
The relationship between Residual Kidney Function and Cognitive Function in Adults Receiving Peritoneal Dialysis: A Systematic Review and Meta-Analysis
*Corresponding Author(s):
Osasuyi IyasereJohn Walls Renal Unit, University Hospitals Of Leicester NHS Trust, Gwendolen Road, Leicester, United Kingdom
Email:Osasuyi.iyasere@nhs.net
Abstract
Background
Cognitive impairment is common in adults receiving peritoneal dialysis (PD) and has been linked to adverse outcomes. Residual kidney function (RKF) is a key determinant of clinical outcomes in dialysis patients but the evidence on whether it affects cognitive outcomes is unclear. The aim of this review was to evaluate the effect of RKF on cognitive function in adults receiving PD.
Methods
Studies up until April 30th, 2023, were identified from databases including MEDLINE and Embase, using a pre-specified protocol (PROSPERO – CRD42023403924). Studies evaluating adults on PD were included. Data pertaining to RKF and cognitive function were extracted. A meta- analysis of the eligible studies was conducted.
Results
15 observational studies (9 cohort; 6 cross-sectional) were included, after reviewing 2622 abstracts. RKF was not a significant risk factor for cognitive impairment [pooled OR = 0.98(0.90 -1.06), p = 0.96]. It did not correlate with cognitive scores [pooled r = - 0.005 (- 0.07 – 0.06), p = 0.88] and did not differ between those with or without cognitive impairment [standardized mean difference = - 0.01(- 0.09 – 0.07), p =1.00)]. Two other studies, which were ineligible for meta-analysis, reported a significant association between RKF and cognitive function. Most of the included studies had a high risk of bias.
Conclusion
RKF may not be associated with cognitive outcomes in adult patients receiving PD. However, the findings require cautious interpretation due to the low quality of evidence. Studies that evaluate longitudinal trends in RKF alongside cognitive measures, would help address the limitations identified.
Keywords
Cognitive function; Dementia; Peritoneal dialysis; Residual kidney function
Introduction
For adults with End-Stage Kidney Disease (ESKD), Peritoneal Dialysis (PD) enables a home-based form of Kidney Replacement Therapy (KRT). This enables flexible treatment that is planned around daily activities. About 11% of people on dialysis are thought to receive PD worldwide [1], albeit with significant international variation in the proportion of the KRT population receiving PD [2]. Despite the benefits of PD, people receiving this therapy are faced with challenges including PD peritonitis, burnout and carer burden [3]. A substantial proportion of people receiving PD are older. In the UK, 45.8% of the prevalent PD population are over the age of 65 [4]. There is therefore a preponderance of geriatric syndromes in this cohort, including frailty, falls and cognitive impairment. The risk of cognitive impairment increases with progressive chronic kidney disease and more so with the onset of dialysis [5,6].
Cognitive impairment is common in the PD population. Shea et al undertook a systematic review and meta-analysis of cognitive impairment in PD patients ( n = 1736) and reported a pooled prevalence of 28.7% (95% CI 15.9-46%) [7]. Dementia, the more severe form of cognitive impairment, has a reported cumulative incidence of approximately 4% at 3 years, in those receiving PD [8]. Increasing age, female gender, educational level, hyponatraemia, time on therapy, diabetes and depressive status have been identified as risk factors for cognitive impairment in people receiving PD [9-11]. Cognitive impairment has been linked to adverse outcomes in people receiving PD, including PD catheter related infections. In a prospective cohort study of 458 PD patients, immediate memory dysfunction was associated with 74% increase in risk of PD peritonitis [HR = 1.736 (95%CI = 1.064 - 2.834, p < 0.05] [12]. Cognitive impairment has also been associated with a higher risk of mortality and hospitalisation [13].
Cognitive impairment is thought to have a cerebrovascular basis in dialysis patients. Functional MRI studies have reported evidence of significant ischaemic insult in PD patients, including deep white matter changes and microbleeds [14]. Cerebral blood flow is also altered in this group of patients, albeit less so than those receiving HD [15]. This difference is also reflected in the relative risk of mild cognitive impairment and dementia, according to dialysis modality. In a registry study of 121,623 ESKD patients, the cumulative risk of dementia was lower in those initiated on PD versus those initiated on HD (HR = 0.74 [0.64, 0.86]) [8]. A recent meta-analysis also reported lower odds of cognitive dysfunction in PD patients versus HD [16]. Intradialytic haemodynamic changes, which are more pronounced in HD patients, are thought to explain the differential risk of cognitive impairment between modalities. Alternatively, it has been speculated that the impact of dialysis modality on Residual Kidney Function (RKF) could be contributory [17]. There is evidence to suggest that RKF declines more rapidly with HD in comparison to PD [18].
RKF is an important determinant of outcomes in PD patients, including peritonitis, mortality and quality of life [19, 20]. It is unclear whether RKF is associated with cognitive dysfunction in this group of patients. This systematic review was conducted to assess the relationship between RKF and cognitive outcomes in adults receiving PD.
Materials and Methods
Search strategy and study selection
This systematic review was conducted in accordance with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) work group. A prespecified protocol was registered prior to the start of the literature review (PROSPERO – CRD42023403924). MEDLINE (PubMed interphase from 1948 onwards, EMBASE (Ovid interphase), CINAHL, PsycINFO and the Cochrane databases were searched from inception to the 30th of April 2023. The search was limited to English language papers. The search strategy is available in the supplemental information (supplementary figure 1). The bibliographies for included papers were searched manually, to identify additional studies.
The criterion for inclusion was any study that reported on cognitive impairment and RKF in adult PD patients. The eligible studies were randomised studies, prospective and retrospective cohort studies, case control and cross-sectional studies. Unpublished abstracts from conference proceedings and letters to editors meeting the other eligibility criteria were also included but case series and case reports were excluded. RKF, defined as urinary clearance of urea and creatinine, was the primary exposure of interest. It was considered to include the following measures: Glomerular filtration rate (GFR), renal solute clearance measures including Kt/V and creatinine clearance, 24-hour urine volume.
Cognitive function was the outcome of interest and was considered to include the following:
- Cognitive function measured cognitive screening tools e.g. Montreal Cognitive assessment tool (MoCA)
- Cognitive function measured by a neuropsychological battery.
- Cognitive function measured by evoked potential.
- Clinical diagnosis of cognitive impairment or dementia
The title and abstract of studies identified from the search were assessed by at least two independent reviewers. If there was not agreement between the two reviewers, the studies were included for full text review. The full text papers of eligible abstracts deemed to have met the inclusion criteria were then evaluated, with all eligible papers included for subsequent data extraction. Disagreements after full text review were resolved by a third reviewer prior to data extraction.
Data extraction
This was carried out using a standardized template. The reviewers collected data relating to patient characteristics (age, gender, ethnicity, diabetes status, level of education); dialysis characteristics - modality (Automated PD or Continuous Ambulatory PD), time on PD intervention, urine volume; study characteristics - study design, sample size, duration of follow-up, and cognitive outcomes. Data pertaining to the association between RKF and cognitive function were extracted in the form of risk ratio, odds ratio, correlation coefficients, linear regression coefficients as well as summary measures of RKF between PD patients with cognitive impairment and those without. These were calculated from patient level data if available, when not otherwise published. Where outcome or exposure data were not available, the corresponding author was contacted by email. Studies with insufficient data, were excluded from the meta-analysis. Risk of bias assessments were independently carried out by 2 reviewers, using the Cochrane Risk of Bias In Non-randomized Studies – of Exposure (ROBINS-E) tool [21]. Conflicts were resolved by discussion between the two reviewers. Where conflicts remained, this was resolved by a third reviewer.
Statistical analysis
A meta- analysis of the effect of RKF on cognitive impairment was conducted where there were two or more studies with the relevant information. The analyses were undertaken in line with predetermined groups, as follows:
- Relative risk (RR) of cognitive impairment as a dichotomous outcome
- Odds ratio (OR) of cognitive impairment as a dichotomous outcome
- Hazard ratio (HR) of cognitive impairment as a dichotomous outcome
- Regression or correlation coefficient of RKF on cognitive function, as a continuous variable
- mean difference in RKF between patients with and without cognitive impairment
Where a standard error of the mean was reported, this was converted to standard deviation for consistency. Where effect estimates were reported as Relative Risk (RR), Odds Ratio (OR) or regression coefficients, unadjusted effects were evaluated separately from adjusted effects. They were defined as adjusted if the analysis considered at least one of the following independent variables - age, gender, educational level, time on dialysis and sodium imbalance. The a priori preference was to report adjusted effects were available.
A random effects model using the inverse variance restricted maximum likelihood approach, was used in the meta-analysis, as heterogeneity was anticipated. Where the effect estimates related to differences in RKF between patients with cognitive impairment versus those without cognitive impairment, these were expressed as the standardized mean difference as the outcomes for included studies were measured on the different scales (e.g. GFR and residual KT/v). Heterogeneity was assessed using the I2 statistic. Sensitivity analysis for the meta-analysis methods was performed using fixed effect approaches. Where the same study had published results for an outcome variable in more than one manuscript, the paper with the most complete data was used. A subgroup analysis was conducted of studies evaluating cognitive subdomains including executive function and memory. All statistical analysis was performed using Revman version 5.3 and MedCalc version 22.009. The quality of evidence was evaluated using the GRADE framework.
Results
2622 studies were identified from the electronic search. After reviewing the title and abstracts, 181 of these studies were deemed eligible for full text review. 15 studies were finally included in the systematic review, all of which were non-randomised (9 cohort and 6 cross-sectional). No additional studies were found after the manual search. Figure 1 shows the screening process and reasons for exclusion.
Table 1 shows the characteristics for the included studies. 4889 participants were enrolled across the included studies. The median follow-up period for the cohort studies was 24 months (range 12 to 36 months).
|
Study ID |
Country |
Year of publication |
Study design |
Follow up duration (months) |
Number of PD participants |
Age (years)a |
Sex |
Ethnicity |
Level of educational attainment |
Diabetes |
PD modality |
PD vintage |
Residual kidney function measure |
Cognitive assessment tool used |
Criteria for cognitive impairment |
Cognitive impairment (prevalence) |
|
Xu 2015(10) |
China |
2015 |
Cross sectional |
0 |
476 |
51.9 ± 14.3 |
51.4% male |
Asian |
Elementary -19.3%, Middle School-30.4%, High school -29.6%; above high school -20.7% |
23.7% |
|
26.3 (12.2–49.9) |
GFR |
3MS |
3MS < 80 |
28.9% |
|
Li 2016(28) |
China |
2016 |
Cross sectional |
0 |
445 |
51.3±14.2 |
52.4% male |
Asian |
Elementary school - 17.5% |
22.7% |
|
25.1 (11.1–49) |
GFR |
3MS |
3MS<75a 3MS<80b |
23.6% |
|
Shea 2016(22) |
China |
2016 |
Cohort |
12 |
151 |
50 ±1 5.0 |
45% female |
Asian |
Less than secondary school -25.8% |
41.7% |
CAPD -91.2%; Assisted PD -19.2% |
|
GFR |
cMMSE |
< 21( 2 years of schooling) |
Immediate memory - 65.4%; Visuospatial -47%; Executive dysfunction -29.9% |
|
Shea 2016(23) |
China |
2016 |
Cohort study |
12 |
114 |
59 ± 15.0 |
47% female |
Asian |
|
59.6% |
CAPD -91.2% |
|
CrCL |
MoCA |
MoCA <23 |
28.9% |
|
Iyasere 2017(17) |
UK |
2017 |
Cohort study |
12(6 - 18) |
25 |
72.8 ± 1.6 |
76% Male |
White -64%, Afro-Caribbean -8%, Asian -24%, Other -4% |
All >12 years education |
44% |
|
8 (5-32) |
CrCL |
MoCA |
MoCA < 26 |
64.3% |
|
Zhang 2018(33) |
China |
2018 |
Cohort study |
24 |
458 |
51.6 |
51.9% Male |
Asian |
Elementary - 20.8% |
23.6% |
|
25.1 - mean |
GFR |
3MS TMT- A and B, RBANS |
3MS <80 |
19.8% baseline |
|
Yi 2018(26) |
China |
2018 |
Cohort study |
30.7 |
784 |
48.8±14.6 |
40.8% Female |
Asian |
1-6 years -15.7%; 6-12 years -57%; > 12 years - 27.3% |
20% |
CAPD |
30.7 [8.9~54.3 |
GFR |
MoCA |
MoCA <26 |
55.5% |
|
Shea 2019(24) |
China |
2019 |
Cohort study |
24 |
206 |
59 ± 13.7 |
45.6% Female |
Asian |
illiterate -10.2%; primary -21.4%; Secondary -40.8%; Tertiary 27.7% |
47.6% |
CAPD - 92.7% |
|
GFR |
MoCA (Hong Kong version) |
Multiple cut offs based on age and educational attainment |
22.3% |
|
Liao 2019(12) |
China |
2019 |
Cohort study |
30 months |
458 |
CI - 60 ± 11.5; no CI 49.6 ± 14.1 |
53.1% Male |
Asian |
Middle school -29.5% High school -29.9% > High school -22.5% |
|
CAPD |
CI -23.8 (10.0–49.1)
No CI -25.3 (11.1–49.1) |
eGFR |
3MS |
3MS<75a 3MS<80b
|
19.7% |
|
Li 2020(41) |
China |
2020 |
Cohort |
24 months |
222 |
56.1 ± 12.8 |
Male -46.8% |
Asian
|
Elementary school - 7.66% |
32.4% |
|
30.9 |
Kt/V, CrCL, renal Beta-2 microglobulin |
3MS, TMT, RBANS |
Standardised score 1SD < published population mean |
12.3% |
|
Iyasere 2020(25) |
UK |
2020 |
Cohort |
|
83 |
Median - 73 (70 -79) |
|
|
|
28.9% |
|
22(10 – 36) |
CrCL |
Clinical diagnosis |
Clinical diagnosis |
8.4% |
|
Huang 2021(13) |
China |
2021 |
Cohort |
Up to 36 months |
913 |
48.7 ± 14.6 |
Male - 60.4% |
Asian |
|
20.4% |
CAPD |
25.5 (6.0-52.6) |
GFR |
MoCA |
MoCA < 26 |
72.7% |
|
Salazar-Felix 2021(29) |
Mexico |
2021 |
Cross sectional |
|
71 |
42.6 ± 16 |
Male - 79% |
|
34% |
APD |
17 (7–32) |
CrCL, urine volume |
MoCA; MMSE |
MMSE < 24 MoCA < 26 |
7% - MMSE 63% -MoCA |
|
|
Yi 2021(26) |
China |
2021 |
Cross sectional |
|
643 |
Median - 45 (37-57.4) |
42.1 % female |
Asian |
16.3% |
Not specified |
27.8 (8.7–56.4) |
GFR |
MoCA |
MoCA <26 |
49.9% |
|
|
Gamage 2022(27) |
Australia |
2022 |
Cross sectional |
|
149 |
60.9 ± 15.1 |
Female - 32.9% |
|
|
48.3% |
APD - 97.2% |
16.8 (16.4) |
Urine volume (mls) |
ACE-R |
Not specified |
36.9% |
Table 1: Characteristics of included studies. a - Mean unless otherwise specified.ACE-R -Addenbrooke's Cognitive Examination-Revised; CI – cognitive impairment; cMMSE – Mini-mental state examination (Chinese version); CrCL -Creatinine clearance; MoCA- Montreal cognitive assessment tool; MMSE - Mini-mental state examination; 3MS – modified Mini-mental state examination; NPB- neuropsychological battery; R-BANS - Repeatable Battery for the Assessment of Neuropsychological Status; SD – standard deviation; TMT- trail making test.
Association between RKF and cognitive impairment
4 studies (17, 22-24) reported on the effect of RKF on cognitive impairment as unadjusted odd ratios. The forest plot from the meta-analysis is shown in figure 2. There was no statistically significant association between RKF and the odds of cognitive impairment after pooled analysis [Pooled OR = 0.98(0.90 -1.06), p = 0.96]. In contrast, RKF was found to be associated with a lower hazard of clinician reported cognitive impairment after adjusting for age and dialysis vintage, in a retrospective cohort study of 83 adult PD patients [HR = 0.37(0.15 -0,93), p = 0.03] (Conference proceeding) [25].
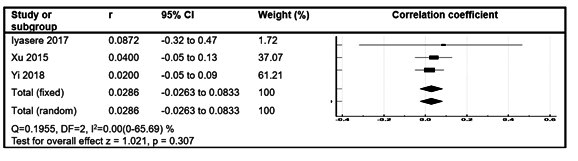 Figure 1: Forest plot showing effect of RKF on the odds of cognitive impairment.
Figure 1: Forest plot showing effect of RKF on the odds of cognitive impairment.
Correlation between measures of RKF and cognitive scores
4 studies (10,17,26,27) evaluated the correlation between RKF and cognitive scores in adult PD patients. 3 of these were included in a meta-analysis (Figure 3). There was no significant correlation identified [Pooled correlation coefficient = 0.0286 (-0.0263 to 0.0833), p = 0.31].
In contrast, Gamage et al found that there was significant positive correlation using polynomial regression modelling, between cognitive (ACE-R) scores and urine output beyond 1500mls per day, in a cross-sectional study of 149 PD patients [27-33]. There was also no significant correlation between RKF and executive function scores [Pooled correlation coefficient = - 0.05(- 0.12 – 0.25), p = 0.19] (supplementary figure 2) or memory scores [Pooled correlation coefficient = - 0.005 (- 0.07 – 0.06), p = 0.88] (supplementary figure 3).
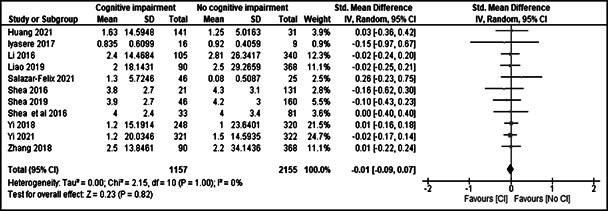 Figure 2: Forest plot of correlation between measures of RKF and cognitive scores.
Figure 2: Forest plot of correlation between measures of RKF and cognitive scores.
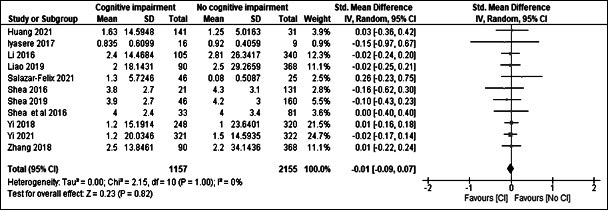 Figure 3: Standardised mean difference in RKF between those with cognitive impairment and those without.
Figure 3: Standardised mean difference in RKF between those with cognitive impairment and those without.
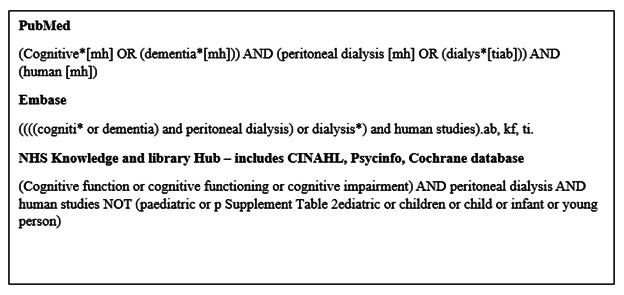 Supplementary Figure 1: Search strategies.
Supplementary Figure 1: Search strategies.
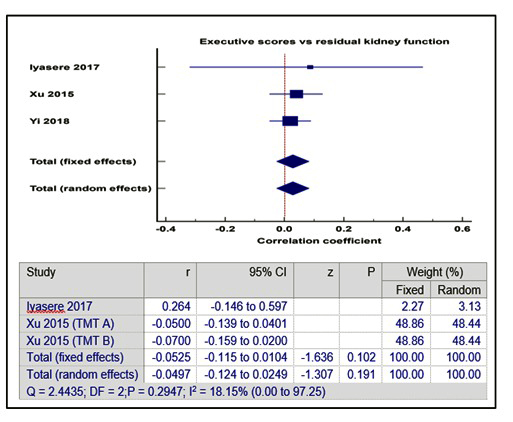 Supplementary Figure 2: Correlation between RKF and executive function.
Supplementary Figure 2: Correlation between RKF and executive function.
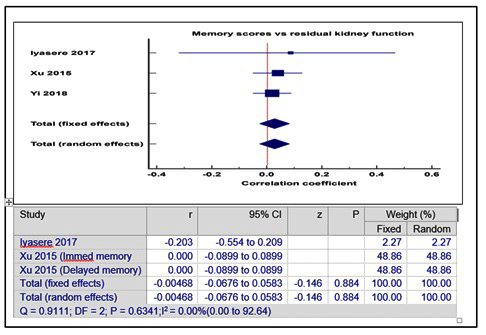 Supplementary Figure 3: Correlation between memory scores and RKF.
Supplementary Figure 3: Correlation between memory scores and RKF.
RKF according to cognitive status in adult PD patients
11 studies [17,22-24, 26,28-33] reported the mean RKF according to cognitive status. A meta-analysis of these studies showed that there was no significant difference in RKF between adult PD patients with cognitive impairment and those without cognitive impairment [standardized mean difference = - 0.01(- 0.09 – 0.07), p =1.00)]. The forest plot is shown in figure 3.
Risk of Bias and Quality of included studies
The risk of bias for most of the studies was rated as either “some concern or high risk of bias, predominantly because of confounding or selection bias (Supplementary Table 1). Confidence in the effect estimates from the meta-analysis was graded as low to moderate (Supplementary Table 2). The funnel plots, which are shown in the supplement, file (Supplementary figures 4-6), were not indicative of publication bias.
|
Study ID |
Confounding |
Measurement with exposure |
Participant selection |
Post exposure interventions |
Missing data |
Measurement of outcomes |
Selection of reported results |
Risk of bias |
|
Li 2016 |
High |
Low |
High |
Low |
Very high |
Low |
Low |
Very High |
|
Shea 2016 |
Low |
Some concerns |
Some concerns |
Low |
Some concerns |
Low |
Low |
Some concerns |
|
Shea 2016* |
High |
Low |
Some concerns |
Low |
Some concerns |
Low |
High |
High |
|
Iyasere 2017 |
High |
Low |
High |
Low |
Very High |
Low |
Low |
Very High |
|
Li 2020 |
Low |
Low |
Some concerns |
Low |
Some concerns |
Low |
Low |
Some concerns |
|
Shea 2019 |
Low |
Some concerns |
Some concerns |
Low |
Some concerns |
Low |
Low |
Some concerns |
|
Zhang 2018 |
High |
Low |
Some concerns |
High |
Some concerns |
Low |
Low |
High |
|
Liao 2019 |
High |
Low |
High |
High |
High |
Low |
Low |
High |
|
Huang 2021 |
Low |
Low |
Some concerns |
Low |
High |
Low |
Low |
High |
|
Yi 2018 |
Low |
Low |
Some concerns |
Low |
Some concerns |
Low |
Low |
Some concerns |
|
Gamage 2022 |
High |
Low |
Some concerns |
High |
High |
Low |
Low |
High |
|
Iyasere 2020 |
High |
Low |
Some concerns |
Low |
Some concerns |
Low |
High |
High |
|
Salazar-Felix 2021 |
Some concerns |
Low
|
Some concerns |
Low |
High |
Low |
Some concerns |
High |
|
Xu 2015 |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low except for residual confounding concerns |
Supplementary Table 1: Risk of Bias.
|
Outcomes |
Effect estimate |
Number of participants (studies) |
Quality of evidence (GRADE) |
|
Odds ratioa |
0.98 (0.90 -1.06) |
496 (4) |
⊕⊕?? |
|
Correlationb |
0.029 (-0.26 to 0.083) |
1285 (3) |
⊕⊕?? |
|
SMDc |
- 0.01 (-0.09 to 0.07) |
1157 (11) |
⊕⊕?? |
Supplementary Table 2: GRADE – Certainty of effect estimates of RKF on cognitive function. a odds of cognitive impairment for every unit rise in RKF; b correlation between cognitive scores and RKF; c mean difference in RKF between patients with cognitive impairment and those without. SMD – standardised mean difference.
Funnel plots
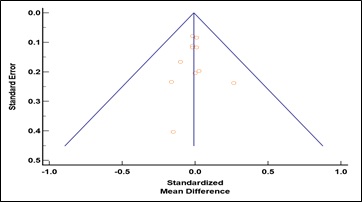 Supplementary Figure 4: Funnel plot for studies reporting on the difference in residual kidney function according to cognitive status. Egger’s test p = 0.90.
Supplementary Figure 4: Funnel plot for studies reporting on the difference in residual kidney function according to cognitive status. Egger’s test p = 0.90.
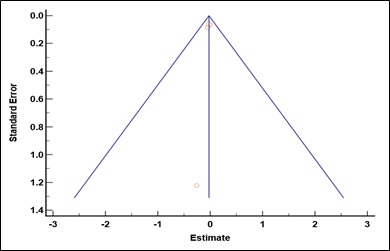 Supplementary Figure 5: Funnel plot for studies reporting the odds of cognitive impairment per unit change in residual kidney function. Egger’s test: p = 0.52.
Supplementary Figure 5: Funnel plot for studies reporting the odds of cognitive impairment per unit change in residual kidney function. Egger’s test: p = 0.52.
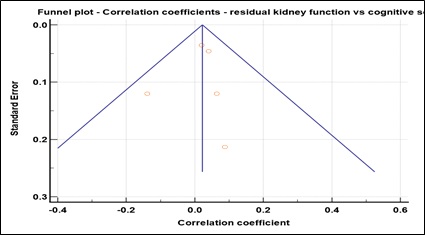 Supplementary Figure 6: Funnel plot for studies reporting the correlation between cognitive scores and residual kidney function. Egger’s test : p = 0.74.
Supplementary Figure 6: Funnel plot for studies reporting the correlation between cognitive scores and residual kidney function. Egger’s test : p = 0.74.
Discussion
This systematic review evaluated the effect of RKF on cognitive impairment in adult PD patients. While 2 observational studies suggested that there was an association between RKF and cognitive function, the pooled analysis from the 13 other studies was not suggestive of a significant association. RKF was also not associated with the subdomains of memory and executive function.
The prevalence of cognitive impairment increases with advancing stages of CKD and cognitive function deteriorates with the onset of dialysis [5,6]. There is a complex interplay of risk factors that contribute to the development of cognitive impairment in dialysis patients [11]. These include traditional risk factors, kidney specific and uraemic factors, which are thought to lead to brain injury through various intermediary pathways [34]. Kurella Tamura et al evaluated the relationship between uraemic solutes and executive function in a cohort of 321 dialysis patients, 10 of which were on PD. A metabolomic approach identified 4 metabolites (4-hydroxyphenylacetate, Phenylacetylglutamine, Hippurate, and Prolyl-hydroxyproline), associated with cognitive impairment. There was a near twofold increase in the relative risk of impaired executive function in dialysis patients with an increase in 3 or more of these metabolites compared to those without. For some of these uraemic solutes, which also undergo tubular secretion, renal clearance is better than dialytic clearance on HD [35]. This has similarly been speculated in comparison to peritoneal clearance [36]. There is therefore a plausible mechanism by which RKF could influence cognitive outcomes in patients with PD. Yet the existing evidence is not corroborative. In contrast, RKF may be linked to cognitive outcomes in HD patients. Elgendy et al evaluated quality of life and cognitive function in 78 HD patients and found that statistically significant relationship between RKF and MoCA scores after adjusting for demographic and clinical covariates [37].
There is also some uncertainty about the effect of dialysis adequacy (peritoneal and residual solute clearance) on cognitive impairment in PD patients. Shin et al evaluated the relationship between dialysis adequacy and cognitive impairment, in a cross-sectional study of 59 PD patients [38]. While there was a correlation between executive function scores and relative overhydration ( assessed using body composition monitoring), there was no association between total Kt/V and global cognitive impairment. This has been similarly reported in HD patients [39]. The two studies which reported an association between RKF and cognitive function(25, 27) suggests that a threshold may exist beyond which the relationship becomes significant and potentially clinically relevant. It is recognized that clinicians are more likely to recognize moderate to severe cognitive dysfunction when compared to cognitive screening tools, which was the predominant method of cognitive assessment in the meta-analysis. Furthermore, Gamage et al reported a non-linear relationship between cognitive scores and urine output, with significant correlation above the threshold of 1500mls per day. Alternatively, one may speculate that the urine output was a surrogate for the fluid status of the participants, and thus may correlate more with sodium balance [40,41], another risk factor for cognitive impairment.
This systematic review has several limitations. There was some variation in how cognitive impairment was defined, even when the same tools were used (Table 1). All included studies were observational with the attendant limitation relating to causality and most reported unadjusted effects. While heterogeneity was not a major concern, most studies had a high risk of bias. While all the included studies provided relevant data, some of them were not designed with the specific objective of assessing the effect of RKF on cognitive outcomes. They may therefore not have been powered to detect a significant association. RKF was measured at a single timepoint in all included studies. Therefore, this review does not provide evidence on how trends in RKF influence cognitive outcomes.
Conclusion
This meta-analysis suggests that RKF may not be associated with cognitive outcomes in adult patients receiving PD. Two other studies suggest otherwise, with consequent uncertainty in the summarized evidence. Due to the low quality of existing evidence, suitably designed studies that take into consideration longitudinal trends in RKF are required to determine the true effect of RKF on cognitive function in adult PD patients.
Declaration of Conflicting Interest
The authors declare that there is no conflict of interest.
Funding
The author(s) received no financial support for the research, authorship and/or publication of this article.
Ethical Approval (include full name of committee approving the research and if available mention reference number of that approval)
Not applicable.
Informed Consent to Participate
Not applicable.
Informed Consent to Publish
Not applicable.
Trial Registration (where applicable)
Not applicable because research pertains to systematic review.
Authorship
OI conceived the study and was responsible for project administration, data-analysis and writing of the original draft. All authors participated in the acquisition, interpretation, and validation of data. All authors reviewed and revised the original manuscript. The final version of this manuscript was approved by all the authors.
Acknowledgements
We would like to thank University Hospitals of Leicester NHS Trust library staff for their assistance in this research.
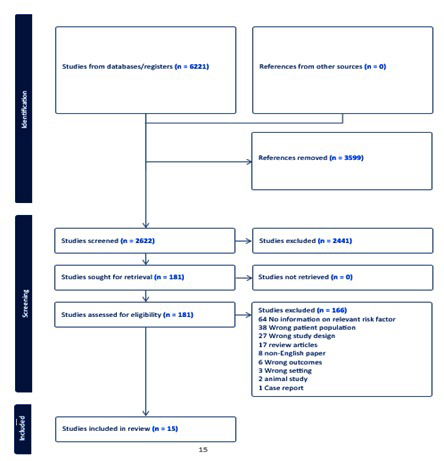 Figure 4: PRISMA Flowchart.
Figure 4: PRISMA Flowchart.
References
- Lambie M, Zhao J, McCullough K, Davies SJ, Kawanishi H, et al. (2022) Variation in Peritoneal Dialysis Time on Therapy by Country: Results from the Peritoneal Dialysis Outcomes and Practice Patterns Study. Clin J Am Soc Nephrol 17: 861-871.
- Briggs V, Davies S, Wilkie M (2019) International Variations in Peritoneal Dialysis Utilization and Implications for Practice. Am J Kidney Dis 74: 101-110.
- Oveyssi J, Manera KE, Baumgart A, Cho Y, Forfang D, et al. (2021) Patient and caregiver perspectives on burnout in peritoneal dialysis. Perit Dial Int 41: 484-493.
- Registry UR (2023) UK Renal Registry 25th Annual Report – data to 31/12/2021.
- Kurella Tamura M, Wadley V, Yaffe K, McClure LA, et al. (2008) Kidney function and cognitive impairment in US adult: the Reasons for Geographic and Racial Differences in stroke (REGARDS) Study. Am J Kidney Dis 52:227-234.
- Kurella Tamura M, Vittinghoff E, Hsu CY, Tam K, Seliger SL, et al. (2016) Cognitive function before and after dialysis initiation in adults with chronic kidney disease. Kidney Int.
- Shea YF, Lee MC, Mok MM, Chan FHW, Chan TM (2019) Prevalence of cognitive impairment among peritoneal dialysis patients: a systematic review and meta-analysis. Clin Experi Nephrol 23: 1221-1234.
- Wolfgram D, Szabo A, Murray AM, Whittle J (2015) Risk of Dementia in Peritoneal Dialysis Patients Compared with Hemodialysis Patients. Perit Dial Int 35: 189-198.
- Dong J, Pi HC, Xiong ZY, Liao JL, Hao L, et al. (2016) Depression and Cognitive Impairment in Peritoneal Dialysis: A Multicenter Cross-sectional Study. Am J Kidney Dis 67: 111-118.
- Xu R, Pi HC, Xiong ZY, Liao JL, Hao L, et al. (2015) Hyponatremia and Cognitive Impairment in Patients Treated with Peritoneal Dialysis. Clin J Am Soc Nephrol 10: 1806-1813.
- Kurella Tamura M, Yaffe K (2011) Dementia and cognitive impairment in ESRD: diagnostic and therapeutic strategies. Kidney Int 79: 14-22.
- Liao JL, Zhang YH, Xiong ZB, Hao L, Liu GL, et al. (2019) The Association of Cognitive Impairment with Peritoneal Dialysis-Related Peritonitis. Perit Dial Int 39: 229-235.
- Huang X, Yi C, Wu M, Qiu Y, Wu H, et al. (2021) Risk Factors and Clinical Outcomes of Cognitive Impairment in Diabetic Patients Undergoing Peritoneal Dialysis. Kidney Blood Press Res 46:531-540.
- Qian Y, Zheng K, Wang H, You H, Han F, et al. (2021) Cerebral microbleeds and their influence on cognitive impairment in dialysis patients. Brain Imaging Behav 15: 85-95.
- McIntyre CW, Goldsmith DJ (2015) Ischemic brain injury in hemodialysis patients: which is more dangerous, hypertension or intradialytic hypotension?. Kidney Int 87: 1109-1115.
- Ali H, Soliman K, Mohamed MM, Daoud A, Shafiq T, et al. (2021) The effects of dialysis modality choice on cognitive functions in patients with end-stage renal failure: a systematic review and meta-analysis. Int urol nephrol 53: 155-163.
- Iyasere O, Okai D, Brown E (2017) Cognitive function and advanced kidney disease: longitudinal trends and impact on decision-making. Clin Kidney J 10: 89-94.
- Jansen MA, Hart AA, Korevaar JC, Dekker FW, Boeschoten EW, et al. (2002) Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int 62: 1046-1053.
- Bargman JM, Thorpe KE, Churchill DN (2001) Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol 12: 2158-2162.
- Han SH, Lee SC, Ahn SV, Lee JE, Kim DK, et al. (2007) Reduced residual renal function is a risk of peritonitis in continuous ambulatory peritoneal dialysis patients. Nephrol Dial Transplant 22: 2653-2658.
- Sterne JA, Hernán MA, Reeves BC, Savovic J, Berkman ND, et al. (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355: i4919.
- Shea YF, Lam MF, Lee MS, Mok MY, Lui SL, et al. (2016) Prevalence of CMMSE defined cognitive impairment among peritoneal dialysis patients and its impact on peritonitis. Clin Exp Nephrol 20:126-133.
- Shea YF, Lam MF, Lee MSC, Mok MYM, Lui SL, et al. (2016) Prevalence of Cognitive Impairment Among Peritoneal Dialysis Patients, Impact on Peritonitis and Role of Assisted Dialysis. Peritoneal dialysis international : journal of the International Society for Peritoneal Dialysis 36: 284-290.
- Shea YF, Lee MSC, Mok MYM, Lam MF, Chu LW, et al. (2019) Self-Care Peritoneal Dialysis Patients with Cognitive Impairment Have a Higher Risk of Peritonitis in the Second Year. Peritoneal dialysis international : journal of the International Society for Peritoneal Dialysis 39: 51-58.
- Iyasere O, Odumosu A, Oseya B, Hainsworth N, Stinson J, et al. (2020) Clinician Reported Cognitive Impairment and Residual Renal Function in Older Patients on Peritoneal Dialysis: A retrospective analysis. UK Kidney Week Virtual.
- Yi C, Lin J, Cao P, Chen J, Zhou T, et al. (2018) Prevalence and Prognosis of Coexisting Frailty and Cognitive Impairment in Patients on Continuous Ambulatory Peritoneal Dialysis. Scientific Reports.
- Gamage I, Dhar A, Tregaskis P, Wilson S (2022) Frequency and risk factors for cognitive dysfunction in peritoneal dialysis patients. Nephrology (Carlton) 27: 945-952.
- Li Y, Tian X, Xiong ZY, Liao JL, Hao L, et al. (2016) Performance of the Modified Mini-Mental State Examination (3MS) in assessing specific cognitive function in patients undergoing peritoneal dialysis. PLoS ONE 11.
- Salazar-Félix NA, Martin-Del-Campo F, Cueto-Manzano AM, Romo-Flores ML, Velázquez-Vidaurri AL, et al. (2021) Prevalence of mild cognitive impairment in automated peritoneal dialysis patients. Nephrol Dial Transplant 36: 2106-2111.
- Liao JL, Xiong ZY, Yang ZK, Hao L, Liu GL, et al. (2017) An association of cognitive impairment with diabetes and retinopathy in end stage renal disease patients under peritoneal dialysis. PLoS ONE 12: 1-13.
- Yi C, Zhang W, Ye H, Wu H, Huang X, et al. (2021) Association of brachial-ankle pulse wave velocity with cognitive impairment in peritoneal dialysis patients. Renal Failure 43: 934-941.
- Huang X, Yi C, Wu M, Qiu Y, Wu H, et al. (2021) Risk Factors and Clinical Outcomes of Cognitive Impairment in Diabetic Patients Undergoing Peritoneal Dialysis. Kidney and Blood Pressure Research 46: 531-540.
- Zhang YH, Yang ZK, Wang JW, Xiong ZY, Liao JL, et al. (2018) Cognitive Changes in Peritoneal Dialysis Patients: A Multicenter Prospective Cohort Study. American journal of kidney diseases: the official journal of the National Kidney Foundation 72: 691-700.
- Miglinas M, Cesniene U, Janusaite MM, Vinikovas A (2020) Cerebrovascular Disease and Cognition in Chronic Kidney Disease Patients. Front Cardiovasc Med 7:96.
- Sirich TL, Funk BA, Plummer NS, Hostetter TH, Meyer TW (2014) Prominent accumulation in hemodialysis patients of solutes normally cleared by tubular secretion. J Am Soc Nephrol 25: 615-622.
- Meyer TW, Bargman JM (2023) The Removal of Uremic Solutes by Peritoneal Dialysis. J Am Soc Nephrol 34: 1919-1927.
- Elgendy A, Abdelsalam AI, Mansour M, Nassar MK (2022) Can residual kidney function affect quality of life and cognitive function in hemodialysis patients?. BMC Nephrol 23: 263.
- Shin DJ, Kim T, Jung DU, Moon JJ, Jeon DW, et al. (2020) Association between Dialysis Adequacy and Cognition in Patients with Peritoneal Dialysis. Psychiatry Investig 17: 1143-1148.
- Giang LM, Weiner DE, Agganis BT, Scott T, Sorensen EP, et al. (2011) Cognitive function and dialysis adequacy: no clear relationship. Am J Nephrol 33: 33-38.
- Rhee CM, Ayus JC, Kalantar-Zadeh K (2019) Hyponatremia in the Dialysis Population. Kidney Int Rep 4: 769-80.
- Li Y, Pi HC, Yang ZK, Dong J (2020) Associations between small and middle molecules clearance and the change of cognitive function in peritoneal dialysis. J Nephrol 33: 839-48.
Citation: Iyasere O, Ahmed H, Chattah F, Abd elMagid K (2024) The relationship between Residual Kidney Function and Cognitive Function in Adults Receiving Peritoneal Dialysis: A Systematic Review and Meta-Analysis. J Nephrol Renal Ther 10: 088.
Copyright: © 2024 Osasuyi Iyasere, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

