
Journal of Nanotechnology Nanomedicine & Nanobiotechnology Category: Medical
Type: Review Article
The Use of Nanotechnology to Treat Cancers
*Corresponding Author(s):
Xiaoming LiKey Laboratory For Biomechanics And Mechanobiology Of Ministry Of Education, School Of Biological Science And Medical Engineering, Beihang University, Beijing 100191, China
Tel:+86 1082316467,
Email:x.m.li@hotmail.com
Yubo Fan
Key Laboratory For Biomechanics And Mechanobiology Of Ministry Of Education, School Of Biological Science And Medical Engineering, Beihang University, Beijing 100191, China
Tel:+86 1082339428,
Email:yubofan@buaa.edu.cn
Received Date: Jul 25, 2014
Accepted Date: Dec 03, 2014
Published Date: Dec 17, 2014
Abstract
Cancers are threats to human’s lives and hard to diagnose and treat. In today’s fights against cancers, it has been shown that traditional treatments cannot meet the requirements. Nanotechnology, as a new and productive tool, has appeared in this world and given new hopes to researchers and patients of cancers. In this article, we highlight the significance of the applications of nanotechnology in the area of cancer therapy?especially in the drug delivery and drug resistance. For example, how to accurately and thoroughly eliminate cancer cells is one of central problems in treatment of cancers, some kind of nanoparticles have been widely used in the drug delivery and drug assistance based on their special properties to solve the problems.
INTRODUCTION
Nanotechnology, as a cutting-edge field, has a wide range of application and clear industrial prospect in monitoring and treatment of cancer, especially in the aspect of drug delivery and drug resistance.
The nanotechnology was defined as research with materials at the nanoscales, which are at dimensions between approximately 1 and 100nm and had been moved forward through multidisciplinary collaborations among many disciplines including chemistry, physics, biology and engineering. Now nanotechnology has played an important role in generating innovative solutions of the central issues of cancer treatment, for example, how to detect tumors earlier, how to target cancer cells accurately and how to improve the radiotherapy treatments. Nanotechnology can also be used to develop highly sensitive diagnostic devices, allowing treatment to begin before the onset of metastasis and resulting in improved outcomes for the patients [1].
Nanotechnology mainly contains seven branches: nanometer system of physics, nanochemical, nanomaterials science, nanobiology, nanoelectronics, nanoprocessing and nanomechanics. In the case of nanomaterials?because of their size, nanoscale materials have some different chemical and physical properties from bulk material. Researchers have capitalized on these qualities of nanomaterials for effective improvements in drug delivery, cancer cell targeting via protein and small molecule binding, intracellular drug release, ex vivo diagnostic applications, imaging, and combination therapies including theranostic integration of molecular imaging with drug delivery [2].
Nanoparticle formulations can reduce or eliminate systemic toxicities by delivering drugs specifically to cancerous tissues through size mediated passive targeting and physiologically mediated active targeting; they can improve early detection through targeted delivery of molecular imaging agents to tumors for improved diagnostic imaging and intraoperative imaging to guide surgical removal of cancerous cells; they can address drug resistance through the delivery of multiple drugs or complimentary treatments such as RNA interference [1].
Nanomedicines have been investigated for their application in anticancer therapies to improve drug delivery, increase the efficacy of treatment, reduce side effects, and overcome drug resistance. The number of studies published under the research topics of “nanomedicine”, “nanoscience” and “nanotechnology” has increased exponentially over the past decade. As more nanostructures were discovered and their potentials were better understood, the number of publications increased and reached its peak in 2011. Currently, the knowledge base of nanoparticles is still expanding with an emphasis on safety and efficacy [3].
The nanotechnology was defined as research with materials at the nanoscales, which are at dimensions between approximately 1 and 100nm and had been moved forward through multidisciplinary collaborations among many disciplines including chemistry, physics, biology and engineering. Now nanotechnology has played an important role in generating innovative solutions of the central issues of cancer treatment, for example, how to detect tumors earlier, how to target cancer cells accurately and how to improve the radiotherapy treatments. Nanotechnology can also be used to develop highly sensitive diagnostic devices, allowing treatment to begin before the onset of metastasis and resulting in improved outcomes for the patients [1].
Nanotechnology mainly contains seven branches: nanometer system of physics, nanochemical, nanomaterials science, nanobiology, nanoelectronics, nanoprocessing and nanomechanics. In the case of nanomaterials?because of their size, nanoscale materials have some different chemical and physical properties from bulk material. Researchers have capitalized on these qualities of nanomaterials for effective improvements in drug delivery, cancer cell targeting via protein and small molecule binding, intracellular drug release, ex vivo diagnostic applications, imaging, and combination therapies including theranostic integration of molecular imaging with drug delivery [2].
Nanoparticle formulations can reduce or eliminate systemic toxicities by delivering drugs specifically to cancerous tissues through size mediated passive targeting and physiologically mediated active targeting; they can improve early detection through targeted delivery of molecular imaging agents to tumors for improved diagnostic imaging and intraoperative imaging to guide surgical removal of cancerous cells; they can address drug resistance through the delivery of multiple drugs or complimentary treatments such as RNA interference [1].
Nanomedicines have been investigated for their application in anticancer therapies to improve drug delivery, increase the efficacy of treatment, reduce side effects, and overcome drug resistance. The number of studies published under the research topics of “nanomedicine”, “nanoscience” and “nanotechnology” has increased exponentially over the past decade. As more nanostructures were discovered and their potentials were better understood, the number of publications increased and reached its peak in 2011. Currently, the knowledge base of nanoparticles is still expanding with an emphasis on safety and efficacy [3].
CANCER AND CANCER’S THERAPY
Over the past twenty years the incidence of cancer has a gradually upward trend because of many factors like environment pollution, air, water and some chemical products. But a significant reduction in the death rates associated with cancer is due to the improvements of early detection and treatment.
However, the treatment strategy for cancer has remained essentially unchanged: surgical resection of the tumor followed by either chemotherapy, radiotherapy or a combination of the two, both of which often cause unselective damage to healthy tissue [1]. Additionally, treatment failure can be due to a number of factors, such as the presence of residual cells left after surgical removal of the tumor, resistance to chemotherapies, physiological obstructions to treatments such as the blood brain barrier [4], cellular barriers limiting access to drug targets, debilitating systemic toxicities, and poor bioavailability or poor pharmacokinetics of the chemotherapeutic [5].
So now, new therapies of cancer must be found to overcome the treatment failure in the other areas like the tumor microenvironment, cancer genomics, the evolution of metastasis and proteomics.
Nanotechnology is one of the most popular research areas, especially with regard to biomedical applications (in this article, it refers to cancer). Nanoparticles have very good applications in the form of targeted drug therapies and time-release drugs [6]. A potent dose of drugs could be delivered to a specific area but engineered to release over a planned period to ensure maximum effectiveness and the patient’s safety. The strong light absorbing properties of AuNPs makes it suitable as heat mediating objects; the absorbed light energy is dissipated into the surroundings of the particles’, generating an elevated temperature in their vicinity. This effect can be used to open polymer microcapsules, for example, for drug delivery purpose sand even destroys the cancerous cells. The nanoparticles are functionalized with antibody specific to the cancerous cells. The functionalized nanoparticles specifically bind with the targeting cells, which was then killed by hyper thermal therapy through heating the particle-loaded tissue [7]. However, for such in vivo applications, the potential cytotoxicity of the nanoparticles might become an issue and should be investigated with care.
However, the treatment strategy for cancer has remained essentially unchanged: surgical resection of the tumor followed by either chemotherapy, radiotherapy or a combination of the two, both of which often cause unselective damage to healthy tissue [1]. Additionally, treatment failure can be due to a number of factors, such as the presence of residual cells left after surgical removal of the tumor, resistance to chemotherapies, physiological obstructions to treatments such as the blood brain barrier [4], cellular barriers limiting access to drug targets, debilitating systemic toxicities, and poor bioavailability or poor pharmacokinetics of the chemotherapeutic [5].
So now, new therapies of cancer must be found to overcome the treatment failure in the other areas like the tumor microenvironment, cancer genomics, the evolution of metastasis and proteomics.
Nanotechnology is one of the most popular research areas, especially with regard to biomedical applications (in this article, it refers to cancer). Nanoparticles have very good applications in the form of targeted drug therapies and time-release drugs [6]. A potent dose of drugs could be delivered to a specific area but engineered to release over a planned period to ensure maximum effectiveness and the patient’s safety. The strong light absorbing properties of AuNPs makes it suitable as heat mediating objects; the absorbed light energy is dissipated into the surroundings of the particles’, generating an elevated temperature in their vicinity. This effect can be used to open polymer microcapsules, for example, for drug delivery purpose sand even destroys the cancerous cells. The nanoparticles are functionalized with antibody specific to the cancerous cells. The functionalized nanoparticles specifically bind with the targeting cells, which was then killed by hyper thermal therapy through heating the particle-loaded tissue [7]. However, for such in vivo applications, the potential cytotoxicity of the nanoparticles might become an issue and should be investigated with care.
NANOPARTICLE DELIVERY
Drug delivery
AuNP:Because of non-toxicity and non-immunogenicity, AuNPs is ideal for preparation of drug delivery scaffold. Functionalization property of AuNP also makes it an excellent potential vehicle for the drug delivery. Functionalized AuNP represent highly attractive and promising capacities in the applications of drug delivery.
Dong Nyoung Heo and his members recently describe in this study whether the gold Nanoparticle (AuNP) surface-functionalized with PEG, biotin, Paclitaxel (PTX) and rhodamine B linked beta-Cyclodextrin (b-CD) (AuNP-50) can be useful as a theranostic agent for cancer therapy without the cytotoxic effect on normal cells [8]. Aubin etc., recently developed drug delivery system with AuNPs and infrared light. This delivery system released multiple drugs in a controlled fashion. They demonstrated that nanoparticles of different shapes respond to different infrared wavelengths. For example, nanobones and nanocapsules melt at light wavelengths of 1100 and 800nm, respectively. Thus excitation at one wavelength could selectively melt one type of Aunanorods and selectively release one type of DNA strand [9]. Chen Wei-Hai and his members developed a novel strategy to construct a therapeutic system based on functionalized AuNPs which can specifically respond to tumor microenvironment was reported. Brown etc., also reported AuNPs for the improved anticancer drug delivery of the active component of oxaliplatin [10]. Naked AuNPs were functionalized with a thiolated Poly (Ethylene Glycol) (PEG) mono layer capped with a carboxylate group. [Pt (1R, 2R-diaminocyclohexane) (H2O)2]2NO3 was added to the PEG surface and yielding a supra molecular complex with drug molecules. The platinum-tethered nanoparticles showed significant improvement in cytotoxicity than oxaliplatin alone in all of the cell lines and an unusual ability to penetrate the nucleus in the lung cancer cells [7,11].
Due to biocompatibility, hyper thermal activity AuNPs find wide application now-a-days in killing of malignant cancerous cells [12].
Recently, Melancon and his team demonstrated the destruction of cancerous cell by photo thermal effect of AuNPs. The hollow gold nanoshells (HAuNS; average diameter, ∼30nm) were covalently attached to monoclonal antibody directed to the Epidermal Growth Factor Receptor (EGFR). The resulting anti-EGFR-HAuNS exhibited excellent colloidal stability and efficient photo thermal effect in the near-infrared region. Anti-EGFR-HAuNS then bound in EGFR-positive A431 tumor cells. Irradiation of A431 cells treated with anti-EGFR-HAuNS with near-infrared laser resulted in selective destruction of these cells [13].
AuNPs has also been applied to amplify the biorecognition of the anticancer drug [14]. Dacarbazine [5-(3, 3-dimethy-1-triazenyl) imidazole-4-carboxamide; DTIC] is a commonly used anticancer drug. AuNPs were stabilized by PPh3 with negative charge. The oxidized DTIC is positive charged. Thus, DTIC could be easily assembled onto the surface of AuNPs. The specific interactions between anticancer drug DTIC and DNA or DNA bases were facilitated by AuNPs.
In 2010, Kyuri Lee and his members modified the AnNPs surface with fluorescent dye labeled heparin molecules to detect a metastatic stage of cancer cells that over-express heparin-degrading enzymes. This kind of AnNPs was as a new class of theragnostic nanomaterials for metastatic cancer cell imaging and apoptosis [15].
The functionalized AuNPs with tumor-triggered drug release stealth behavior show a great potential in application for cancer therapy and diagnosis.Other nano-sized materials:From some researches, we know that more and more kinds of nanomaterials were used in the treatment of cancer and had promising prospect. And recently?nano-system was the research point, like pH-sensitive nano-systems, including advances in drug delivery, mechanisms of drug release, and possible improvements in drug absorption [16].
In 2013, a new nano-sized Hydroxyapatite (HAP) based drug-delivery system was successfully developed by Biswanath Kundua and his team with nano-sized Hydroxyapatite (HAp) (sizes 5-30nm) and synthesized with a Ca/P molar ratio of 1.67. After thorough in vitro characterization, these nano-HAP particles were loaded/intercalated with DOX (50-60% encapsulation efficiency), and thorough characterization of the size, shape and morphology of the particles was performed. The results indicate that this new formulation is an efficient, safe and reliable treatment method for HCC [17].
In 2014, Ye Wang and his co-workers reported a new nanocarrier called Anodic Alumina Nanotubes (AANTs) for potential cancer therapy. AANTs were electrochemically engineered by a unique pulse anodization process, which enables precise control of the nanotube geometry, and used here as nanocarriers for drug delivery [18].
While Jin-Zhi Du and his team recently found a kind of nanoparticle, pHe-activated NPs. And they put the special emphasis on pHe-activated surface charge reversal NPs, for drug and siRNA delivery to tumors [19].
Dong Nyoung Heo and his members recently describe in this study whether the gold Nanoparticle (AuNP) surface-functionalized with PEG, biotin, Paclitaxel (PTX) and rhodamine B linked beta-Cyclodextrin (b-CD) (AuNP-50) can be useful as a theranostic agent for cancer therapy without the cytotoxic effect on normal cells [8]. Aubin etc., recently developed drug delivery system with AuNPs and infrared light. This delivery system released multiple drugs in a controlled fashion. They demonstrated that nanoparticles of different shapes respond to different infrared wavelengths. For example, nanobones and nanocapsules melt at light wavelengths of 1100 and 800nm, respectively. Thus excitation at one wavelength could selectively melt one type of Aunanorods and selectively release one type of DNA strand [9]. Chen Wei-Hai and his members developed a novel strategy to construct a therapeutic system based on functionalized AuNPs which can specifically respond to tumor microenvironment was reported. Brown etc., also reported AuNPs for the improved anticancer drug delivery of the active component of oxaliplatin [10]. Naked AuNPs were functionalized with a thiolated Poly (Ethylene Glycol) (PEG) mono layer capped with a carboxylate group. [Pt (1R, 2R-diaminocyclohexane) (H2O)2]2NO3 was added to the PEG surface and yielding a supra molecular complex with drug molecules. The platinum-tethered nanoparticles showed significant improvement in cytotoxicity than oxaliplatin alone in all of the cell lines and an unusual ability to penetrate the nucleus in the lung cancer cells [7,11].
Due to biocompatibility, hyper thermal activity AuNPs find wide application now-a-days in killing of malignant cancerous cells [12].
Recently, Melancon and his team demonstrated the destruction of cancerous cell by photo thermal effect of AuNPs. The hollow gold nanoshells (HAuNS; average diameter, ∼30nm) were covalently attached to monoclonal antibody directed to the Epidermal Growth Factor Receptor (EGFR). The resulting anti-EGFR-HAuNS exhibited excellent colloidal stability and efficient photo thermal effect in the near-infrared region. Anti-EGFR-HAuNS then bound in EGFR-positive A431 tumor cells. Irradiation of A431 cells treated with anti-EGFR-HAuNS with near-infrared laser resulted in selective destruction of these cells [13].
AuNPs has also been applied to amplify the biorecognition of the anticancer drug [14]. Dacarbazine [5-(3, 3-dimethy-1-triazenyl) imidazole-4-carboxamide; DTIC] is a commonly used anticancer drug. AuNPs were stabilized by PPh3 with negative charge. The oxidized DTIC is positive charged. Thus, DTIC could be easily assembled onto the surface of AuNPs. The specific interactions between anticancer drug DTIC and DNA or DNA bases were facilitated by AuNPs.
In 2010, Kyuri Lee and his members modified the AnNPs surface with fluorescent dye labeled heparin molecules to detect a metastatic stage of cancer cells that over-express heparin-degrading enzymes. This kind of AnNPs was as a new class of theragnostic nanomaterials for metastatic cancer cell imaging and apoptosis [15].
The functionalized AuNPs with tumor-triggered drug release stealth behavior show a great potential in application for cancer therapy and diagnosis.Other nano-sized materials:From some researches, we know that more and more kinds of nanomaterials were used in the treatment of cancer and had promising prospect. And recently?nano-system was the research point, like pH-sensitive nano-systems, including advances in drug delivery, mechanisms of drug release, and possible improvements in drug absorption [16].
In 2013, a new nano-sized Hydroxyapatite (HAP) based drug-delivery system was successfully developed by Biswanath Kundua and his team with nano-sized Hydroxyapatite (HAp) (sizes 5-30nm) and synthesized with a Ca/P molar ratio of 1.67. After thorough in vitro characterization, these nano-HAP particles were loaded/intercalated with DOX (50-60% encapsulation efficiency), and thorough characterization of the size, shape and morphology of the particles was performed. The results indicate that this new formulation is an efficient, safe and reliable treatment method for HCC [17].
In 2014, Ye Wang and his co-workers reported a new nanocarrier called Anodic Alumina Nanotubes (AANTs) for potential cancer therapy. AANTs were electrochemically engineered by a unique pulse anodization process, which enables precise control of the nanotube geometry, and used here as nanocarriers for drug delivery [18].
While Jin-Zhi Du and his team recently found a kind of nanoparticle, pHe-activated NPs. And they put the special emphasis on pHe-activated surface charge reversal NPs, for drug and siRNA delivery to tumors [19].
Delivery of interfering RNA (siRNA)
Using siRNA to treat cancer is a productive and promising approach and siRNA has shown great promise in vitro, but delivery in vivo is challenging because siRNA is negatively charged and prone to degradation. Successful siRNA therapy requires a carrier which is both nontoxic and non-immunogenic to deliver the siRNA to cells and to affect internalization into the cells for release into the cytosol, the site of action. A variety of delivery method shave been attempted to overcome these barriers, including polyplex and lipoplex formulations, polymeric nanoparticles and liposomal formulations. These methods have had only partial success due to difficulties releasing the siRNA in the cytosol, low knock down efficiency and, in the case of the liposomes, problems with reproducibility, manufacturing and drug administration. Nanoparticle delivery vehicles for siRNA have been shown to deliver functional siRNA to the cytosol, resulting in dose dependent knockdown on gene targets [20], and multiple promising nanotechnology platforms for siRNA delivery are under development.
In 2014, Jinjun Shi etc., present an NP platform with sustained siRNA-release properties, which can be self-assembled using biodegradable and biocompatible polymers and lipids. The hybrid lipid-polymer NPs showed excellent silencing efficacy and the temporal release of siRNA from the NPs continued for over one month. When tested on luciferase-expressed HeLa cells and A549 lung carcinoma cells after short-term transfection, the siRNA NPs showed greater sustained silencing activity than lipofectamine 2000-siRNA complexes. More importantly, the NP-mediated sustained silencing of Prohibitin 1 (PHB1) generates more effective tumor cell growth inhibition in vitro and in vivo than the lipofectamine complexes [21].
Since 2004, carbon nanomaterials were widely applied in gene therapy. Based on the Prato reaction, the cationic ammonium-functionalized carbon nanotubes could effectively condense oligodeoxy nucleotides for gene delivery [22]. The carbon nanotubes use a nanoneedle endocytosis-independent mechanism for cell membrane penetration and show little toxic effect on the treated cells [23,24].
King Sun Siu etc., demonstrate that a non-covalently functionalized SWCNT with IS/C for topical siRNA delivery was developed. This polymer has the potential for delivering siRNA in vitro and topically in vivo. They utilized this CNT for RNAi therapy was further tested with Braf siRNA on a melanoma model and observed significant tumor progression reduction in a 25 day interval [25].
In 2014, Jinjun Shi etc., present an NP platform with sustained siRNA-release properties, which can be self-assembled using biodegradable and biocompatible polymers and lipids. The hybrid lipid-polymer NPs showed excellent silencing efficacy and the temporal release of siRNA from the NPs continued for over one month. When tested on luciferase-expressed HeLa cells and A549 lung carcinoma cells after short-term transfection, the siRNA NPs showed greater sustained silencing activity than lipofectamine 2000-siRNA complexes. More importantly, the NP-mediated sustained silencing of Prohibitin 1 (PHB1) generates more effective tumor cell growth inhibition in vitro and in vivo than the lipofectamine complexes [21].
Since 2004, carbon nanomaterials were widely applied in gene therapy. Based on the Prato reaction, the cationic ammonium-functionalized carbon nanotubes could effectively condense oligodeoxy nucleotides for gene delivery [22]. The carbon nanotubes use a nanoneedle endocytosis-independent mechanism for cell membrane penetration and show little toxic effect on the treated cells [23,24].
King Sun Siu etc., demonstrate that a non-covalently functionalized SWCNT with IS/C for topical siRNA delivery was developed. This polymer has the potential for delivering siRNA in vitro and topically in vivo. They utilized this CNT for RNAi therapy was further tested with Braf siRNA on a melanoma model and observed significant tumor progression reduction in a 25 day interval [25].
DRUG RESISTANCE
Mechanisms of drug resistance
Multidrug Resistance (MDR) is the term used to describe the resistance of cancer to related and unrelated classes of chemotherapeutic drugs and is currently one of the biggest challenges to overcome. Initially, patients may have either a partial or complete response to the first line of treatment but eventually exhibit cancer progression or recurrence. With repeated treatment, tumors often become resistant not only to the specific chemotherapeutic agent being employed, but cross-resistant to both similar and structurally unrelated classes of cytotoxic drugs [26-28].
The drug resistance of cancer stem cells
Cancer stem cells, also known as tumor initiating cells, are cells that have the capacity to self-renew and to give rise to the heterogeneous lineages that are found within a tumor [29]. Evidence for cancer stem cells dates back to 1971 when it was shown that only 1 in 100 to 1 in 10,000 mouse myeloma cells were able to form colonies [30]. This was confirmed 6 years later in humans when only 1 in 1000 to 1 in 5000 lung cancer, ovarian cancer, or neuroblastoma cells formed colonies in soft agar [31]. However, a fundamental question on whether all cancer cells had a low probability to behave as stem cells or if only a small subset had the ability to proliferate rapidly and form tumors remained [32]. One essential study to answer this question was performed in a group of Acute Myeloid Leukemia (AML) patients in 1997. Dick et al., showed that only a very small subset of cells that were CD34+ CD38- had the ability to cause AML in NOD/SCID mice, indicating that subpopulations of cells had differential abilities to proliferate and transfer disease [33]. Tumor initiating cells were later isolated in breast carcinomas, and it was shown that only a subset of cells could be serially passed and gave rise to both phenotypically identical and diverse cells consistent with those found in the initial tumor [34]. Cancer stem cells have also been isolated from medulloblastoma, glioblastoma [35], ependymoma [36], colon cancer [37,38], chronic and acute myeloid leukemia [39], pancreatic cancer [40], and head and neck squamous cell carcinomas [41]. However, the origins of these CSC’s, i.e., from normal stem cells versus progenitor cells, is still not clear and may vary from tumor to tumor or by tumor type [41]. One key feature of CSC’s is the role that they play in resistance to therapy and recurrence. Because chemotherapeutic drugs typically affect frequently dividing cells, CSC’s, which are primarily quiescent and have active DNA repair mechanisms, are not harmed. They also express high levels of specific ABC drug transporters, namely ABCB1, ABCG2, and ABCC1, which are known MDR genes in tumor cells, allowing for increased survival. Whereas chemotherapy may be effective against committed tumor cells, the resistant CSC’s may survive and repopulate the tumor with self-renewing cells and variably differentiated offspring, as shown in (Figure 1) [42]. The ability to eradicate these CSC’s with specific drugs is crucial to prevent tumor repopulation and recurrence.
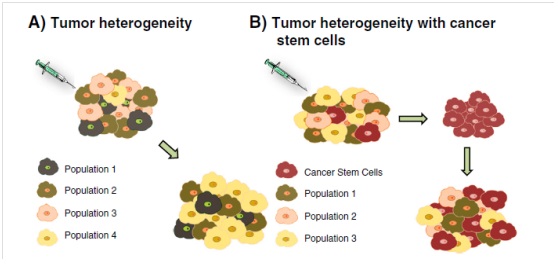
Figure 1: Two alternate drug resistance mechanisms.
A: A heterogeneous population of cancer cells is shown in the top left. Following administration of treatment, Population 3 is completely eliminated while a subpopulation, Population 4, emerges as a dominant clone (bottom right.
B: A heterogeneous population of cancer cells, including cancer stem cells, is shown in the top left. After administration of treatment, only the resistant cancer stem cells are seen (top right). After time, the cancer stem cells are able to repopulate the tumor with all previous cell populations present before treatment (bottom right) [42].

Figure 1: Two alternate drug resistance mechanisms.
A: A heterogeneous population of cancer cells is shown in the top left. Following administration of treatment, Population 3 is completely eliminated while a subpopulation, Population 4, emerges as a dominant clone (bottom right.
B: A heterogeneous population of cancer cells, including cancer stem cells, is shown in the top left. After administration of treatment, only the resistant cancer stem cells are seen (top right). After time, the cancer stem cells are able to repopulate the tumor with all previous cell populations present before treatment (bottom right) [42].
The nano-drug delivery mechanisms’ potential to treat cancers
The medicine or materials in nanosize have the potential to solve the drug resistance problem. There are some examples to support it.
Hua Guo etc., prepared a pH-sensitive pullulan-based nanoparticle carrier for ADR and investigated its reversal effect against drug-resistance of MCF-7/ADR cells. The results showed that URPA nanoparticles possessed high ADR loading capability and exhibited pH-sensitive in vitro ADR release. In addition, URPA nanoparticles significantly enhanced the cellular uptake of ADR inMCF-7/ADR cells and delivered ADR to the cell nucleus by avoiding the export effect of P-gp, thus could effectively overcome the drug resistance of MCF-7/ADR cells [43].
The development of Multidrug Resistance (MDR) in cancer cells is one of major obstacles to the effective cancer chemotherapy. Then the nano-delivery systems have emerged as the novel cancer therapeutics to overcome MDR. Thierry and his co-workers developed DOX-encapsulate liposomes with Cardiolipin/Phosphatidylcholine/Cholesterol (CL/PC/CHOL) and demonstrated that both DOX-encapsulated liposomes and free DOX spiked into a suspension of empty liposomes could reverse MDR and had comparable activity in MDR Chinese hamster LZ cells [44]. Qiu and co-workers designed a delivery system of novel β-CD-centered star-shaped amphiphilic polymers (sPEL/CD) for DOX [45]. Limin Pan and his co-workers reported the effective circumvention of multidrug resistance in cancer cells by an active nuclear-targeted drug delivery system that was constructed by conjugating TAT peptide onto the surface of Mesoporous Silica Nanoparticles (MSNs-TAT) [46]. They found an effective strategy for overcoming the multidrug resistance of cancer cells (MCF-7/ADR cells) by the direct drug (DOX) delivery into the nuclei using a TAT-conjugated MSNs based DDS.
Hua Guo etc., prepared a pH-sensitive pullulan-based nanoparticle carrier for ADR and investigated its reversal effect against drug-resistance of MCF-7/ADR cells. The results showed that URPA nanoparticles possessed high ADR loading capability and exhibited pH-sensitive in vitro ADR release. In addition, URPA nanoparticles significantly enhanced the cellular uptake of ADR inMCF-7/ADR cells and delivered ADR to the cell nucleus by avoiding the export effect of P-gp, thus could effectively overcome the drug resistance of MCF-7/ADR cells [43].
The development of Multidrug Resistance (MDR) in cancer cells is one of major obstacles to the effective cancer chemotherapy. Then the nano-delivery systems have emerged as the novel cancer therapeutics to overcome MDR. Thierry and his co-workers developed DOX-encapsulate liposomes with Cardiolipin/Phosphatidylcholine/Cholesterol (CL/PC/CHOL) and demonstrated that both DOX-encapsulated liposomes and free DOX spiked into a suspension of empty liposomes could reverse MDR and had comparable activity in MDR Chinese hamster LZ cells [44]. Qiu and co-workers designed a delivery system of novel β-CD-centered star-shaped amphiphilic polymers (sPEL/CD) for DOX [45]. Limin Pan and his co-workers reported the effective circumvention of multidrug resistance in cancer cells by an active nuclear-targeted drug delivery system that was constructed by conjugating TAT peptide onto the surface of Mesoporous Silica Nanoparticles (MSNs-TAT) [46]. They found an effective strategy for overcoming the multidrug resistance of cancer cells (MCF-7/ADR cells) by the direct drug (DOX) delivery into the nuclei using a TAT-conjugated MSNs based DDS.
Specific resistance mechanisms overcome by nanomedicine
Prevention of the cross talk between cancer cells and supporting stroma and vasculature, which promotes cell growth and prevents apoptosis, is an attractive strategy for overcoming resistance to therapy [47].
Fei Peng and his co-workers demonstrated a kind of Silicon Nanowires (SiNWs)-based drug nanocarriers (SiNW-DOX), which is high-efficacy for treatment of drug-resistant cancer cells. Typically, drug-resistance cancer cells (e.g., MCF-7/ADR cells) can be significantly inhibited by the SiNWs based nanocarriers, exhibiting ~10% cell viability during 72-h incubation with the SiNWs-DOX (80μgmL-1 DOX), which is in sharp contrast to free DOX-treated cells preserving ~40% cell viability. Remarkably, the RF value of SiNW-DOX is as low as ~2.0, which is much better than that (~300) of free DOX under the same experiment conditions [48].
Huihui Bu and his co-workers developed a TPGS incorporating Nanoemulsion of Paclitaxel (NE-PTX) to circumvent the drug resistance in breast cancer. The severe PTX resistance in resistant MCF-7/ADR cells was greatly reduced by NE-PTX, which could mainly result from the inhibition of P-gp activity, enhancement of anti-cancer activity and improvement of drug concentration in tumor tissue [49].
Fei Peng and his co-workers demonstrated a kind of Silicon Nanowires (SiNWs)-based drug nanocarriers (SiNW-DOX), which is high-efficacy for treatment of drug-resistant cancer cells. Typically, drug-resistance cancer cells (e.g., MCF-7/ADR cells) can be significantly inhibited by the SiNWs based nanocarriers, exhibiting ~10% cell viability during 72-h incubation with the SiNWs-DOX (80μgmL-1 DOX), which is in sharp contrast to free DOX-treated cells preserving ~40% cell viability. Remarkably, the RF value of SiNW-DOX is as low as ~2.0, which is much better than that (~300) of free DOX under the same experiment conditions [48].
Huihui Bu and his co-workers developed a TPGS incorporating Nanoemulsion of Paclitaxel (NE-PTX) to circumvent the drug resistance in breast cancer. The severe PTX resistance in resistant MCF-7/ADR cells was greatly reduced by NE-PTX, which could mainly result from the inhibition of P-gp activity, enhancement of anti-cancer activity and improvement of drug concentration in tumor tissue [49].
BIOMARKER
Biomarkers are another area of high interest in cancer research where nanotechnology could have great performance. Biomarkers are used as a mechanism to non-invasively identify, diagnose and characterize disease states in order to improve treatment selection and outcomes for patients. Many different categories of biomarkers have been identified such as metabolites, peptides, proteins, cell-free nucleic acids, exosomes and circulating tumor cells [50-57]. However, these biomarkers tend to circulate in low concentrations [53,58,59] and often only combinations of multiple biomarkers have predictive value, making the development of highly sensitive devices capable of simultaneous measurement of multiple markers necessary [60]. For endogenous biomarkers, there are also innate barriers to clinical utility, including very low production in early stage disease [53,58,59] and serious issues with sample to sample variability, sample collection variability, the complex composition of protein biomarkers in plasma, and the rapid degradation of these biomarker in vivo [57]. This suggests there would be value in developing new ways to identify alternative endogenous biomarkers or in designing new approaches to biomarkers, such as creation of synthetic or exogenous markers [61]. Recently, a report reported that Cpg Island Methylator Phenotype (CIMP) was a novel biomarker for clinical classification of breast cancer.
CONCLUSION AND FUTURE PROSPECT
Over the past few years, a great progress has been made in treatment of cancer.
In this review, we describe some specific methods that can improve the influence of nanotechnology on cancer treatment, especially in the drug delivery, drug resistance and biomarker. AuNPs are widely used as nanocarriers that have been tested for a number of cancer treatment applications, including drug and gene delivery and photo thermal therapy. And many nanoparticles can help deliver siRNA for the treatment of cancer.
Despite these successes in the field of nanotechnological cancer treatment, there are unique difficulties that need to be overcome to accelerate translation of this research to clinical applications. Although nano-delivery systems are promising in cancer therapy, there remain many challenges for these systems. Further innovative ideas will need to be developed and explored to develop an in vivo-stable, bio-safe, multi-drug carrier system that has the ability to deliver simultaneously both chemotherapy and siRNA agents to cancer targets, and release these compounds in a controlled manner at two different timings upon cellular internalization, for producing a maximum effect of the combination therapy for treatment of drug resistant tumors.
Looking into the future, some considerations or directions require a concerted effort for success for scientists in developing nanotechnology cancer therapeutics. The first direction is the optimal design of nanoparticles to be disease-specific [62]. The second direction is making the best use of nanomaterials physico-chemical properties to treat cancers. The last direction is to emerge new concepts from interdisciplinary collaborations to design nanotechnology with multiple functions, more than those functions mentioned above.
Nevertheless, the future of nanotechnology application in cancer therapy is exciting and will certainly help to relieve the pain of patient’s.
In this review, we describe some specific methods that can improve the influence of nanotechnology on cancer treatment, especially in the drug delivery, drug resistance and biomarker. AuNPs are widely used as nanocarriers that have been tested for a number of cancer treatment applications, including drug and gene delivery and photo thermal therapy. And many nanoparticles can help deliver siRNA for the treatment of cancer.
Despite these successes in the field of nanotechnological cancer treatment, there are unique difficulties that need to be overcome to accelerate translation of this research to clinical applications. Although nano-delivery systems are promising in cancer therapy, there remain many challenges for these systems. Further innovative ideas will need to be developed and explored to develop an in vivo-stable, bio-safe, multi-drug carrier system that has the ability to deliver simultaneously both chemotherapy and siRNA agents to cancer targets, and release these compounds in a controlled manner at two different timings upon cellular internalization, for producing a maximum effect of the combination therapy for treatment of drug resistant tumors.
Looking into the future, some considerations or directions require a concerted effort for success for scientists in developing nanotechnology cancer therapeutics. The first direction is the optimal design of nanoparticles to be disease-specific [62]. The second direction is making the best use of nanomaterials physico-chemical properties to treat cancers. The last direction is to emerge new concepts from interdisciplinary collaborations to design nanotechnology with multiple functions, more than those functions mentioned above.
Nevertheless, the future of nanotechnology application in cancer therapy is exciting and will certainly help to relieve the pain of patient’s.
ACKNOWLEDGEMENTS
The authors acknowledge the financial supports from the National Basic Research Program of China (973 Program, No. 2011CB710901), the National Natural Science Foundation of China (No. 31370959), Fok Ying Tong Education Foundation (No. 141039), Beijing Natural Science Foundation (No. 7142094), Program for New Century Excellent Talents (NCET) in University from Ministry of Education of China, State Key Laboratory of New Ceramic and Fine Processing (Tsinghua University), International Joint Research Center of Aerospace Biotechnology and Medical Engineering, Ministry of Science and Technology of China, and the 111 Project (No. B13003).
REFERENCES
- Hull LC, Farrell D, Grodzinski P (2014) Highlights of recent developments and trends in cancer nanotechnology research--view from NCI Alliance for Nanotechnology in Cancer. Biotechnol Adv 32: 666-678.
- Koutsopoulos S (2012) Molecular fabrications of smart nanobiomaterials and applications in personalized medicine. Adv Drug Deliv Rev 64: 1459-1476.
- Markman JL, Rekechenetskiy A, Holler E, Ljubimova JY (2013) Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv Drug Deliv Rev 65: 1866-1879.
- Alexis F, Pridgen EM, Langer R, Farokhzad OC (2010) Nanoparticle technologies for cancer therapy. Handb Exp Pharmacol 55-86.
- Zamboni WC, Torchilin V, Patri AK, Hrkach J, Stern S, et al. (2012) Best practices in cancer nanotechnology: perspective from NCI nanotechnology alliance. Clin Cancer Res 18: 3229-3241.
- Ghosh P, Han G, De M, Kim CK, Rotello VM (2008) Gold nanoparticles in delivery applications. Adv Drug Deliv Rev 60: 1307-1315.
- Nath D, Banerjee P (2013) Green nanotechnology - a new hope for medical biology. Environ Toxicol Pharmacol 36: 997-1014.
- Heo DN, Yang DH, Moon HJ, Lee JB, Bae MS, et al. (2012) Gold nanoparticles surface-functionalized with paclitaxel drug and biotin receptor as theranostic agents for cancer therapy. Biomaterials 33: 856-866.
- Aubin-Tam ME, Hamad-Schifferli K (2008) Structure and function of nanoparticle-protein conjugates. Biomed Mater 3: 034001.
- Chen WH, Xu XD, Jia HZ, Lei Q, Luo GF, et al. (2013) Therapeutic nanomedicine based on dual-intelligent functionalized gold nanoparticles for cancer imaging and therapy in vivo. Biomaterials 34: 8798-8807.
- Brown SD, Nativo P, Smith JA, Stirling D, Edwards PR, et al. (2010) Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J Am Chem Soc 132: 4678-4684.
- Dickerson EB, Dreaden EC, Huang X, El-Sayed IH, Chu H, et al. (2008) Gold nanorod assisted near-infrared Plasmonic Photothermal Therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett 269: 57-66.
- Melancon MP, Lu W, Yang Z, Zhang R, Cheng Z, et al. (2008) In vitro and in vivo targeting of hollow gold nanoshells directed at epidermal growth factor receptor for photothermal ablation therapy. Mol Cancer Ther 7: 1730-1739.
- Shen Q, Wang X, Fu D (2008) The amplification effect of functionalized gold nanoparticles on the binding of cancer drug decarbazine to DNA and DNA bases. Applied Surface Science 255: 577-580.
- Lee K, Lee HJ, Bae KH, Park TG (2010) Heparin immobilized gold nanoparticles for targeted detection and apoptotic death of metastatic cancer cells. Biomaterials 31: 6530-6536.
- Liu J, Huang Y, Kumar A, Tan A, Jin S, et al. (2014) pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol Adv 32: 693-710.
- Biswanath Kundu, Debasree Ghosh, Mithlesh Kumar Sinha, Partha Sarathi Sen, VamsiKrishna Balla, et al. (2013) Doxorubicin-intercalated nano-hydroxyapatite drug-delivery system for liver cancer: An animal model. Ceramics International 39: 9557-9566.
- Wang Y, Santos A, Kaur G, Evdokiou A, Losic D (2014) Structurally engineered anodic alumina nanotubes as nano-carriers for delivery of anticancer therapeutics. Biomaterials 35: 5517-5526.
- Du JZ, Mao CQ, Yuan YY, Yang XZ, Wang J (2014) Tumor extracellular acidity-activated nanoparticles as drug delivery systems for enhanced cancer therapy. Biotechnol Adv 32: 789-803.
- Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, et al. (2010) Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464: 1067-1070.
- Shi J, Xu Y, Xu X, Zhu X, Pridgen E, et al. (2014) Hybrid lipid-polymer nanoparticles for sustained siRNA delivery and gene silencing. Nanomedicine 10: 897-900.
- Bianco A, Hoebeke J, Godefroy S, Chaloin O, Pantarotto D, et al. (2005) Cationic carbon nanotubes bind to CpG oligodeoxynucleotides and enhance their immunostimulatory properties. J Am Chem Soc 127: 58-59.
- Cheung W, Pontoriero F, Taratula O, Chen AM, He H (2010) DNA and carbon nanotubes as medicine. Adv Drug Deliv Rev 62: 633-649.
- Singh R, Pantarotto D, McCarthy D, Chaloin O, Hoebeke J, et al. (2005) Binding and condensation of plasmid DNA onto functionalized carbon nanotubes: toward the construction of nanotube-based gene delivery vectors. J Am Chem Soc 127: 4388-4396.
- Siu KS, Chen D, Zheng X, Zhang X, Johnston N, et al. (2014) Non-covalently functionalized single-walled carbon nanotube for topical siRNA delivery into melanoma. Biomaterials 35: 3435-3442.
- Xue X, Liang XJ (2012) Overcoming drug efflux-based multidrug resistance in cancer with nanotechnology. Chin J Cancer 31: 100-109.
- Dong X, Mumper RJ (2010) Nanomedicinal strategies to treat multidrug-resistant tumors: current progress. Nanomedicine (Lond) 5: 597-615.
- Shapira A, Livney YD, Broxterman HJ, Assaraf YG (2011) Nanomedicine for targeted cancer therapy: towards the overcoming of drug resistance. Drug Resist Updat 14: 150-163.
- MF Clarke, JE Dick, PB Dirks, CJ Eaves, CH Jamieson, et al. (2006) Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 66: 9339-9344.
- Park CH, Bergsagel DE, McCulloch EA (1971) Mouse myeloma tumor stem cells: a primary cell culture assay. J Natl Cancer Inst 46: 411-422.
- Hamburger AW, Salmon SE (1977) Primary bioassay of human tumor stem cells. Science 197: 461-463.
- Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414: 105-111.
- Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3: 730-737.
- Marsden CG, Wright MJ, Pochampally R, Rowan BG (2009) Breast tumor-initiating cells isolated from patient core biopsies for study of hormone action. Methods Mol Biol 590: 363-375.
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, et al. (2004) Identification of human brain tumour initiating cells. Nature 432: 396-401.
- Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, et al. (2005) Radial glia cells are candidate stem cells of ependymoma. Cancer Cell 8: 323-335.
- O'Brien CA, Pollett A, Gallinger S, Dick JE (2007) A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445: 106-110.
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, et al. (2007) Identification and expansion of human colon-cancer-initiating cells. Nature 445: 111-115.
- Hope KJ, Jin L, Dick JE (2004) Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol 5: 738-743.
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, et al. (2007) Identification of pancreatic cancer stem cells. Cancer Res 67: 1030-1037.
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, et al. (2007) Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA 104: 973-978.
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646-674.
- Guo H, Liu Y, Wang Y, Wu J, Yang X, et al. (2014) pH-sensitive pullulan-based nanoparticle carrier for adriamycin to overcome drug-resistance of cancer cells. Carbohydr Polym 111: 908-917.
- Thierry AR, Vigé D, Coughlin SS, Belli JA, Dritschilo A, et al. (1993) Modulation of doxorubicin resistance in multidrug-resistant cells by liposomes. FASEB J 7: 572-579.
- Qiu LY, Wang RJ, Zheng C, Jin Y, Jin le Q (2010) Beta-cyclodextrin-centered star-shaped amphiphilic polymers for doxorubicin delivery. Nanomedicine (Lond) 5: 193-208.
- Pan L, Liu J, He Q, Wang L, Shi J (2013) Overcoming multidrug resistance of cancer cells by direct intranuclear drug delivery using TAT-conjugated mesoporous silica nanoparticles. Biomaterials 34: 2719-2730.
- Burger JA, Peled A (2009) CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia 23: 43-52.
- Peng F, Su Y, Ji X, Zhong Y, Wei X, et al. (2014) Doxorubicin-loaded silicon nanowires for the treatment of drug-resistant cancer cells. Biomaterials 35: 5188-5195.
- Bu H, He X, Zhang Z, Yin Q, Yu H, et al. (2014) A TPGS-incorporating nanoemulsion of paclitaxel circumvents drug resistance in breast cancer. Int J Pharm 471: 206-213.
- Findeisen P, Neumaier M (2012) Functional protease profiling for diagnosis of malignant disease. Proteomics Clin Appl 6: 60-78.
- Hanash SM, Pitteri SJ, Faca VM (2008) Mining the plasma proteome for cancer biomarkers. Nature 452: 571-579.
- Moon PG, You S, Lee JE, Hwang D, Baek MC (2011) Urinary exosomes and proteomics. Mass Spectrom Rev 30: 1185-1202.
- Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, et al. (2007) Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450: 1235-1239.
- Sawyers CL (2008) The cancer biomarker problem. Nature 452: 548-552.
- Schwarzenbach H, Hoon DS, Pantel K (2011) Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 11: 426-437.
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, et al. (2009) Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 457: 910-914.
- Surinova S, Schiess R, Hüttenhain R, Cerciello F, Wollscheid B, et al. (2011) On the development of plasma protein biomarkers. J Proteome Res 10: 5-16.
- Hori SS, Gambhir SS (2011) Mathematical model identifies blood biomarker-based early cancer detection strategies and limitations. Sci Transl Med 3: 109ra116.
- Lutz AM, Willmann JK, Cochran FV, Ray P, Gambhir SS (2008) Cancer screening: a mathematical model relating secreted blood biomarker levels to tumor sizes. PLoS Med 5: 170.
- Alhasan AH, Kim DY, Daniel WL, Watson E, Meeks JJ, et al. (2012) Scano metric microRNA array profiling of prostate cancer markers using spherical nucleic acid-gold nanoparticle conjugates. Anal Chem 84: 4153-4160.
- Kwong GA, von Maltzahn G, Murugappan G, Abudayyeh O, Mo S, et al. (2013) Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nat Biotechnol 31: 63-70.
- Gao Y, Xie J, Chen H, Gu S, Zhao R, et al. (2014) Nanotechnology-based intelligent drug design for cancer metastasis treatment. Biotechnol Adv 32: 761-777.
Citation: Li X, Wang Z, Fan Y, Feng Q, Cui FZ, et al. (PIC) The Use of Nanotechnology to Treat Cancers. J Nanotechnol Nanomed Nanobiotechnol, 1: 002.
Copyright: © 2014 Xiaoming Li, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Journal Highlights
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!
