
The Utility of the CardioMEMS Device in Pediatrics: A Case of Innovation and Compassion Studied 4 Years after Device Implantation for Remote Pulmonary Artery Monitoring
*Corresponding Author(s):
Federico Gutiérrez-Larraya AguadoPediatric Cardiology Service, La Paz University Hospital, Madrid, Spain
Email:flarraya@yahoo.es
Introduction
Advanced heart failure and pulmonary hypertension significantly compromise patients' quality of life. This has led to the development of technologies such as the CardioMEMS device for their management. Although its use is widely approved for adults with advanced heart failure [1], its application in pediatrics [2], especially outside conventional heart failure scenarios [3,4], is innovative and considered compassionate due to the lack of approval by regulatory entities such as the FDA and CE marking for this age group. On the other hand, cardiac catheterizations in pediatric patients, especially diagnostic procedures to assess pulmonary pressures, are not without risks [5].
Case Study
We present the case of a 4-year-old child (17.3 kg) born with a congenital aortic stenosis and a failed Ross procedure because of intraoperative acute myocardial infarction and severe left ventricular dysfunction so “A left ventricular assist device (LVAD)” (Berlin Heart®) was implanted in June 2020. The CardioMEMS device was implanted while the patient was supported by the LVAD in October 2020. The device aimed to remotely monitor pulmonary artery pressure as the patient awaited a heart transplant under severe heart failure management, which included extensive pharmacological treatment and had been complicated by three previous catheterizations. After detailed discussions with the care team and explaining the situation to the parents, the recommendation for compassionate implantation of the CardioMEMS device was made. Despite initial displacement due to extensive manipulation of the thorax during heart transplantation (including a right diaphrama plication for a palsy), there was no migration to another pulmonary branch, and the device continued to transmit accurate data, facilitating detailed monitoring of the patient's status.
Methodology And Results
Catheterization was naturally indicated for managing his condition. We first performed an ultrasonic evaluation of his femoral veins to ensure they had at least a 3 mm axial diameter. After securing vascular access, angiographic verification of the right femoral vein patency and diameter was performed. We conducted a standard diagnostic hemodynamic catheterization under fluoroscopic control in a biplane system (Artis Zee, Siemens®) with a low dose protocol. Then, pump injection with diluted contrast (0.5 ml of isosmolar contrast per kg of body weight, diluted with isotonic saline solution in a 70% contrast to 30% saline ratio) into the left pulmonary artery was achieved with biplanar acquisition at 15 images per second (Figures 1 & 2). This process was to examine the left lung pulmonary arterial architecture to find an ideal implantation site, requiring a length of at least 30 mm with initial and final diameters of 7 and 5 mm, respectively, and a bifurcation pattern showing branches larger than 3 mm in diameter. Once the target site was selected, the angiographic catheter was exchanged over a conventional 0.035 guidewire, which served as a support for successive dilations of the venous access point until the 12 F introducer advanced without resistance. A multipurpose 5F catheter was then advanced over the guidewire, followed by a 0.35 hydrophilic guidewire to position the catheter in the selected segment. After removing the hydrophilic guidewire, a small manual contrast injection was performed to evaluate the distal anatomy before advancing a 0.018” guidewire through the catheter, ensuring the floppy segment did not distort or angle and that a non-floppy portion was present at the device implantation site. With these considerations meticulously observed, and the catheter removed, the catheter housing the CardioMEMS was advanced, ensuring smooth and natural progression until reaching the destination and proceeding with its release. Following this, the catheter was removed, and the previous multipurpose catheter was reinserted to a position proximal to the device's release point. A manual contrast injection was then performed through the catheter via a Y-connector without removing the guidewire, and biplanar images were acquired to assess the occupied volume of the device, its loops, and any potential interference with the distal filling of that branch or collateral vessels. High-quality pulmonary pressures were obtained through CardioMEMS®, so the procedure was concluded without any problems related to the device or vascular access. Successful monitoring of the pulmonary pressures facilitated the management of this challenging patient, and a heart transplant was successfully performed 13 months later. The surgery required extensive manipulation of the patient's thorax, which, as evidenced in radiographs taken in the following days, led to displacement of the device that did not interfere with measurements. The patient underwent subsequent catheterizations for post-transplant protocolized control without complications in vascular access, performed through the same right femoral vein. On two occasions, an excellent correlation was observed between the invasive measurements of pulmonary pressures and the results from the CardioMEMS check. During a recent catheterization, within the regular control indications with the patient weighing 28 kg, this correlation was confirmed, and an angiography was performed on the left pulmonary branch showing the arterial vascular architecture (Figures 3 & 4). Despite the displaced device, there is no interference or obstruction to the permeability of the lobar and sub segmental branches in angiography nor in pressures distal or proximal and an excellent correlation was obtained between invasive (Figure 5) and CardioMEMS® measurements (Figure 6). The procedure confirmed the permeability of the pulmonary branches, reaffirming the utility of the device for continuous and non-invasive monitoring in a pediatric context.
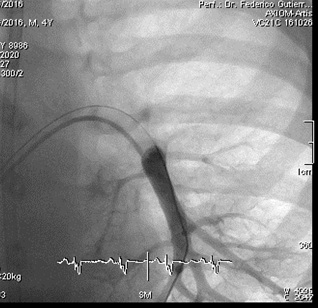 Figure 1: Posteroanterior angiography of left inferior lobar pulmonary artery pre-CardioMEMS® Implantation.
Figure 1: Posteroanterior angiography of left inferior lobar pulmonary artery pre-CardioMEMS® Implantation.
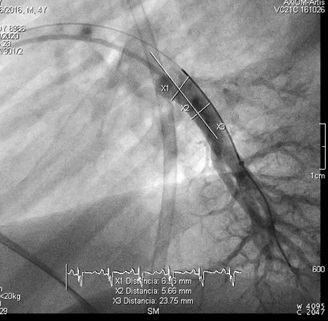 Figure 2: Lateral angiography of left inferior lobar pulmonary artery pre-CardioMEMS® Implantation.
Figure 2: Lateral angiography of left inferior lobar pulmonary artery pre-CardioMEMS® Implantation. 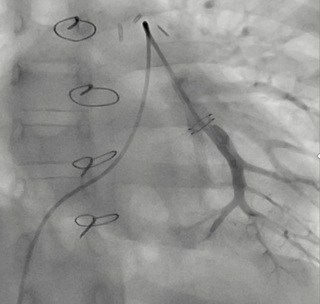
Figure 3: Posteroanterior angiography of left inferior lobar pulmonary artery 4 years post-CardioMEMS® Implantation.
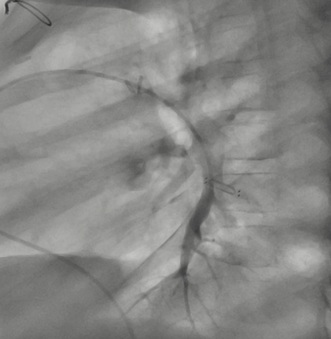 Figure 4: Lateral angiography of left inferior lobar pulmonary artery 4 years post-CardioMEMS® Implantation.
Figure 4: Lateral angiography of left inferior lobar pulmonary artery 4 years post-CardioMEMS® Implantation.
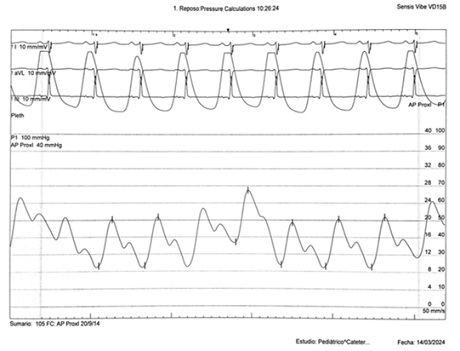 Figure 5: Invasive left pulmonary artery trace 4 years post-CardioMEMS® Implantation.
Figure 5: Invasive left pulmonary artery trace 4 years post-CardioMEMS® Implantation.
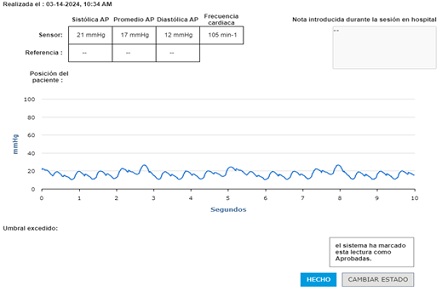 Figure 6: CardioMEMS® interrogation during catheterization 4 years post implantation.
Figure 6: CardioMEMS® interrogation during catheterization 4 years post implantation.
Discussion
This case illustrates the potential extension of the use of the CardioMEMS device beyond the populations and conditions for which it was originally designed, opening new avenues for the management of pediatric patients with complex cardiac conditions. The experience gained with this case suggests that, with the proper precautions and follow-up, the device can offer a valuable tool for monitoring and managing patients outside conventional indications, supporting clinical decision-making with accurate and updated data with no significant problems related to arterial obstruction.
Conclusion
The compassionate application of the CardioMEMS device in pediatrics represents a significant advancement in the care of patients with complex cardiac conditions, demonstrating that technological innovation, when applied thoughtfully and ethically, can transcend regulatory barriers to improve the quality of life for younger patients. This case underscores the importance of exploring new applications for existing technologies to fill gaps in.
References
- Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, et al. (2016) Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 387: 453-461.
- Orr WB, Colombo JN, Roberts B, Avari Silva JN, Balzer D (2022) Incorporation of the CardioMEMS™ System During an Exercise Physiology Test in a Pediatric Congenital Heart Disease Patient Contributing to Medical Decision-Making. Pediatric Cardiology. 43: 695-699.
- Assmus B, Angermann CE, Alkhlout B, Asselbergs FK, Schnupp S, et al. (2022) Effects of remote haemodynamic-guided heart failure management in patients with different subtypes of pulmonary hypertension: insights from the MEMS-HF study. Eur J of Heart Failure. 24: 2320-2330.
- Bradley EA, Jassal A, Moore-Clingenpeel M, Abrahama WT, Berman D, et al. (2019) Ambulatory Fontan pressure monitoring: Results from the implantable hemodynamic monitor Fontan feasibility cohort (IHM-FFC). Int J Cardiol. 284: 22-27.
- O’Byrne ML, Glatz AC, Hanna BD, Shinohara RT, Gillespie MJ, et al. (2015) Predictors of Catastrophic Adverse Outcomes in Children With Pulmonary Hypertension Undergoing Cardiac Catheterization: A Multi-Institutional Analysis From the Pediatric Health Information Systems Database. J Am Coll Cardiol. 66: 1261-1269.
Citation: Aguado FGL, de Lera CL, Pardeiro CA, Domingo EB, Romero AL (2024) The Utility of the CardioMEMS Device in Pediatrics: A Case of Innovation and Compassion Studied 4 Years after Device Implantation for Remote Pulmonary Artery Monitoring. J Clin Stud Med Case Rep 11:234
Copyright: © 2024 Federico Gutiérrez-Larraya Aguado, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

