46,105,106,107]. An adequate evaluation should be performed at the time of IBD diagnosis, regardless of the presence of ocular manifestations, to allow having a baseline picture of the ophthalmologic condition.
Episcleritis
Episcleritis is a benign inflammation of the episclera, a thin hypervascular layer that covers the sclera. It is the most common ocular manifestation and has the least complications. It causes eye discomfort, acute redness in one or both eyes, and episcleral diffuse or localized edema [105,106].
This condition is more often associated with IBD activity than other ocular manifestations. It occurs during exacerbation of BDI and regresses with effective treatment of intestinal inflammatory activity [46,107]. It usually has a recurrent pattern and may progress to scleritis. The distinction between scleritis and episcleritis may be difficult for a non specialist, as well as between episcleritis and conjunctivitis, a condition that is common among the general population and that may coincide with IBD. Because episcleritis is a benign disease, a specific treatment is rarely indicated; however, the use of a cold compress, eye lubricant, topical NSAID, or corticosteroid may be necessary [105,106].
Scleritis
Scleritis is an inflammation of the sclera, the opaque and protective outermost layer of the eye. Scleritis can be categorized as anterior or posterior scleritis, according to the anatomy of the primary focus of the inflammation. Anterior scleritis is subdivided into diffuse scleritis, nodular scleritis (with or without necrosis), and scleromalacia. In scleromalacia, there is significant thinning of the scleral layers.
A symptom of scleritis is pain radiating to the face and head that typically worsens at night, which is associated with ocular hyperemia and visual loss. It may progress to permanent loss of vision if not diagnosed and treated early. Necrotizing scleritis is strongly associated with autoimmune diseases and requires immediate immunosuppressive treatment, with pulse therapy often being necessary. The risk of ocular perforation is imminent in severe and hyper acute cases.
Systemic treatment with an NSAID is indicated, but caution is advised regarding its use in the presence of IBD. Systemic corticosteroids or immunosuppressants may be necessary in more severe cases. The control of intestinal inflammatory activity is important to prevent recurrence [105,106].
Uveitis
Uveitis is a general term that includes all inflammations of the uveal tract, which extends from the iris to the optic nerve, including the retina and the vitreous body. It is classified, according to the predominantly affected site, into anterior, intermediate, posterior, and diffuse uveitis (panuveitis) [108]. Anterior uveitis is the most frequent and the most associated with autoimmune diseases. It is further classified as granulomatous and nongranulomatous uveitis [108,109].
Anterior uveitis is the presentation most commonly related to IBD. It is chronic, is not related to intestinal disease activity, and has an insidious onset and bilateral involvement [12].
The symptoms comprise visual discomfort, with or without conjunctival hyperemia, eye pain with intensity depending on the degree of inflammation and the involvement of ciliary body, and low acuity that depends on the amount of cells in the anterior chamber or on concomitant complications. Thus, even in the absence of signs and symptoms of alarm such as moderate to intense eye pain, photophobia, blurred vision, and reduced visual acuity; an evaluation by a specialist is warranted for a better staging of the uveitis.
An early start of treatment avoids complications and visual loss. Management of anterior uveitis is relatively simple, consisting in the use of topical corticosteroids, and the response is almost always complete. Mydriatics are used to relax the ciliary body and the pupil, decreasing pain and posterior synechiae. The chronic use of corticosteroids may lead to important complications such as cataracts and glaucoma. These complications are also observed in uveitis not well managed or when there is chronification.
Systemic drugs may be used in cases of therapeutic failure or chronification of the clinical presentation, such as immunosuppressants or biological agents for a better control [110]. In selected cases, periocular or intraocular injection of a corticosteroid may be used as adjuvant therapy [105,106].
The anti-TNF immunobiologics adalimumab and infliximab have been shown to be effective in the control of severe cases [111]. Control of uveitis with ustekinumab has also been reported in a limited number of cases and may be considered in cases refractory to anti-TNF drugs [38].
Thromboembolic Manifestations
The acute or chronic inflammatory state seen in IBD is a prothrombotic condition caused by an imbalance between pro- and anticoagulant factors. This imbalance results in venous thromboembolism (VTE) as a frequent EIM in IBD [112,113,114], with an estimated risk of pulmonary thromboembolism (PTE) and deep vein thrombosis (DVT) three times higher than in the general population [111].
In hospitalized patients, the most commonly reported thrombotic event is portal vein thrombosis, with a risk of 1.77 and 1.24 times greater in UC and CD respectively compared to the population without IBD. PTE is also reported to have a higher incidence in UC (0.53%), but inpatients without IBD and with CD, the differences were small, with an incidence of 0.38% and 0.28%, respectively [113].
In retrospective analysis of discharge data from the National Inpatient Sample in U.S between 2009 and 2014, Cohen et al. analyzed more than 45 million hospital discharges of patients with and without IBD, associated or not with thrombotic events. The prevalence of thrombosis among patients with IBD was 1.7 times higher than among patients without IBD (7.51% versus 4.53%, p <0.0001) [112].
Mesenteric and portal venous thrombosis were the most common sites of venous thrombosis in IBD patients, but PTE and DVT were more common among hospitalized patients without IBD. The risk factors associated with the thromboembolic complications for IBD patients were the presence of a central venous catheter, malignancy, malnutrition, and chronic use of corticosteroids [112]. The risk of acute arterial events in IBD is uncertain, and the findings are conflicting [112,115].
Besides exposure to acquired and genetic risk factors, pharmacological therapy should also be considered a factor in the pro- and anti-inflammatory imbalance, modifying the risk of VTE. The majority of the drug options reduce inflammatory activity in IBD, an important risk factor for VTE; however, drugs such as corticosteroids, methotrexate, and tofacitinib should be used cautiously or even avoided in the groups at risk for VTE, even if there is proven benefit in the clinical treatment of IBD [116].
Corticosteroids induce a procoagulant state of clinical relevance, especially when administered chronically. Methotrexate may induce thromboembolic events by causing folate deficiency, an independent risk factor for hyperhomocysteinemia.
Tofacitinib, of the class of Janus kinase inhibitors, previously approved for the treatment of rheumatoid arthritis, psoriatic arthritis, and psoriasis, was shown to increase the risk of developing VTE, and therefore, the European Medicines Agency recommends against its use by patients aged over 65 years, at a maintenance dose of 10 mg twice daily for cardiac patients, by high-risk patients or patients with a previous history of thromboembolic events, after a major surgery and prolonged immobilization, and by patients with obesity (BMI>30), diabetes, hypertension, or smoking habits.
The use of tofacitinib by UC patients is more recent, and data on the adverse events of this treatment are still limited. However, its use should be avoided in these clinical situations, or the drug should be used cautiously when other options are not available. Potential adverse drug events and individual risk factors should therefore be considered in patients with IBD [116].
Despite rectal bleeding being a common symptom in IBD, anticoagulation is safe and should be promoted for all hospitalized patients, regardless of clinical severity and the reason for hospitalization [117] (Table 4).
Anticoagulation should be maintained throughout the duration of hospitalization, and although there is evidence that thromboembolic events may occur in the immediate post-discharge period, there are no data on the extension of post-discharge prophylaxis. Nevertheless, its use may be justified inpatients at high risk for thrombosis [118].
The association of mechanical measures with prophylaxis with heparin is indicated for patients who are at a higher risk for thrombosis. In the presence of severe bleeding or other conditions with an increased risk of bleeding, only mechanical measures should be used [119].
|
Patients with IBD hospitalized with moderate to severe activity without severe bleeding: anticoagulant prophylaxis with LMWH, low-dose unfractionated heparin, or fondaparinux is recommended. |
|
Patients with IBD undergoing abdominopelvic surgery or major general surgery: anticoagulant prophylaxis during hospitalization is recommended. |
|
Outpatients with active IBD and without previous VTE: anticoagulant prophylaxis is not recommended. |
|
Patients with VTE: the coexistence of IBD is not an indication for investigating hereditary or acquired hypercoagulation conditions. |
|
Patients with clinically inactive IBD in the first episode of VTE in the presence of a reversible risk factor unrelated to the disease: anticoagulant therapy for at least 3 months and for at least 1 month after resolution of the risk factor is recommended. |
|
Patients with symptomatic acute splanchnic vein thrombosis (portal, mesenteric, and/or splenic vein): anticoagulant therapy is recommended. |
|
Patients with clinically inactive IBD with symptomatic acute splanchnic vein thrombosis in the presence of a reversible risk factor unrelated to the disease: anticoagulant therapy for at least 3 months and for at least 1 month after resolution of the risk factor is recommended. |
Table 4: Recommendations for the prevention and management of venous thromboembolism in inflammatory bowel disease.
IBD: Inflammatory bowel disease; LMWH: Low-molecular-weight heparin; VTE: Venous Thromboembolism. Adapted from Nguyen GC, 2014 [120].
Summary
In this review, we emphasize that extraintestinal manifestations are frequent in inflammatory bowel disease, have a broad clinical spectrum, varying from mild forms to severe cases, sometimes with serious complications. The complexity of the therapeutic approach requires the involvement of different specialties, as well as the elaboration of systematic protocols for adequate diagnosis and case management, improving the quality of life of patients and reducing the risk of complications.
References
- Gionchetti P, Dignass A, Danese S, Magro Dias FJ, Rogler G, et al. (2017) 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 2: Surgical Management and Special Situations. J Crohns Colitis 11: 135-149.
- Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, et al. (2017) Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis 11: 649-670.
- Park SK, Wong Z, Park SH, Vu KV, Bang KB, et al. (2021) Extraintestinal manifestation of inflammatory bowel disease in Asian patients: A multinational study. Dig Liver Dis 53: 196-201.
- Algaba A, Guerra I, Ricart E, Iglesias E, Mañosa M, et al. (2020) Extraintestinal Manifestations in Patients with Inflammatory Bowel Disease: Study Based on the ENEIDA Registry. Dig Dis Sci
- Perez-Alamino R, Maldonado-Ficco H, Maldonado-Cocco JA (2016) Rheumatic manifestations in inflammatory bowel diseases: a link between GI and rheumatology. Clin Rheumatol 35: 291-296.
- Garber A, Regueiro M (2019) Extraintestinal Manifestations of Inflammatory Bowel Disease: Epidemiology, Etiopathogenesis, and Management. Curr Gastroenterol Rep 21: 31.
- Martinelli VF, de Brito CAA, Gomes RG, Domingues ALC, Jucá NT, et al. (2020) Clinical and Epidemiological Characteristics of Patients with Intestinal Inflammatory Disease in Pernambuco, Northeast of Brazil. J Gastroenterol Hepatol 9: 3202-3208.
- Kotze PG, Steinwurz F, Francisconi C, Zaltman C, Pinheiro M, et al. (2020) Review of the epidemiology and burden of ulcerative colitis in Latin America. Therap Adv Gastroenterol 13: 1756284820931739.
- Chimenti MS, Conigliaro P, Triggianese P, Canofari C, Cedola F, et al. (2019) Use of synthetic and biological DMARDs in patients with enteropathic spondyloarthritis: a combined gastro-rheumatological approach. Clin Exp Rheumatol 37: 723-730.
- Conigliaro P, Chimenti MS, Ascolani M, Triggianese P, Novelli L, et al. (2016) Impact of a multidisciplinary approach in enteropathic spondyloarthritis patients. Autoimmun Rev 15: 184-190.
- Alnaqbi KA, Touma Z, Passalent L, Johnson SR, Tomlinson GA, et al. (2013) Development, sensibility, and reliability of the Toronto Axial Spondyloarthritis Questionnaire in inflammatory bowel disease. J Rheumatol 40: 1726-1735.
- Di Carlo M, Luchetti MM, Benfaremo D, Di Donato E, Mosca P, et al. (2018) The DETection of Arthritis in Inflammatory boweL diseases (DETAIL) questionnaire: development and preliminary testing of a new tool to screen patients with inflammatory bowel disease for the presence of spondyloarthritis. Clin Rheumatol 37: 1037-1044.
- Queiro R, Rodríguez-Caminero S, Riestra S, de Francisco R, Pérez-Martínez I, et al. (2018) Performance of Two Screening Questionnaires for Inflammatory Arthritis in Patients with Inflammatory Bowel Disease. Biomed Res Int 2018: 8618703.
- Peluso R, Di Minno MN, Iervolino S, Manguso F, Tramontano G, et al. (2013) Enteropathic spondyloarthritis: from diagnosis to treatment. Clin Dev Immunol 2013: 631408.
- Ben Nessib D, Ferjani H, Maatallah K, Rahmouni S, Kaffel D, Hamdi W (2020) Update on therapeutic management of spondyloarthritis associated with inflammatory bowel disease. Clin Rheumatol 39: 3543-3553.
- van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27: 361-368.
- Sieper J, Rudwaleit M, Baraliakos X, Brandt J, Braun J, et al. (2009) The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 68: ii1-44.
- Rudwaleit M, van der Heijde D, Landewé R, Akkoc N, Brandt J, et al. (2011) The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 70: 25-31.
- Ward MM, Deodhar A, Akl EA, Lui A, Ermann J, et al. (2016) American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol 68: 282-298.
- van der Heijde D, Ramiro S, Landewé R, Baraliakos X, Van den Bosch F, et al. (2017) 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 76: 978-991.
- Smolen JS, Schöls M, Braun J, Dougados M, FitzGerald O, et al. (2018) Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis 77: 3-17.
- Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, et al. (2019) British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 68: s1-s106.
- Sandborn WJ, Hanauer SB, Katz S, Safdi M, Wolf DG, et al. (2001) Etanercept for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology 121: 1088-1094.
- Dallocchio A, Canioni D, Ruemmele F, Duquesne A, Scoazec JY, et al. (2010) Occurrence of inflammatory bowel disease during treatment of juvenile idiopathic arthritis with etanercept: a French retrospective study. Rheumatology (Oxford) 49: 1694-1698.
- O'Toole A, Lucci M, Korzenik J (2016) Inflammatory Bowel Disease Provoked by Etanercept: Report of 443 Possible Cases Combined from an IBD Referral Center and the FDA. Dig Dis Sci 61: 1772-1774.
- Dubinsky MC, Cross RK, Sandborn WJ, Long M, Song X, et al. (2018) Extraintestinal Manifestations in Vedolizumab and Anti-TNF-Treated Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis 24: 1876-1882.
- Chateau T, Bonovas S, Le Berre C, Mathieu N, Danese S, et al. (2019) Vedolizumab Treatment in Extra-Intestinal Manifestations in Inflammatory Bowel Disease: A Systematic Review. J Crohns Colitis 13: 1569-1577.
- Feagan BG, Sandborn WJ, Colombel JF, Byrne SO, Khalid JM, et al. (2019) Incidence of Arthritis/Arthralgia in Inflammatory Bowel Disease with Long-term Vedolizumab Treatment: Post Hoc Analyses of the GEMINI Trials. J Crohns Colitis 13: 50-57.
- Ramos GP, Dimopoulos C, McDonald NM, Janssens LP, Hung KW, et al. (2020) The Impact of Vedolizumab on Pre-Existing Extraintestinal Manifestations of Inflammatory Bowel Disease: A Multicenter Study. Inflamm Bowel Dis 9: izaa293.
- Guillo L, D'Amico F, Danese S, Peyrin-Biroulet L (2020) Ustekinumab for extra-intestinal manifestations of inflammatory bowel disease: a systematic literature review. J Crohns Colitis 24: jjaa260.
- Roth N, Biedermann L, Fournier N, Butter M, Vavricka SR, et al. (2019) Occurrence of skin manifestations in patients of the Swiss Inflammatory Bowel Disease Cohort Study. PLoS One 14: e0210436.
- Travis S, Innes N, Davies MG, Daneshmend T, Hughes S (1997) Sweet's syndrome: an unusual cutaneous feature of Crohn's disease or ulcerative colitis. The South West Gastroenterology Group. Eur J Gastroenterol Hepatol 9: 715-720.
- Truchuelo MT, Alcántara J, Vano-Galván S, Jaén P, Moreno C (2013) Bowel-associated dermatosis-arthritis syndrome: another cutaneous manifestation of inflammatory intestinal disease. Int J Dermatol 52: 1596-1598.
- Farhi D, Cosnes J, Zizi N, Chosidow O, Seksik P, et al. (2008) Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2402 patients. Medicine (Baltimore) 87: 281-293.
- Lakatos L, Pandur T, David G, Balogh Z, Kuronya P, et al. (2003) Association of extraintestinal manifestations of inflammatory bowel disease in a province of western Hungary with disease phenotype: results of a 25-year follow-up study. World J Gastroenterol 9: 2300-2307.
- Burgdorf W (1981) Cutaneous manifestations of Crohn's disease. J Am Acad Dermatol 5: 689-695.
- Greuter T, Navarini A, Vavricka SR (2017) Skin Manifestations of Inflammatory Bowel Disease. Clin Rev Allergy Immunol 53: 413-427.
- Harbord M, Annese V, Vavricka SR, Allez M, Barreiro-de Acosta M, et al. (2016) The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J Crohns Colitis 10: 239-254.
- Vavricka SR, Gubler M, Gantenbein C, Spoerri M, Froehlich F, et al. (2017) Anti-TNF Treatment for Extraintestinal Manifestations of Inflammatory Bowel Disease in the Swiss IBD Cohort Study. Inflamm Bowel Dis 23: 1174-1181.
- Peyrin-Biroulet L, Van Assche G, Gómez-Ulloa D, García-Álvarez L, Lara N, et al. (2017) Systematic Review of Tumor Necrosis Factor Antagonists in Extraintestinal Manifestations in Inflammatory Bowel Disease. Clin Gastroenterol Hepatol 15: 25-36.e27.
- Löfberg R, Louis EV, Reinisch W, Robinson AM, Kron M, et al. (2012) Adalimumab produces clinical remission and reduces extraintestinal manifestations in Crohn's disease: results from CARE. Inflamm Bowel Dis 18: 1-9.
- Clayton TH, Walker BP, Stables GI (2006) Treatment of chronic erythema nodosum with infliximab. Clin Exp Dermatol 31: 823-834.
- Ortego-Centeno N, Callejas-Rubio JL, Sanchez-Cano D, Caballero-Morales T (2007) Refractory chronic erythema nodosum successfully treated with adalimumab. J Eur Acad Dermatol Venereol 21: 408-410.
- Bernstein CN (2001) Extraintestinal manifestations of inflammatory bowel disease. Curr Gastroenterol Rep 3: 477-483.
- States V, O'Brien S, Rai JP, Roberts HL, Paas M, et al. (2020) Pyoderma Gangrenosum in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci 65: 2675-2685.
- Orchard T (2003) Extraintestinal complications of inflammatory bowel disease. Curr Gastroenterol Rep 5: 512-517.
- Freeman HJ (2005) Erythema nodosum and pyoderma gangrenosum in 50 patients with Crohn's disease. Can J Gastroenterol 19: 603-606.
- Agarwal A, Andrews JM (2013) Systematic review: IBD-associated pyoderma gangrenosum in the biologic era, the response to therapy. Aliment Pharmacol Ther 38: 563-572.
- Ashchyan HJ, Butler DC, Nelson CA, Noe MH, Tsiaras WG, et al. (2013)The Association of Age With Clinical Presentation and Comorbidities of Pyoderma Gangrenosum. JAMA Dermatol 154: 409-413.
- Maverakis E, Ma C, Shinkai K, Fiorentino D, Callen JP, et al. (2018) Diagnostic Criteria of Ulcerative Pyoderma Gangrenosum: A Delphi Consensus of International Experts. JAMA Dermatol 154: 461-466.
- Ahn C, Negus D, Huang W (2018) Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol 14: 225-233.
- Weenig RH, Davis MD, Dahl PR, Su WP (2002) Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med 347: 1412-1418.
- Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, et al. (2015) Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm Bowel Dis 21: 1982-1992.
- Rodríguez-Zúñiga MJM, Heath MS, Gontijo JRV, Ortega-Loayza AG (2019) Pyoderma gangrenosum: a review with special emphasis on Latin America literature. An Bras Dermatol 94: 729-743.
- Dos Santos CHM, Koga GA (2021) The Challenge of Treating Pyoderma Gangrenosum in a Patient With Crohn Disease Based on Poor Scientific Evidence. Inflamm Bowel Dis 27: e23-e24.
- Thomas KS, Ormerod AD, Craig FE, Greenlaw N, Norrie J, et al. (2016) Clinical outcomes and response of patients applying topical therapy for pyoderma gangrenosum: A prospective cohort study. J Am Acad Dermatol 75: 940-949.
- Baltazar D, Haag C, Gupta AS, Marzano AM, Ortega Loayza AG (2019) A Comprehensive Review of Local Pharmacologic Therapy for Pyoderma Gangrenosum. Wounds 31: 151-157.
- Herberger K, Dissemond J, Hohaus K, Schaller J, Anastasiadou Z, et al. (2016) Treatment of pyoderma gangrenosum: retrospective multicentre analysis of 121 patients. Br J Dermatol 175: 1070-1072.
- Ormerod AD, Thomas KS, Craig FE, Mitchell E, Greenlaw N, et al. (2015) Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ 350: h2958.
- Aseni P, Di Sandro S, Mihaylov P, Lamperti L, De Carlis LG (2008) Atypical presentation of pioderma gangrenosum complicating ulcerative colitis: rapid disappearance with methylprednisolone. World J Gastroenterol 14: 5471-5473.
- Brooklyn TN, Dunnill MG, Shetty A, Bowden JJ, Williams JD, et al. (2006) Infliximab for the treatment of pyoderma gangrenosum: a randomized, double blind, placebo controlled trial. Gut 55: 505-509.
- Hubbard VG, Friedmann AC, Goldsmith P (2005) Systemic pyoderma gangrenosum responding to infliximab and adalimumab. Br J Dermatol 152: 1059-1061.
- Cariñanos I, Acosta MB, Domènech E (2011) Adalimumab for pyoderma gangrenosum associated with inflammatory bowel disease. Inflamm Bowel Dis 17: E153-E154.
- Goldminz AM, Botto NC, Gottlieb AB (2012) Severely recalcitrant pyoderma gangrenosum successfully treated with ustekinumab. J Am Acad Dermatol 67: e237-e238.
- Benzaquen M, Monnier J, Beaussault Y, Rouby F, Berbis P (2017) Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: Effectiveness of ustekinumab. Australas J Dermatol 58: e270-e271.
- Guenova E, Teske A, Fehrenbacher B, Hoerber S, Adamczyk A, et al. (2011) Interleukin 23 expression in pyoderma gangrenosum and targeted therapy with ustekinumab. Arch Dermatol 147: 1203-1205.
- Laird ME, Tong LX, Lo Sicco KI, Kim RH, Meehan SA, et al. (2017) Novel use of apremilast for adjunctive treatment of recalcitrant pyoderma gangrenosum. JAAD Case Rep 3: 228-229.
- Wollina U (2017) Emerging treatments for pyoderma gangrenosum. Exp Opin Orphan Drugs 5: 827–832.
- Song H, Lahood N, Mostaghimi A (2018) Intravenous immunoglobulin as adjunct therapy for refractory pyoderma gangrenosum: systematic review of cases and case series. Br J Dermatol 178: 363-368.
- Barbosa NS, Tolkachjov SN, El-Azhary RA, Davis MD, Camilleri MJ, et al. (2016) Clinical features, causes, treatments, and outcomes of peristomal pyoderma gangrenosum (PPG) in 44 patients: The Mayo Clinic experience, 1996 through 2013. J Am Acad Dermatol 75: 931-939.
- Afifi L, Sanchez IM, Wallace MM, Braswell SF, Ortega-Loayza AG, et al. (2018) Diagnosis and management of peristomal pyoderma gangrenosum: A systematic review. J Am Acad Dermatol 78: 1195-1204.
- Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO (2002)Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc 56: 48-54.
- Zheng HH, Jiang XL. (2016) Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis of 16 observational studies. Eur J Gastroenterol Hepatol 28: 383-390.
- Fung BM, Lindor KD, Tabibian JH (2019) Cancer risk in primary sclerosing cholangitis: Epidemiology, prevention, and surveillance strategies. World J Gastroenterol 25: 659-671.
- Lindor KD, Kowdley KV, Harrison ME, American College of Gastroenterology. (2015) ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am J Gastroenterol 110: 646-659.
- Kwo PY, Cohen SM, Lim JK (2017) ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol 112: 18-35.
- Chapman MH, Thorburn D, Hirschfield GM, Webster GGJ, Rushbrook SM, et al. (2019) British Society of Gastroenterology and UK-PSC guidelines for the diagnosis and management of primary sclerosing cholangitis. Gut 68: 1356-1378.
- European Society of Gastrointestinal Endoscopy; European Association for the Study of the Liver. (2017) European Association for the Study of the Liver. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. J Hepatol 66: 1265-1281.
- Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, et al. (2010) Diagnosis and management of primary sclerosing cholangitis. Hepatology 51: 660-678.
- Wunsch E, Trottier J, Milkiewicz M, Raszeja-Wyszomirska J, Hirschfield GM, et al. (2014) Prospective evaluation of ursodeoxycholic acid withdrawal in patients with primary sclerosing cholangitis. Hepatology 60: 931-940.
- Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, et al. (2009) High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology 50: 808-814.
- Eaton JE, Silveira MG, Pardi DS, Sinakos E, Kowdley KV, et al. (2011) High-dose ursodeoxycholic acid is associated with the development of colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Am J Gastroenterol 106: 1638-1645.
- Tabibian JH, Lindor KD (2014) Ursodeoxycholic acid in primary sclerosing cholangitis: if withdrawal is bad, then administration is good (right?). Hepatology 60: 785-788.
- Hansen JD, Kumar S, Lo WK, Poulsen DM, Halai UA, et al. (2013) Ursodiol and colorectal cancer or dysplasia risk in primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis. Dig Dis Sci 58: 3079-3087.
- Singh S, Khanna S, Pardi DS, Loftus EV Jr, Talwalkar JA (2013) Effect of ursodeoxycholic acid use on the risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 19: 1631-1638.
- Isayama H, Tazuma S, Kokudo N, Tanaka A, Tsuyuguchi T, et al. (2018) Clinical guidelines for primary sclerosing cholangitis 2017. J Gastroenterol 53: 1006-1034.
- Giljaca V, Poropat G, Stimac D, Gluud C (2010) Glucocorticosteroids for primary sclerosing cholangitis. Cochrane Database Syst Rev 2010: CD004036.
- Shah A, Crawford D, Burger D, Martin N, Walker M, et al. (2019) Effects of Antibiotic Therapy in Primary Sclerosing Cholangitis with and without Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Semin Liver Dis 39: 432-441.
- Hedin CRH, Sado G, Ndegwa N, Lytvyak E, Mason A, et al. (2020) Effects of Tumor Necrosis Factor Antagonists in Patients With Primary Sclerosing Cholangitis. Clin Gastroenterol Hepatol 18: 2295-2304.
- Tse CS, Loftus EV Jr, Raffals LE, Gossard AA, Lightner AL (2018) Effects of vedolizumab, adalimumab and infliximab on biliary inflammation in individuals with primary sclerosing cholangitis and inflammatory bowel disease. Aliment Pharmacol Ther 48: 190-195.
- Zhu Z, Mei Z, Guo Y, Wang G, Wu T, et al. (2018) Reduced Risk of Inflammatory Bowel Disease-associated Colorectal Neoplasia with Use of Thiopurines: a Systematic Review and Meta-analysis. J Crohns Colitis 12: 546-558.
- Abdalla SM, Dejman A, Clark V, Levy C (2019) Use of Fenofibrate for patients with primary Sclerosing Cholangitis. Clin Res Hepatol Gastroenterol 43: e33-e36.
- Biedermann L, Renz L, Fournier N, Rossel JB, Butter M, et al. (2019) Uveitis manifestations in patients of the Swiss Inflammatory Bowel Disease Cohort Study. Therap Adv Gastroenterol 12: 1756284819865142.
- Thomas AS, Lin P (2016) Ocular manifestations of inflammatory bowel disease. Curr Opin Ophthalmol 27: 552-560.
- Das KM (1999) Relationship of extraintestinal involvements in inflammatory bowel disease: new insights into autoimmune pathogenesis. Dig Dis Sci 44: 1-13.
- Hedin CRH, Vavricka SR, Stagg AJ, Schoepfer A, Raine T, et al. (2019) The Pathogenesis of Extraintestinal Manifestations: Implications for IBD Research, Diagnosis, and Therapy. J Crohns Colitis 13: 541-554.
- Troncoso LL, Biancardi AL, de Moraes HV Jr, Zaltman C. (2017) Ophthalmic manifestations in patients with inflammatory bowel disease: A review. World J Gastroenterol 23: 5836-5848.
- Jansen FM, Vavricka SR, den Broeder AA, de Jong EMGJ, Hoentjen F, et al. (2020) Clinical management of the most common extra-intestinal manifestations in patients with inflammatory bowel disease focused on the joints, skin and eyes. United European Gastroenterology Journal 8: 1031-1044.
- Brazilian Study Group of Inflammatory Bowel Diseases. (2010) Consensus guidelines for the management of inflammatory bowel disease. Arq Gastroenterol 47: 313-325.
- Jabs DA, Nussenblatt RB, Rosenbaum JT (2005) Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 140: 509-516.
- Diaz-Valle D, Mendez R, Arriola P, Cuina R, Arino M (2008) Non-infectious systemic diseases and uveitis. An Sist Sanit Navar 3: 97-110.
- Jabs DA, Rosenbaum JT, Foster CS, Holland GN, Jaffe GJ, et al. (2000) Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol 130: 492-513.
- Peyrin-Biroulet L, Van Assche G, Gómez-Ulloa D, García-Álvarez L, Lara N, et al. (2017) Systematic Review of Tumor Necrosis Factor Antagonists in Extraintestinal Manifestations in Inflammatory Bowel Disease. Clin Gastroenterol Hepatol 15: 25-36.e27.
- Cohen JB, Comer DM, Yabes JG, Ragni MV (2020) Inflammatory Bowel Disease and Thrombosis: A National Inpatient Sample Study. TH Open 4: e51-e58.
- Saleh T, Matta F, Yaekoub AY, Danescu S, Stein PD (2011) Risk of venous thromboembolism with inflammatory bowel disease. Clin Appl Thromb Hemost 17: 254-258.
- Yuhara H, Steinmaus C, Corley D, Koike J, Igarashi M, et al. (2013) Meta-analysis: the risk of venous thromboembolism in patients with inflammatory bowel disease. Aliment Pharmacol Ther 37: 953-962.
- Kirchgesner J, Beaugerie L, Carrat F, Andersen NN, Jess T, et al. (2018) Increased risk of acute arterial events in young patients and severely active IBD: a nationwide French cohort study. Gut 67: 1261-1268.
- Papa A, Tursi A, Danese S, Rapaccini G, Gasbarrini A, et al. (2020) Venous Thromboembolism in Patients with Inflammatory Bowel Disease: The Role of Pharmacological Therapy and Surgery. J Clin Med 9: 2115.
- Grainge MJ, West J, Card TR (2010) Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet 375: 657-663.
- Bryant RV, Jairath V, Curry N, Travis SP (2014) Thrombosis in inflammatory bowel disease: are we tailoring prophylaxis to those most at risk? J Crohns Colitis 8: 166-171.
- Zezos P, Kouklakis G, Saibil F (2014) Inflammatory bowel disease and thromboembolism. World J Gastroenterol 20: 13863-13878.
- Nguyen GC, Bernstein CN, Bitton A, Chan AK, Griffiths AM, et al. (2014) Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology 146: 835-848.
Copyright: © 2021 Carlos Alexandre Antunes de Brito, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.